Ionic liquid electrolytes for magnesium metal batteries
OCT 14, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Mg Battery Electrolyte Evolution and Research Objectives
The evolution of magnesium battery technology has been marked by significant challenges, particularly in developing suitable electrolytes that enable reversible magnesium deposition and stripping. Early research in the 1990s primarily focused on Grignard reagent-based electrolytes, which demonstrated the feasibility of rechargeable magnesium batteries but suffered from limited electrochemical stability and compatibility with cathode materials.
The 2000s witnessed a paradigm shift with the introduction of organohaloaluminate electrolytes by Aurbach and colleagues, which offered improved electrochemical performance. However, these systems were plagued by corrosivity, sensitivity to moisture, and narrow electrochemical windows, limiting practical applications. This period established fundamental understanding of the complex chemistry involved in magnesium electrodeposition processes.
From 2010 onwards, research diversified into non-nucleophilic electrolyte systems, including magnesium bis(trifluoromethanesulfonyl)imide (Mg(TFSI)2) based solutions and various boron-based complexes. These developments aimed to overcome the limitations of earlier generations while maintaining efficient magnesium transport properties.
The most recent evolution (2015-present) has seen increasing focus on ionic liquid-based electrolytes for magnesium batteries. Ionic liquids offer attractive properties including negligible volatility, high thermal stability, and wide electrochemical windows. The combination of magnesium salts with ionic liquids presents a promising direction for developing safer and more efficient electrolyte systems.
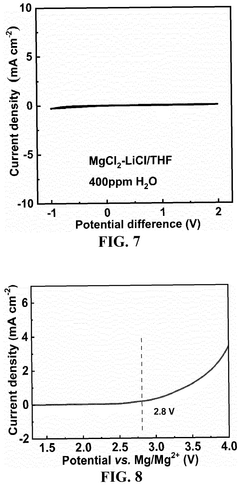
Current research objectives in this field are multifaceted. Primary goals include developing electrolytes with enhanced ionic conductivity (>5 mS/cm at room temperature) and wider electrochemical stability windows (>3V vs. Mg/Mg²⁺) to enable higher energy density systems. Researchers are also focused on improving the reversibility of magnesium deposition/dissolution processes to achieve coulombic efficiencies exceeding 99%.
Another critical objective is mitigating the formation of passivation layers on the magnesium anode surface, which has been a persistent challenge hindering practical applications. This requires fundamental understanding of interfacial phenomena and development of electrolyte formulations that form favorable solid-electrolyte interphases.
Additionally, research aims to enhance compatibility with high-voltage cathode materials, particularly those based on sulfur and oxygen, which offer theoretical energy densities comparable to or exceeding lithium-ion systems. This necessitates electrolytes that remain stable against both highly reducing magnesium metal anodes and oxidizing cathode environments.
Long-term objectives include developing electrolyte systems that enable practical magnesium batteries with energy densities >400 Wh/kg, cycle life >1000 cycles, and operational temperature ranges from -20°C to 60°C, positioning magnesium as a viable alternative to lithium-ion technology for next-generation energy storage applications.
The 2000s witnessed a paradigm shift with the introduction of organohaloaluminate electrolytes by Aurbach and colleagues, which offered improved electrochemical performance. However, these systems were plagued by corrosivity, sensitivity to moisture, and narrow electrochemical windows, limiting practical applications. This period established fundamental understanding of the complex chemistry involved in magnesium electrodeposition processes.
From 2010 onwards, research diversified into non-nucleophilic electrolyte systems, including magnesium bis(trifluoromethanesulfonyl)imide (Mg(TFSI)2) based solutions and various boron-based complexes. These developments aimed to overcome the limitations of earlier generations while maintaining efficient magnesium transport properties.
The most recent evolution (2015-present) has seen increasing focus on ionic liquid-based electrolytes for magnesium batteries. Ionic liquids offer attractive properties including negligible volatility, high thermal stability, and wide electrochemical windows. The combination of magnesium salts with ionic liquids presents a promising direction for developing safer and more efficient electrolyte systems.
Current research objectives in this field are multifaceted. Primary goals include developing electrolytes with enhanced ionic conductivity (>5 mS/cm at room temperature) and wider electrochemical stability windows (>3V vs. Mg/Mg²⁺) to enable higher energy density systems. Researchers are also focused on improving the reversibility of magnesium deposition/dissolution processes to achieve coulombic efficiencies exceeding 99%.
Another critical objective is mitigating the formation of passivation layers on the magnesium anode surface, which has been a persistent challenge hindering practical applications. This requires fundamental understanding of interfacial phenomena and development of electrolyte formulations that form favorable solid-electrolyte interphases.
Additionally, research aims to enhance compatibility with high-voltage cathode materials, particularly those based on sulfur and oxygen, which offer theoretical energy densities comparable to or exceeding lithium-ion systems. This necessitates electrolytes that remain stable against both highly reducing magnesium metal anodes and oxidizing cathode environments.
Long-term objectives include developing electrolyte systems that enable practical magnesium batteries with energy densities >400 Wh/kg, cycle life >1000 cycles, and operational temperature ranges from -20°C to 60°C, positioning magnesium as a viable alternative to lithium-ion technology for next-generation energy storage applications.
Market Analysis for Next-Generation Battery Technologies
The global battery market is experiencing a significant shift towards next-generation technologies, with magnesium metal batteries emerging as a promising alternative to conventional lithium-ion systems. The market for advanced battery technologies is projected to reach $240 billion by 2030, growing at a CAGR of 18% from 2023 to 2030, driven primarily by increasing demand for electric vehicles, renewable energy storage systems, and portable electronics.
Ionic liquid electrolytes for magnesium metal batteries represent a particularly dynamic segment within this market. Unlike traditional battery electrolytes, ionic liquids offer enhanced safety profiles, wider electrochemical windows, and improved thermal stability—attributes that address critical market demands for safer and more efficient energy storage solutions.
Market research indicates that the demand for magnesium-based battery technologies is accelerating due to several factors. First, magnesium is substantially more abundant than lithium, with reserves approximately 3,000 times greater in the Earth's crust. This abundance translates to potentially lower raw material costs and reduced supply chain vulnerabilities, addressing concerns about lithium resource constraints.
Second, the safety advantages of magnesium-based systems are attracting significant attention from automotive manufacturers and grid storage developers. With thermal runaway and fire risks representing major barriers to widespread adoption of current battery technologies, the inherent safety of magnesium systems could unlock new market segments previously hesitant to adopt battery storage solutions.
Regional market analysis reveals varying adoption patterns. Asia-Pacific currently dominates the research and development landscape for magnesium battery technologies, with China, Japan, and South Korea leading commercial development efforts. North America and Europe are rapidly expanding their research initiatives, particularly in the ionic liquid electrolyte domain, supported by substantial government funding programs aimed at reducing dependency on lithium supply chains.
Industry forecasts suggest that ionic liquid electrolytes for magnesium batteries could capture 12% of the next-generation battery electrolyte market by 2028, representing a significant opportunity for early movers in this technology space. This growth is expected to be particularly strong in applications requiring high safety standards and long cycle life, such as stationary energy storage and commercial electric vehicles.
Consumer electronics represents another promising market segment, with manufacturers seeking higher energy density solutions that can extend device operation times while maintaining strict safety requirements. The potential for magnesium metal batteries with ionic liquid electrolytes to deliver twice the energy density of current lithium-ion technologies positions them favorably in this competitive landscape.
Ionic liquid electrolytes for magnesium metal batteries represent a particularly dynamic segment within this market. Unlike traditional battery electrolytes, ionic liquids offer enhanced safety profiles, wider electrochemical windows, and improved thermal stability—attributes that address critical market demands for safer and more efficient energy storage solutions.
Market research indicates that the demand for magnesium-based battery technologies is accelerating due to several factors. First, magnesium is substantially more abundant than lithium, with reserves approximately 3,000 times greater in the Earth's crust. This abundance translates to potentially lower raw material costs and reduced supply chain vulnerabilities, addressing concerns about lithium resource constraints.
Second, the safety advantages of magnesium-based systems are attracting significant attention from automotive manufacturers and grid storage developers. With thermal runaway and fire risks representing major barriers to widespread adoption of current battery technologies, the inherent safety of magnesium systems could unlock new market segments previously hesitant to adopt battery storage solutions.
Regional market analysis reveals varying adoption patterns. Asia-Pacific currently dominates the research and development landscape for magnesium battery technologies, with China, Japan, and South Korea leading commercial development efforts. North America and Europe are rapidly expanding their research initiatives, particularly in the ionic liquid electrolyte domain, supported by substantial government funding programs aimed at reducing dependency on lithium supply chains.
Industry forecasts suggest that ionic liquid electrolytes for magnesium batteries could capture 12% of the next-generation battery electrolyte market by 2028, representing a significant opportunity for early movers in this technology space. This growth is expected to be particularly strong in applications requiring high safety standards and long cycle life, such as stationary energy storage and commercial electric vehicles.
Consumer electronics represents another promising market segment, with manufacturers seeking higher energy density solutions that can extend device operation times while maintaining strict safety requirements. The potential for magnesium metal batteries with ionic liquid electrolytes to deliver twice the energy density of current lithium-ion technologies positions them favorably in this competitive landscape.
Ionic Liquid Electrolytes: Current Status and Technical Barriers
Despite significant advancements in ionic liquid (IL) electrolytes for magnesium metal batteries, several critical technical barriers continue to impede their widespread commercial adoption. The primary challenge remains the formation of a passivation layer on the magnesium anode surface, which significantly hinders Mg2+ ion transport and leads to high interfacial resistance. Unlike lithium-ion systems, this passivation layer in magnesium batteries is typically non-conductive for Mg2+ ions, severely limiting reversible plating and stripping processes.
Ionic liquids, while offering excellent thermal stability and wide electrochemical windows, often exhibit prohibitively high viscosity, particularly at room temperature. This high viscosity directly translates to reduced ionic conductivity, typically ranging from 10^-4 to 10^-3 S/cm, which is significantly lower than conventional organic electrolytes. The consequence is increased internal resistance and diminished rate capability in practical battery applications.
Another substantial barrier is the limited compatibility between ionic liquid electrolytes and high-voltage cathode materials. Many promising cathode materials for magnesium batteries operate at potentials where most ionic liquids undergo oxidative decomposition, leading to capacity fading and safety concerns during cycling. This electrochemical instability narrows the practical voltage window for magnesium battery operation.
The coordination chemistry between Mg2+ ions and ionic liquids presents additional complications. The strong coulombic interactions between magnesium ions and the anions in ionic liquids often result in the formation of complex coordination structures that impede Mg2+ mobility. This phenomenon, known as ion clustering, significantly reduces the effective transference number for magnesium ions, limiting overall battery performance.
Cost factors also present a significant barrier to commercialization. Current synthesis methods for high-purity ionic liquids suitable for battery applications remain expensive and difficult to scale, with prices often exceeding $100 per kilogram. This represents a substantial cost premium compared to conventional organic electrolytes, making mass production economically challenging.
Water sensitivity remains a persistent issue for many ionic liquid systems. Even trace amounts of moisture can lead to hydrogen evolution at the magnesium anode and formation of Mg(OH)2, compromising both safety and electrochemical performance. This necessitates stringent manufacturing conditions and hermetic sealing technologies, further increasing production complexity and cost.
Lastly, the lack of standardized testing protocols specifically designed for ionic liquid-based magnesium batteries makes performance comparison across different research groups challenging, hindering systematic progress in overcoming these technical barriers.
Ionic liquids, while offering excellent thermal stability and wide electrochemical windows, often exhibit prohibitively high viscosity, particularly at room temperature. This high viscosity directly translates to reduced ionic conductivity, typically ranging from 10^-4 to 10^-3 S/cm, which is significantly lower than conventional organic electrolytes. The consequence is increased internal resistance and diminished rate capability in practical battery applications.
Another substantial barrier is the limited compatibility between ionic liquid electrolytes and high-voltage cathode materials. Many promising cathode materials for magnesium batteries operate at potentials where most ionic liquids undergo oxidative decomposition, leading to capacity fading and safety concerns during cycling. This electrochemical instability narrows the practical voltage window for magnesium battery operation.
The coordination chemistry between Mg2+ ions and ionic liquids presents additional complications. The strong coulombic interactions between magnesium ions and the anions in ionic liquids often result in the formation of complex coordination structures that impede Mg2+ mobility. This phenomenon, known as ion clustering, significantly reduces the effective transference number for magnesium ions, limiting overall battery performance.
Cost factors also present a significant barrier to commercialization. Current synthesis methods for high-purity ionic liquids suitable for battery applications remain expensive and difficult to scale, with prices often exceeding $100 per kilogram. This represents a substantial cost premium compared to conventional organic electrolytes, making mass production economically challenging.
Water sensitivity remains a persistent issue for many ionic liquid systems. Even trace amounts of moisture can lead to hydrogen evolution at the magnesium anode and formation of Mg(OH)2, compromising both safety and electrochemical performance. This necessitates stringent manufacturing conditions and hermetic sealing technologies, further increasing production complexity and cost.
Lastly, the lack of standardized testing protocols specifically designed for ionic liquid-based magnesium batteries makes performance comparison across different research groups challenging, hindering systematic progress in overcoming these technical barriers.
Contemporary Ionic Liquid Electrolyte Formulations for Mg Batteries
01 Ionic liquid compositions for magnesium batteries
Specific ionic liquid compositions can be used as electrolytes in magnesium metal batteries to improve performance. These compositions typically include magnesium salts dissolved in ionic liquids, which provide high ionic conductivity and electrochemical stability. The unique properties of ionic liquids, such as low volatility and wide electrochemical windows, make them suitable alternatives to conventional electrolytes for magnesium batteries.- Ionic liquid compositions for magnesium batteries: Specific ionic liquid compositions can be used as electrolytes in magnesium metal batteries to improve performance. These compositions typically include magnesium salts dissolved in ionic liquids, which provide high ionic conductivity and electrochemical stability. The unique properties of ionic liquids, such as low volatility and wide electrochemical windows, make them suitable alternatives to conventional electrolytes for magnesium batteries.
- Electrolyte additives for enhanced performance: Various additives can be incorporated into ionic liquid electrolytes to enhance the performance of magnesium metal batteries. These additives can improve magnesium ion transport, reduce interfacial resistance, and enhance the stability of the electrolyte-electrode interface. Common additives include specific solvents, salts, and compounds that facilitate magnesium deposition and dissolution processes, leading to improved cycling efficiency and battery life.
- Novel ionic liquid structures for magnesium batteries: Novel ionic liquid structures have been developed specifically for use in magnesium metal batteries. These structures feature tailored cations and anions designed to enhance magnesium ion mobility and reduce coordination strength. By modifying the chemical structure of the ionic liquid components, researchers have created electrolytes with improved compatibility with magnesium metal anodes and higher overall battery performance.
- Manufacturing methods for ionic liquid electrolytes: Specialized manufacturing methods have been developed for producing ionic liquid electrolytes suitable for magnesium metal batteries. These methods focus on achieving high purity, consistent composition, and optimal physical properties. Techniques include controlled synthesis processes, purification steps to remove impurities that could hinder battery performance, and formulation approaches that ensure proper mixing and stability of the electrolyte components.
- Composite electrolyte systems with ionic liquids: Composite electrolyte systems that combine ionic liquids with other materials have been developed for magnesium batteries. These systems may incorporate polymers, ceramic materials, or other components to create hybrid electrolytes with enhanced properties. The composite approach can improve mechanical stability, increase ionic conductivity, and provide better interfacial contact between the electrolyte and electrodes, resulting in magnesium batteries with superior performance characteristics.
02 Electrolyte additives for enhanced performance
Various additives can be incorporated into ionic liquid electrolytes to enhance the performance of magnesium metal batteries. These additives can improve magnesium ion transport, reduce interfacial resistance, and enhance the stability of the electrolyte-electrode interface. Common additives include specific organic compounds, metal salts, and polymers that work synergistically with the ionic liquid to optimize battery performance.Expand Specific Solutions03 Novel ionic liquid structures for magnesium batteries
Novel ionic liquid structures have been developed specifically for use in magnesium metal batteries. These include custom-designed cations and anions that facilitate magnesium ion transport while maintaining high electrochemical stability. The molecular design of these ionic liquids focuses on reducing viscosity, enhancing magnesium salt solubility, and improving the reversibility of magnesium deposition and dissolution processes.Expand Specific Solutions04 Manufacturing methods for ionic liquid electrolytes
Specialized manufacturing methods have been developed for producing ionic liquid electrolytes for magnesium batteries. These methods focus on ensuring high purity, controlling water content, and optimizing the mixing process of magnesium salts with ionic liquids. Advanced techniques for removing impurities and water are crucial as they can significantly affect the electrochemical performance and stability of the resulting electrolyte system.Expand Specific Solutions05 Hybrid and composite electrolyte systems
Hybrid and composite electrolyte systems combine ionic liquids with other materials to create enhanced electrolytes for magnesium batteries. These systems may incorporate polymers, solid-state components, or other solvents to form gel, quasi-solid, or composite electrolytes. Such hybrid approaches aim to combine the advantages of ionic liquids (such as high ionic conductivity) with the benefits of other materials (such as mechanical stability or improved interfacial properties).Expand Specific Solutions
Leading Research Groups and Industrial Players in Mg Batteries
The magnesium metal battery market with ionic liquid electrolytes is in an early growth phase, characterized by intensive R&D rather than mass commercialization. The global market size remains relatively small but is projected to expand significantly as energy storage demands increase. Technologically, this field is still developing, with key players demonstrating varying levels of advancement. Toyota Motor Corp. leads commercial research with substantial patent portfolios, while academic institutions like Tsinghua University and Shanghai Jiao Tong University contribute fundamental research breakthroughs. Research organizations including KIST, Dai Nippon Printing, and Furukawa Battery are developing specialized electrolyte formulations. The technology shows promise but requires further development in electrolyte stability, conductivity, and compatibility with magnesium anodes before widespread commercial adoption.
Toyota Motor Corp.
Technical Solution: Toyota has developed advanced ionic liquid electrolytes for magnesium metal batteries featuring high ionic conductivity and wide electrochemical stability windows. Their proprietary formulations combine magnesium bis(trifluoromethanesulfonyl)imide (Mg(TFSI)2) with ionic liquids based on imidazolium and pyrrolidinium cations. Toyota's approach includes the addition of magnesium chloride (MgCl2) as a critical component to enhance the reversible deposition and dissolution of magnesium. Their electrolytes demonstrate stable cycling at room temperature with coulombic efficiencies exceeding 95% and support operation across a wide temperature range (-20°C to 60°C). Toyota has also engineered these electrolytes to be compatible with various cathode materials, including Chevrel phases and sulfur-based compounds, enabling practical energy densities above 400 Wh/kg at the cell level.
Strengths: Superior electrochemical stability window (>3V) allowing higher voltage operation; excellent thermal stability reducing safety concerns; compatibility with multiple cathode chemistries enabling versatile applications. Weaknesses: Higher viscosity than conventional electrolytes potentially limiting rate capability; relatively expensive production costs compared to lithium-ion battery electrolytes; challenges with long-term stability during extended cycling.
Dalian Institute of Chemical Physics of CAS
Technical Solution: The Dalian Institute has developed innovative ionic liquid electrolytes for magnesium batteries based on their proprietary "coordination chemistry" approach. Their technology utilizes carefully designed ionic liquids incorporating magnesium bis(hexafluoroisopropyloxy)borate (Mg[B(hfip)4]2) salt combined with N-methyl-N-propylpiperidinium bis(fluorosulfonyl)imide ([PP13][FSI]) ionic liquid. This unique combination creates a weakly coordinating anion environment that facilitates magnesium ion transport while preventing passivation layer formation. Their research demonstrates that controlling the solvation structure through specific ionic liquid selection dramatically improves magnesium deposition/dissolution kinetics. The electrolytes show exceptional stability against magnesium metal (>1000 cycles without significant capacity fade) and compatibility with various cathode materials including Chevrel phases and organic cathodes. Notably, their electrolytes maintain functionality at temperatures as low as -30°C with only modest decreases in performance, addressing a key limitation of conventional magnesium battery systems. The institute has also developed scalable synthesis methods for these electrolytes, making them viable candidates for commercial application.
Strengths: Exceptional electrochemical stability enabling long cycle life; excellent low-temperature performance expanding potential applications; compatibility with multiple cathode chemistries. Weaknesses: Complex synthesis process potentially increasing production costs; higher viscosity than conventional electrolytes limiting rate performance; potential challenges with water sensitivity requiring careful handling.
Critical Patents and Scientific Breakthroughs in Ionic Liquid Electrolytes
Chelating ionic liquids for magnesium battery electrolytes and systems
PatentActiveUS20190074548A1
Innovation
- A rechargeable magnesium battery system utilizing a chelating ionic liquid medium with a polyether chain and specific cations and anions, such as N-methoxyPEGm-N-methylpyrrolidinium and bis(trifluoromethylsulfonyl)imide, which facilitates reversible magnesium metal deposition and dissolution by preventing direct interactions between magnesium ions and anions, thereby enhancing oxidative stability and safety.
Magnesium battery electrolyte and preparation method therefor, and magnesium battery
PatentPendingEP4481878A1
Innovation
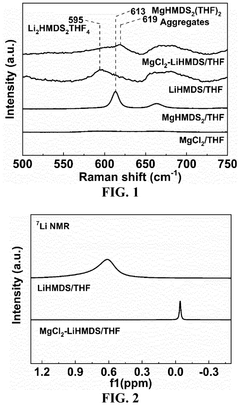
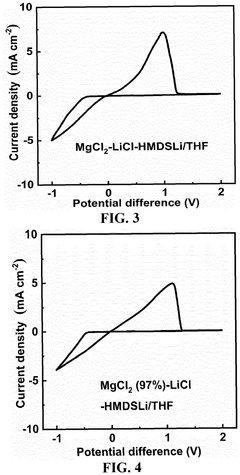
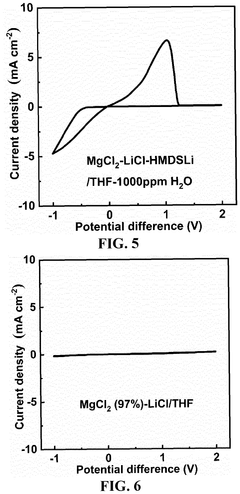
- An electrolytic solution comprising a non-aqueous solvent and an electrolyte salt with a specific chemical formula [Mg m Li n X o (HMDS) 2m+n-o R p ]·M q, where X is a halogen ion or bis(trifluoromethanesulfonyl)imide, HMDS is hexamethyldisilamino, and R is alkyl, fluoroalkyl, or aryl, combined with a method involving mixing anhydrous magnesium and lithium salts in a non-aqueous solvent at controlled temperatures to form a stable and impurity-resistant electrolyte.
Safety and Stability Assessment of Ionic Liquid Electrolytes
Safety assessment of ionic liquid electrolytes for magnesium metal batteries reveals significant advantages over conventional electrolytes. These ionic liquids demonstrate remarkably low flammability and volatility, substantially reducing fire hazards associated with battery thermal runaway events. Thermal stability tests indicate that most magnesium-compatible ionic liquids maintain structural integrity at temperatures exceeding 300°C, compared to conventional carbonate-based electrolytes that decompose around 80-120°C.
Chemical stability analyses show that properly designed ionic liquid electrolytes exhibit minimal reactivity with magnesium metal anodes, addressing a critical challenge in magnesium battery development. The formation of stable interfaces between the electrolyte and magnesium metal prevents continuous electrolyte decomposition, enhancing long-term cycling performance and safety. Electrochemical stability windows of 3.0-4.5V have been documented for several ionic liquid formulations, providing adequate operational range for practical applications.
Environmental safety evaluations indicate reduced toxicity profiles compared to conventional electrolytes containing volatile organic compounds. Many ionic liquids demonstrate biodegradability, though this property varies significantly based on the specific cation-anion combinations. Toxicological studies suggest lower acute toxicity risks, though comprehensive long-term exposure data remains limited.
Stability under operational conditions reveals that ionic liquid electrolytes maintain performance integrity across wider temperature ranges (-20°C to 100°C) than conventional systems. This characteristic is particularly valuable for applications requiring operation in extreme environments. Accelerated aging tests demonstrate that properly formulated ionic liquid electrolytes retain over 85% of their initial conductivity after 1000 hours at elevated temperatures.
Challenges persist regarding moisture sensitivity, as many magnesium-compatible ionic liquids experience performance degradation upon water absorption. Implementation of rigorous moisture control protocols during manufacturing and hermetic sealing technologies are essential for maintaining electrolyte integrity. Additionally, some ionic liquid formulations exhibit increased viscosity at lower temperatures, potentially limiting low-temperature performance.
Regulatory compliance assessment indicates that ionic liquid electrolytes generally align with evolving battery safety standards, including UN 38.3 transportation requirements and IEC 62133 safety specifications. Their reduced flammability particularly addresses concerns outlined in UL 1642 standards for lithium batteries, potentially simplifying certification processes for magnesium battery systems utilizing these electrolytes.
Chemical stability analyses show that properly designed ionic liquid electrolytes exhibit minimal reactivity with magnesium metal anodes, addressing a critical challenge in magnesium battery development. The formation of stable interfaces between the electrolyte and magnesium metal prevents continuous electrolyte decomposition, enhancing long-term cycling performance and safety. Electrochemical stability windows of 3.0-4.5V have been documented for several ionic liquid formulations, providing adequate operational range for practical applications.
Environmental safety evaluations indicate reduced toxicity profiles compared to conventional electrolytes containing volatile organic compounds. Many ionic liquids demonstrate biodegradability, though this property varies significantly based on the specific cation-anion combinations. Toxicological studies suggest lower acute toxicity risks, though comprehensive long-term exposure data remains limited.
Stability under operational conditions reveals that ionic liquid electrolytes maintain performance integrity across wider temperature ranges (-20°C to 100°C) than conventional systems. This characteristic is particularly valuable for applications requiring operation in extreme environments. Accelerated aging tests demonstrate that properly formulated ionic liquid electrolytes retain over 85% of their initial conductivity after 1000 hours at elevated temperatures.
Challenges persist regarding moisture sensitivity, as many magnesium-compatible ionic liquids experience performance degradation upon water absorption. Implementation of rigorous moisture control protocols during manufacturing and hermetic sealing technologies are essential for maintaining electrolyte integrity. Additionally, some ionic liquid formulations exhibit increased viscosity at lower temperatures, potentially limiting low-temperature performance.
Regulatory compliance assessment indicates that ionic liquid electrolytes generally align with evolving battery safety standards, including UN 38.3 transportation requirements and IEC 62133 safety specifications. Their reduced flammability particularly addresses concerns outlined in UL 1642 standards for lithium batteries, potentially simplifying certification processes for magnesium battery systems utilizing these electrolytes.
Scalability and Manufacturing Challenges for Commercial Implementation
The transition from laboratory-scale research to commercial production of ionic liquid electrolytes for magnesium metal batteries faces significant manufacturing challenges. Current production methods for high-purity ionic liquids involve complex synthesis routes with multiple purification steps, resulting in high production costs that can exceed $1,000 per kilogram. This cost structure presents a substantial barrier to widespread adoption, particularly when competing with conventional lithium-ion battery technologies that benefit from established manufacturing ecosystems.
Scale-up processes for ionic liquid electrolytes encounter several technical hurdles. The synthesis reactions are often sensitive to moisture and oxygen, necessitating specialized production environments with controlled atmospheres. Additionally, the purification processes—including multiple recrystallization steps, vacuum drying, and chromatographic separations—are difficult to translate from laboratory to industrial scale without compromising product quality or further increasing costs.
Quality control represents another critical challenge in the manufacturing pipeline. Trace impurities, particularly water, can significantly impact the electrochemical performance of magnesium metal batteries. Developing robust, high-throughput analytical methods for monitoring ionic liquid purity during production remains an ongoing challenge for manufacturers seeking to ensure consistent product performance.
Material compatibility issues further complicate the manufacturing landscape. Ionic liquids can interact with various container materials and processing equipment, potentially leading to contamination or degradation of the electrolyte. Identifying appropriate materials for large-scale handling, storage, and processing requires extensive testing and validation, adding to development timelines and costs.
Supply chain considerations also present obstacles to commercialization. Many ionic liquids require specialized precursors with limited commercial availability. Establishing reliable supply networks for these materials at industrial scales will be necessary to support consistent production and avoid bottlenecks in the manufacturing process.
Environmental and safety regulations introduce additional complexity to manufacturing operations. While ionic liquids are often promoted as "green" alternatives to volatile organic solvents, their environmental impact and toxicity profiles must be thoroughly assessed before large-scale production. Regulatory compliance may require specialized handling procedures and waste management protocols, potentially adding to operational costs.
Addressing these manufacturing challenges will require collaborative efforts between academic researchers, chemical manufacturers, and battery producers. Strategic investments in process intensification, continuous manufacturing technologies, and alternative synthesis routes could significantly reduce production costs and improve scalability, ultimately determining whether ionic liquid electrolytes can transition from promising laboratory materials to commercially viable components of next-generation magnesium battery systems.
Scale-up processes for ionic liquid electrolytes encounter several technical hurdles. The synthesis reactions are often sensitive to moisture and oxygen, necessitating specialized production environments with controlled atmospheres. Additionally, the purification processes—including multiple recrystallization steps, vacuum drying, and chromatographic separations—are difficult to translate from laboratory to industrial scale without compromising product quality or further increasing costs.
Quality control represents another critical challenge in the manufacturing pipeline. Trace impurities, particularly water, can significantly impact the electrochemical performance of magnesium metal batteries. Developing robust, high-throughput analytical methods for monitoring ionic liquid purity during production remains an ongoing challenge for manufacturers seeking to ensure consistent product performance.
Material compatibility issues further complicate the manufacturing landscape. Ionic liquids can interact with various container materials and processing equipment, potentially leading to contamination or degradation of the electrolyte. Identifying appropriate materials for large-scale handling, storage, and processing requires extensive testing and validation, adding to development timelines and costs.
Supply chain considerations also present obstacles to commercialization. Many ionic liquids require specialized precursors with limited commercial availability. Establishing reliable supply networks for these materials at industrial scales will be necessary to support consistent production and avoid bottlenecks in the manufacturing process.
Environmental and safety regulations introduce additional complexity to manufacturing operations. While ionic liquids are often promoted as "green" alternatives to volatile organic solvents, their environmental impact and toxicity profiles must be thoroughly assessed before large-scale production. Regulatory compliance may require specialized handling procedures and waste management protocols, potentially adding to operational costs.
Addressing these manufacturing challenges will require collaborative efforts between academic researchers, chemical manufacturers, and battery producers. Strategic investments in process intensification, continuous manufacturing technologies, and alternative synthesis routes could significantly reduce production costs and improve scalability, ultimately determining whether ionic liquid electrolytes can transition from promising laboratory materials to commercially viable components of next-generation magnesium battery systems.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!