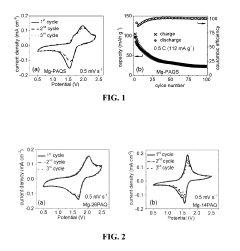
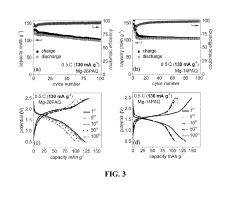
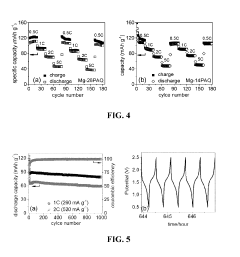
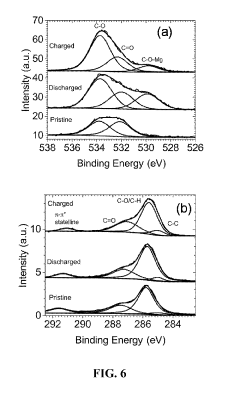
Organic cathode materials for high-energy magnesium batteries
OCT 14, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Mg Battery Organic Cathode Development Background & Objectives
Magnesium batteries have emerged as promising alternatives to lithium-ion batteries due to their potential for higher energy density, improved safety, and lower cost. The development of magnesium batteries traces back to the early 1990s, but significant progress has been made only in the past decade. The evolution of this technology has been primarily driven by the increasing demand for energy storage solutions with higher capacity, longer cycle life, and enhanced safety profiles for applications ranging from portable electronics to electric vehicles and grid-scale energy storage.
The fundamental advantage of magnesium batteries lies in their theoretical capacity of 2205 mAh/g and volumetric capacity of 3833 mAh/cm³, significantly higher than lithium-ion counterparts. Additionally, magnesium is the eighth most abundant element in the Earth's crust, making it economically viable for large-scale production. The absence of dendrite formation during cycling further enhances the safety profile of magnesium batteries compared to lithium-ion systems.
Despite these advantages, the development of magnesium batteries has been hindered by several challenges, particularly in cathode materials. Traditional inorganic cathodes suffer from slow magnesium-ion diffusion kinetics and structural instability during cycling. This has led researchers to explore organic cathode materials as alternatives, which offer advantages such as structural diversity, tunable redox properties, and potentially faster ion transport kinetics.
The technical objectives of research on organic cathode materials for high-energy magnesium batteries include developing materials with high specific capacity (>300 mAh/g), stable cycling performance (>1000 cycles with minimal capacity fade), fast charging capabilities (complete charge in <30 minutes), and compatibility with conventional electrolytes. Additionally, these materials should be environmentally benign, cost-effective, and scalable for commercial production.
Current research trends focus on several classes of organic materials, including conjugated carbonyl compounds, conducting polymers, and organosulfur compounds. These materials can undergo reversible redox reactions with magnesium ions, potentially enabling high-capacity energy storage. The molecular structure of these organic compounds can be tailored to optimize properties such as redox potential, electron conductivity, and ion diffusion pathways.
Looking forward, the field is moving toward multi-functional organic cathodes that combine high capacity with structural stability and fast kinetics. Integration of computational screening methods with experimental validation is accelerating the discovery of novel organic cathode materials. The ultimate goal is to develop magnesium batteries that can outperform current lithium-ion technologies in terms of energy density, safety, and cost, thereby enabling the next generation of energy storage solutions for a sustainable future.
The fundamental advantage of magnesium batteries lies in their theoretical capacity of 2205 mAh/g and volumetric capacity of 3833 mAh/cm³, significantly higher than lithium-ion counterparts. Additionally, magnesium is the eighth most abundant element in the Earth's crust, making it economically viable for large-scale production. The absence of dendrite formation during cycling further enhances the safety profile of magnesium batteries compared to lithium-ion systems.
Despite these advantages, the development of magnesium batteries has been hindered by several challenges, particularly in cathode materials. Traditional inorganic cathodes suffer from slow magnesium-ion diffusion kinetics and structural instability during cycling. This has led researchers to explore organic cathode materials as alternatives, which offer advantages such as structural diversity, tunable redox properties, and potentially faster ion transport kinetics.
The technical objectives of research on organic cathode materials for high-energy magnesium batteries include developing materials with high specific capacity (>300 mAh/g), stable cycling performance (>1000 cycles with minimal capacity fade), fast charging capabilities (complete charge in <30 minutes), and compatibility with conventional electrolytes. Additionally, these materials should be environmentally benign, cost-effective, and scalable for commercial production.
Current research trends focus on several classes of organic materials, including conjugated carbonyl compounds, conducting polymers, and organosulfur compounds. These materials can undergo reversible redox reactions with magnesium ions, potentially enabling high-capacity energy storage. The molecular structure of these organic compounds can be tailored to optimize properties such as redox potential, electron conductivity, and ion diffusion pathways.
Looking forward, the field is moving toward multi-functional organic cathodes that combine high capacity with structural stability and fast kinetics. Integration of computational screening methods with experimental validation is accelerating the discovery of novel organic cathode materials. The ultimate goal is to develop magnesium batteries that can outperform current lithium-ion technologies in terms of energy density, safety, and cost, thereby enabling the next generation of energy storage solutions for a sustainable future.
Market Analysis for High-Energy Mg Battery Applications
The global market for high-energy magnesium batteries is experiencing significant growth potential, driven by increasing demand for sustainable energy storage solutions. Current projections indicate the magnesium battery market could reach several billion dollars by 2030, with organic cathode materials representing a crucial segment of this emerging market.
The primary market drivers for high-energy magnesium batteries include the growing electric vehicle (EV) sector, renewable energy storage systems, and portable electronics. The EV market particularly stands out as a high-potential application area, with magnesium batteries offering theoretical advantages over current lithium-ion technologies in terms of energy density, safety, and resource availability.
Consumer electronics represents another substantial market opportunity, where the higher energy density and improved safety characteristics of magnesium batteries could provide competitive advantages. The renewable energy storage sector also shows promising growth prospects as grid-scale storage solutions become increasingly important for managing intermittent renewable power generation.
Geographically, North America, Europe, and Asia-Pacific regions are expected to lead adoption, with China, Japan, South Korea, and the United States making significant investments in magnesium battery research and development. The European market shows particular interest due to stringent environmental regulations and sustainability goals.
Market barriers include competition from established lithium-ion technology, which benefits from decades of optimization and manufacturing scale. Additionally, the current high production costs of organic cathode materials for magnesium batteries present commercialization challenges that must be overcome to achieve market penetration.
Industry analysts note that the market for magnesium batteries with organic cathodes will likely follow a staged adoption pattern, beginning with niche applications where their specific advantages outweigh cost considerations, before expanding to mass-market applications as manufacturing scales and costs decrease.
The competitive landscape includes both established battery manufacturers exploring magnesium technology as portfolio diversification and specialized startups focused exclusively on next-generation magnesium battery development. Strategic partnerships between material science companies, battery manufacturers, and end-users are becoming increasingly common to accelerate commercialization.
Consumer and industrial demand for batteries with improved sustainability profiles creates a favorable market environment for magnesium batteries with organic cathodes, as they potentially offer reduced environmental impact compared to conventional lithium-ion batteries that rely on critical minerals with supply chain vulnerabilities.
The primary market drivers for high-energy magnesium batteries include the growing electric vehicle (EV) sector, renewable energy storage systems, and portable electronics. The EV market particularly stands out as a high-potential application area, with magnesium batteries offering theoretical advantages over current lithium-ion technologies in terms of energy density, safety, and resource availability.
Consumer electronics represents another substantial market opportunity, where the higher energy density and improved safety characteristics of magnesium batteries could provide competitive advantages. The renewable energy storage sector also shows promising growth prospects as grid-scale storage solutions become increasingly important for managing intermittent renewable power generation.
Geographically, North America, Europe, and Asia-Pacific regions are expected to lead adoption, with China, Japan, South Korea, and the United States making significant investments in magnesium battery research and development. The European market shows particular interest due to stringent environmental regulations and sustainability goals.
Market barriers include competition from established lithium-ion technology, which benefits from decades of optimization and manufacturing scale. Additionally, the current high production costs of organic cathode materials for magnesium batteries present commercialization challenges that must be overcome to achieve market penetration.
Industry analysts note that the market for magnesium batteries with organic cathodes will likely follow a staged adoption pattern, beginning with niche applications where their specific advantages outweigh cost considerations, before expanding to mass-market applications as manufacturing scales and costs decrease.
The competitive landscape includes both established battery manufacturers exploring magnesium technology as portfolio diversification and specialized startups focused exclusively on next-generation magnesium battery development. Strategic partnerships between material science companies, battery manufacturers, and end-users are becoming increasingly common to accelerate commercialization.
Consumer and industrial demand for batteries with improved sustainability profiles creates a favorable market environment for magnesium batteries with organic cathodes, as they potentially offer reduced environmental impact compared to conventional lithium-ion batteries that rely on critical minerals with supply chain vulnerabilities.
Current Status and Challenges in Organic Cathode Materials
The development of organic cathode materials for magnesium batteries has gained significant attention in recent years, yet remains in a relatively nascent stage compared to lithium-ion battery technology. Current research primarily focuses on carbonyl compounds, conducting polymers, organosulfur compounds, and organic radical materials. Among these, quinone derivatives have shown promising electrochemical performance with theoretical capacities exceeding 400 mAh/g and operating voltages around 2.0-2.5V vs. Mg/Mg².
Despite these advances, several critical challenges impede the widespread application of organic cathode materials in magnesium batteries. The most prominent issue is the poor electronic conductivity inherent to most organic materials, which significantly limits rate capability and practical capacity utilization. This conductivity problem is further exacerbated by the sluggish diffusion kinetics of divalent Mg²⁺ ions within organic structures.
Stability concerns present another major hurdle, as many organic cathode materials suffer from dissolution in conventional electrolytes, leading to rapid capacity fading during cycling. This dissolution problem is particularly severe in ether-based electrolytes commonly used in magnesium battery systems, creating a complex compatibility challenge between cathode materials and electrolyte formulations.
The reversibility of magnesium insertion/extraction in organic hosts remains problematic, with many materials showing significant capacity decay after initial cycles. This is often attributed to irreversible structural changes or side reactions occurring during the redox processes, highlighting the need for more robust molecular designs.
From a geographical perspective, research on organic cathode materials for magnesium batteries is primarily concentrated in North America, Europe, and East Asia, with notable contributions from research institutions in the United States, Germany, Japan, and China. The field has seen approximately a 40% increase in published research papers over the past five years, indicating growing interest in this technology.
Current technical limitations also include insufficient understanding of the reaction mechanisms between organic cathodes and magnesium ions, particularly regarding the influence of solvation effects and interfacial phenomena. The development of in-situ characterization techniques specific to organic-magnesium systems lags behind those available for lithium-ion batteries, hampering mechanistic studies.
Energy density remains substantially below theoretical predictions, with most practical demonstrations achieving only 30-50% of calculated values. This gap stems from a combination of factors including incomplete material utilization, high inactive material content required for conductivity enhancement, and voltage hysteresis during cycling.
Despite these advances, several critical challenges impede the widespread application of organic cathode materials in magnesium batteries. The most prominent issue is the poor electronic conductivity inherent to most organic materials, which significantly limits rate capability and practical capacity utilization. This conductivity problem is further exacerbated by the sluggish diffusion kinetics of divalent Mg²⁺ ions within organic structures.
Stability concerns present another major hurdle, as many organic cathode materials suffer from dissolution in conventional electrolytes, leading to rapid capacity fading during cycling. This dissolution problem is particularly severe in ether-based electrolytes commonly used in magnesium battery systems, creating a complex compatibility challenge between cathode materials and electrolyte formulations.
The reversibility of magnesium insertion/extraction in organic hosts remains problematic, with many materials showing significant capacity decay after initial cycles. This is often attributed to irreversible structural changes or side reactions occurring during the redox processes, highlighting the need for more robust molecular designs.
From a geographical perspective, research on organic cathode materials for magnesium batteries is primarily concentrated in North America, Europe, and East Asia, with notable contributions from research institutions in the United States, Germany, Japan, and China. The field has seen approximately a 40% increase in published research papers over the past five years, indicating growing interest in this technology.
Current technical limitations also include insufficient understanding of the reaction mechanisms between organic cathodes and magnesium ions, particularly regarding the influence of solvation effects and interfacial phenomena. The development of in-situ characterization techniques specific to organic-magnesium systems lags behind those available for lithium-ion batteries, hampering mechanistic studies.
Energy density remains substantially below theoretical predictions, with most practical demonstrations achieving only 30-50% of calculated values. This gap stems from a combination of factors including incomplete material utilization, high inactive material content required for conductivity enhancement, and voltage hysteresis during cycling.
Current Organic Cathode Material Solutions for Mg Batteries
01 Organic polymer cathode materials for magnesium batteries
Organic polymer materials can be used as cathode materials in magnesium batteries to improve energy density and cycling performance. These polymers typically contain conjugated structures with redox-active functional groups that can reversibly store and release magnesium ions. The polymeric structure provides stability during charge-discharge cycles while maintaining good electronic conductivity. These materials offer advantages such as structural flexibility, tunable properties, and potentially lower environmental impact compared to inorganic cathodes.- Organic polymer cathode materials for magnesium batteries: Organic polymer materials can be used as cathode materials in magnesium batteries to improve energy density and cycling performance. These polymers typically contain conjugated structures with redox-active functional groups that can reversibly store magnesium ions. The polymer-based cathodes offer advantages such as structural flexibility, tunable electrochemical properties, and environmental friendliness compared to traditional inorganic cathodes.
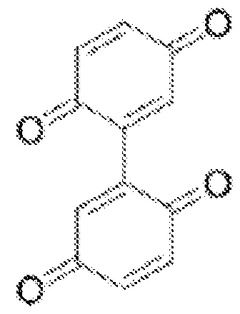
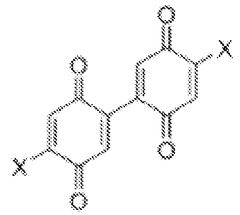
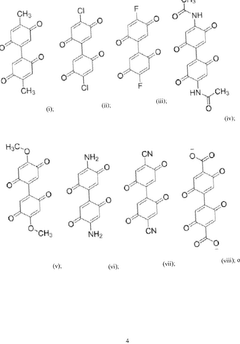
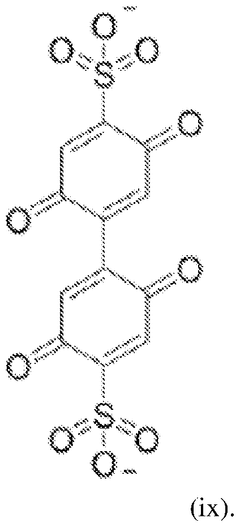
- Quinone-based organic cathode materials: Quinone derivatives are promising organic cathode materials for magnesium batteries due to their high theoretical capacity and redox activity. These compounds contain carbonyl groups that can coordinate with magnesium ions during the charge-discharge process. Various quinone structures, including anthraquinones, benzoquinones, and their derivatives, have been investigated to optimize properties such as solubility, stability, and electron transfer kinetics for improved battery performance.
- Conductive additives and composite electrodes: Organic cathode materials often suffer from poor electrical conductivity, which can be addressed by incorporating conductive additives or forming composite electrodes. Carbon-based materials such as carbon nanotubes, graphene, or conductive polymers are commonly used to enhance electron transport within the electrode. These composite structures improve the utilization of active materials, rate capability, and cycling stability of magnesium batteries with organic cathodes.
- Electrolyte compatibility with organic cathodes: The performance of organic cathode materials in magnesium batteries is significantly influenced by the compatibility with electrolytes. Specialized electrolyte formulations are developed to ensure efficient magnesium ion transport while preventing degradation of the organic cathode materials. Non-nucleophilic electrolytes and those containing specific additives can mitigate side reactions, enhance the reversibility of magnesium deposition/dissolution, and improve the overall electrochemical performance of the battery system.
- Organosulfur compounds as cathode materials: Organosulfur compounds represent another class of organic cathode materials for magnesium batteries, offering high energy density through sulfur-based redox reactions. These materials typically contain disulfide bonds or thiol groups that can undergo reversible redox reactions with magnesium ions. Researchers have explored various molecular designs and composite structures to address challenges such as dissolution into the electrolyte, slow reaction kinetics, and capacity fading during cycling.
02 Quinone-based organic cathode materials
Quinone derivatives serve as promising organic cathode materials for magnesium batteries due to their redox-active carbonyl groups that can coordinate with magnesium ions. These materials undergo reversible redox reactions, accepting electrons during discharge and releasing them during charging. Quinone-based cathodes offer high theoretical capacity, good rate capability, and can be synthesized from renewable resources. Modifications to the quinone structure, such as adding electron-withdrawing or electron-donating groups, can tune the redox potential and improve electrochemical performance.Expand Specific Solutions03 Conductive polymer composites for magnesium battery cathodes
Conductive polymer composites combine organic cathode materials with conductive additives to enhance electron transport and electrochemical performance in magnesium batteries. These composites typically incorporate carbon-based materials (such as carbon nanotubes, graphene, or conductive carbon black) or conductive polymers (like PEDOT:PSS) with the active organic material. The improved conductivity facilitates faster charge transfer kinetics, better utilization of active materials, and enhanced rate capability, addressing one of the key limitations of organic cathode materials.Expand Specific Solutions04 Organosulfur compounds as cathode materials
Organosulfur compounds represent an important class of organic cathode materials for magnesium batteries, utilizing the reversible redox chemistry of sulfur-containing functional groups. These materials typically contain disulfide bonds or thiol groups that can undergo reversible redox reactions with magnesium ions. The high electronegativity of sulfur enables strong interactions with magnesium ions, potentially leading to high energy density. Researchers have explored various organosulfur structures including disulfides, thioethers, and sulfur-containing polymers to optimize electrochemical performance and cycling stability.Expand Specific Solutions05 Electrolyte compatibility with organic cathodes
The compatibility between electrolytes and organic cathode materials is crucial for the performance of magnesium batteries. Conventional magnesium electrolytes often contain nucleophilic components that can react with the redox-active functional groups in organic cathodes, causing degradation and capacity loss. Research focuses on developing non-nucleophilic electrolytes that are compatible with organic cathodes while maintaining good magnesium ion conductivity. Strategies include using magnesium bis(trifluoromethanesulfonyl)imide salts, boron-based electrolytes, or incorporating protective additives that form stable interfaces between the cathode and electrolyte.Expand Specific Solutions
Key Industry Players in Mg Battery Research
The organic cathode materials for high-energy magnesium batteries market is in an early growth stage, characterized by intensive research and development rather than widespread commercialization. The global market size remains relatively small but is projected to expand significantly as magnesium battery technology matures as an alternative to lithium-ion systems. Currently, the technology readiness level is moderate, with key players demonstrating varying degrees of advancement. Toyota, Samsung Electronics, and Panasonic are leading industrial research efforts with substantial patent portfolios, while academic institutions like University of Houston and Xiangtan University contribute fundamental research breakthroughs. Research organizations such as AIST and Hydro-Québec are developing promising prototype materials, though challenges in electrolyte compatibility and cycling stability remain barriers to commercial adoption.
Uchicago Argonne LLC
Technical Solution: Argonne National Laboratory has developed innovative organic cathode materials for magnesium batteries focusing on quinone-based compounds. Their approach utilizes redox-active organic molecules with carbonyl groups that can reversibly accept magnesium ions during charge/discharge cycles. The research team has synthesized and tested various derivatives of anthraquinone and benzoquinone structures, optimizing them for magnesium ion storage. Their materials demonstrate high theoretical capacity (up to 260 mAh/g) and operate at voltages around 2.0-2.5V vs Mg/Mg2+. A key innovation is the incorporation of electron-withdrawing groups to lower the LUMO energy level, enhancing the redox potential and energy density. Argonne's materials also feature tailored molecular structures with appropriate spacing to accommodate the large, divalent magnesium ions, addressing a common challenge in magnesium battery chemistry.
Strengths: High theoretical capacity, tunable molecular structure, and potentially lower cost compared to inorganic alternatives. The organic materials offer better kinetics for magnesium ion insertion/extraction than many inorganic cathodes. Weaknesses: Lower cycling stability compared to inorganic materials, potential dissolution of active material into the electrolyte during cycling, and lower volumetric energy density.
The Regents of the University of California
Technical Solution: The University of California has pioneered research on conjugated organic frameworks (COFs) and polymers as cathode materials for magnesium batteries. Their approach focuses on creating ordered structures with precisely engineered redox-active sites and ion diffusion channels. The research team has developed π-conjugated polymers containing carbonyl groups that serve as magnesium ion coordination sites, achieving reversible magnesium storage with capacities exceeding 180 mAh/g. A significant innovation is their work on tailoring the polymer backbone structure to optimize the balance between electronic conductivity and ion transport. They've also developed novel electrolyte formulations compatible with these organic cathodes, addressing the critical electrolyte-cathode interface challenges. Their materials operate at voltages around 1.8-2.3V vs Mg/Mg2+, with some compounds demonstrating stable cycling for over 500 cycles with minimal capacity fade.
Strengths: Excellent structural tunability, potentially sustainable production from renewable resources, and good rate capability due to engineered ion transport pathways. Weaknesses: Lower volumetric energy density compared to inorganic materials, potential for self-discharge due to solubility issues, and challenges in scaling up synthesis with consistent quality.
Critical Patents and Research on Mg-Compatible Organic Cathodes
Polyanthraquinone-based organic cathode for high-performance rechargeable magnesium-ion batteries
PatentActiveUS10297829B2
Innovation
- The development of a cathode comprising a redox-active polyanthraquinone polymer, specifically 2,6-polyanthraquinone (26PAQ) and 1,4-polyanthraquinone, combined with a carbon material in a binding matrix, which provides improved cycling stability and capacity retention by preventing electrode dissolution and facilitating reversible magnesium ion intercalation.
Organic cathode materials for magnesium ion batteries
PatentWO2025171375A1
Innovation
- Development of bis-benzoquinone compounds for use as cathode materials in magnesium batteries, synthesized through a straightforward method using ceric ammonium nitrate, offering high specific energies and stable cycling rates.
Sustainability and Environmental Impact Assessment
The sustainability aspects of organic cathode materials for magnesium batteries represent a significant advantage over conventional battery technologies. These materials are predominantly derived from abundant natural resources and biomass, offering a renewable alternative to the metal-based cathodes that rely on finite mineral deposits. The carbon-based structures of organic materials can be synthesized from sustainable precursors, potentially reducing the environmental footprint associated with resource extraction and processing.
Life cycle assessment (LCA) studies indicate that organic cathode materials generally exhibit lower environmental impact scores across multiple categories including global warming potential, resource depletion, and ecotoxicity. When compared to traditional cathode materials, organic alternatives typically require less energy-intensive manufacturing processes, operating at lower temperatures and utilizing fewer hazardous chemicals. This translates to reduced greenhouse gas emissions during production phases.
The biodegradability of many organic cathode compounds presents another environmental advantage. Unlike conventional battery materials that persist in the environment for extended periods, properly designed organic materials can decompose under appropriate conditions, minimizing long-term ecological risks. This characteristic aligns with circular economy principles and addresses end-of-life management challenges that plague current battery technologies.
Water consumption and land use impacts also tend to be lower for organic cathode production systems. The synthesis pathways often require less water-intensive processing compared to mining and refining operations necessary for inorganic materials. Additionally, the potential for bio-based precursors opens pathways to integrate battery material production with sustainable agricultural practices.
Toxicity profiles of organic cathode materials generally show reduced human and environmental health risks. The absence of heavy metals and toxic elements commonly found in conventional cathodes eliminates concerns related to bioaccumulation and persistent environmental contamination. However, comprehensive toxicological assessments remain necessary for novel organic compounds to ensure their safety throughout the product lifecycle.
Recycling and material recovery present both opportunities and challenges. While organic materials may be more amenable to certain recycling processes, the diversity of molecular structures could complicate standardized recovery methods. Research into dedicated recycling technologies for organic battery components is essential to fully realize their sustainability potential and ensure closed-loop material flows.
Life cycle assessment (LCA) studies indicate that organic cathode materials generally exhibit lower environmental impact scores across multiple categories including global warming potential, resource depletion, and ecotoxicity. When compared to traditional cathode materials, organic alternatives typically require less energy-intensive manufacturing processes, operating at lower temperatures and utilizing fewer hazardous chemicals. This translates to reduced greenhouse gas emissions during production phases.
The biodegradability of many organic cathode compounds presents another environmental advantage. Unlike conventional battery materials that persist in the environment for extended periods, properly designed organic materials can decompose under appropriate conditions, minimizing long-term ecological risks. This characteristic aligns with circular economy principles and addresses end-of-life management challenges that plague current battery technologies.
Water consumption and land use impacts also tend to be lower for organic cathode production systems. The synthesis pathways often require less water-intensive processing compared to mining and refining operations necessary for inorganic materials. Additionally, the potential for bio-based precursors opens pathways to integrate battery material production with sustainable agricultural practices.
Toxicity profiles of organic cathode materials generally show reduced human and environmental health risks. The absence of heavy metals and toxic elements commonly found in conventional cathodes eliminates concerns related to bioaccumulation and persistent environmental contamination. However, comprehensive toxicological assessments remain necessary for novel organic compounds to ensure their safety throughout the product lifecycle.
Recycling and material recovery present both opportunities and challenges. While organic materials may be more amenable to certain recycling processes, the diversity of molecular structures could complicate standardized recovery methods. Research into dedicated recycling technologies for organic battery components is essential to fully realize their sustainability potential and ensure closed-loop material flows.
Cost Analysis and Commercial Viability
The economic feasibility of organic cathode materials for magnesium batteries represents a critical factor in their potential market adoption. Current cost analysis indicates that organic materials offer significant advantages over traditional inorganic cathodes, with raw material costs potentially 30-50% lower due to the abundance of carbon, hydrogen, oxygen, and nitrogen elements. The synthesis processes for organic cathodes typically require fewer energy-intensive steps and operate at lower temperatures (often below 200°C) compared to inorganic materials that may require processing at 600-800°C, resulting in reduced manufacturing energy consumption and associated costs.
Production scalability presents a promising outlook, as many organic compounds can leverage existing pharmaceutical and fine chemical manufacturing infrastructure. This contrasts favorably with specialized equipment needed for conventional battery material production. Several economic models project that at scale, organic cathode-based magnesium batteries could achieve costs below $150/kWh, approaching the $100/kWh threshold considered necessary for widespread electric vehicle adoption.
Material sustainability further enhances commercial viability through reduced environmental compliance costs. Many organic cathode materials can be derived from renewable resources or even recycled biomass, potentially creating circular economy opportunities that traditional cathode materials cannot match. Life cycle assessments indicate potential carbon footprint reductions of 25-40% compared to conventional battery technologies.
Market entry barriers remain significant despite these advantages. The battery industry's established supply chains and manufacturing processes are heavily optimized for current technologies, creating institutional resistance to new material systems. Initial capital investments for commercial-scale production facilities are estimated at $50-100 million, requiring substantial financial commitment before economies of scale can be realized.
Commercialization timelines suggest organic cathode magnesium batteries may reach specialized markets within 3-5 years, focusing initially on stationary storage applications where energy density requirements are less stringent. Mass market applications, particularly in the automotive sector, face a longer horizon of 7-10 years before achieving cost parity with lithium-ion technologies. This gradual market penetration strategy allows for manufacturing optimization while building consumer confidence in the technology.
Strategic partnerships between material developers, battery manufacturers, and end-users will be essential to overcome the "valley of death" between laboratory success and commercial viability. Several joint ventures between chemical companies and battery manufacturers have already formed to accelerate this transition, with combined investments exceeding $300 million in the past three years.
Production scalability presents a promising outlook, as many organic compounds can leverage existing pharmaceutical and fine chemical manufacturing infrastructure. This contrasts favorably with specialized equipment needed for conventional battery material production. Several economic models project that at scale, organic cathode-based magnesium batteries could achieve costs below $150/kWh, approaching the $100/kWh threshold considered necessary for widespread electric vehicle adoption.
Material sustainability further enhances commercial viability through reduced environmental compliance costs. Many organic cathode materials can be derived from renewable resources or even recycled biomass, potentially creating circular economy opportunities that traditional cathode materials cannot match. Life cycle assessments indicate potential carbon footprint reductions of 25-40% compared to conventional battery technologies.
Market entry barriers remain significant despite these advantages. The battery industry's established supply chains and manufacturing processes are heavily optimized for current technologies, creating institutional resistance to new material systems. Initial capital investments for commercial-scale production facilities are estimated at $50-100 million, requiring substantial financial commitment before economies of scale can be realized.
Commercialization timelines suggest organic cathode magnesium batteries may reach specialized markets within 3-5 years, focusing initially on stationary storage applications where energy density requirements are less stringent. Mass market applications, particularly in the automotive sector, face a longer horizon of 7-10 years before achieving cost parity with lithium-ion technologies. This gradual market penetration strategy allows for manufacturing optimization while building consumer confidence in the technology.
Strategic partnerships between material developers, battery manufacturers, and end-users will be essential to overcome the "valley of death" between laboratory success and commercial viability. Several joint ventures between chemical companies and battery manufacturers have already formed to accelerate this transition, with combined investments exceeding $300 million in the past three years.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!