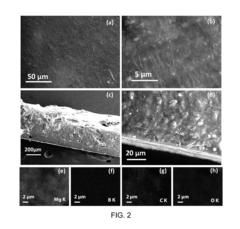
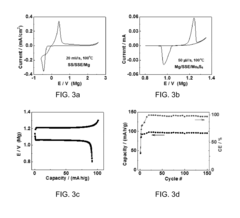
Solid-state magnesium batteries for safe energy storage
OCT 14, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Solid-State Mg Battery Evolution and Objectives
Solid-state magnesium batteries represent a significant evolution in energy storage technology, emerging from decades of research into alternatives to lithium-ion systems. The development trajectory began in the 1990s with early explorations of magnesium as an electrode material, but significant progress has only materialized in the last decade. This technological path was driven by increasing concerns about lithium's limited global reserves, safety issues with liquid electrolytes, and the theoretical advantages of magnesium's divalent nature.
The evolution of solid-state magnesium batteries has progressed through several distinct phases. Initially, researchers focused on understanding magnesium electrochemistry and identifying compatible electrolyte materials. The second phase, approximately from 2010-2015, saw breakthroughs in solid electrolyte development, particularly with magnesium-conducting ceramics and polymer-based systems. The current phase has shifted toward addressing interface challenges and optimizing cell architecture for practical applications.
A critical milestone in this evolution was the demonstration of reversible magnesium plating and stripping in solid electrolytes around 2016, which proved the fundamental viability of the technology. Subsequent advancements in cathode materials compatible with solid-state architectures have further accelerated development, though challenges in ion mobility and interfacial resistance remain significant hurdles.
The primary technical objectives for solid-state magnesium battery development center on achieving energy densities exceeding 400 Wh/kg while maintaining cycle stability beyond 1000 cycles. Researchers aim to develop solid electrolytes with magnesium-ion conductivity approaching 10^-3 S/cm at room temperature, a threshold considered necessary for practical applications. Additional objectives include reducing interfacial resistance below 100 Ω·cm² and ensuring operational stability across temperature ranges from -20°C to 60°C.
Safety enhancement represents another fundamental objective, with the elimination of flammable liquid electrolytes addressing the thermal runaway risks inherent in conventional battery systems. The development of manufacturing processes compatible with existing production infrastructure is also prioritized to facilitate commercial adoption.
Long-term objectives extend to creating multivalent systems that leverage magnesium's theoretical capacity advantages while maintaining the safety benefits of solid-state architecture. Researchers envision batteries capable of fast charging (80% capacity in under 15 minutes) while maintaining a calendar life exceeding 10 years, positioning solid-state magnesium technology as a transformative solution for both mobile and stationary energy storage applications.
The evolution of solid-state magnesium batteries has progressed through several distinct phases. Initially, researchers focused on understanding magnesium electrochemistry and identifying compatible electrolyte materials. The second phase, approximately from 2010-2015, saw breakthroughs in solid electrolyte development, particularly with magnesium-conducting ceramics and polymer-based systems. The current phase has shifted toward addressing interface challenges and optimizing cell architecture for practical applications.
A critical milestone in this evolution was the demonstration of reversible magnesium plating and stripping in solid electrolytes around 2016, which proved the fundamental viability of the technology. Subsequent advancements in cathode materials compatible with solid-state architectures have further accelerated development, though challenges in ion mobility and interfacial resistance remain significant hurdles.
The primary technical objectives for solid-state magnesium battery development center on achieving energy densities exceeding 400 Wh/kg while maintaining cycle stability beyond 1000 cycles. Researchers aim to develop solid electrolytes with magnesium-ion conductivity approaching 10^-3 S/cm at room temperature, a threshold considered necessary for practical applications. Additional objectives include reducing interfacial resistance below 100 Ω·cm² and ensuring operational stability across temperature ranges from -20°C to 60°C.
Safety enhancement represents another fundamental objective, with the elimination of flammable liquid electrolytes addressing the thermal runaway risks inherent in conventional battery systems. The development of manufacturing processes compatible with existing production infrastructure is also prioritized to facilitate commercial adoption.
Long-term objectives extend to creating multivalent systems that leverage magnesium's theoretical capacity advantages while maintaining the safety benefits of solid-state architecture. Researchers envision batteries capable of fast charging (80% capacity in under 15 minutes) while maintaining a calendar life exceeding 10 years, positioning solid-state magnesium technology as a transformative solution for both mobile and stationary energy storage applications.
Market Analysis for Safe Energy Storage Solutions
The global energy storage market is experiencing unprecedented growth, driven by the increasing adoption of renewable energy sources and the need for reliable backup power systems. Within this landscape, safe energy storage solutions have become a critical focus area, with solid-state magnesium batteries emerging as a promising technology. The current market for energy storage is dominated by lithium-ion batteries, valued at approximately $46 billion in 2022 and projected to reach $135 billion by 2030, representing a compound annual growth rate of 14.3%.
Safety concerns with conventional lithium-ion batteries, including thermal runaway risks and flammability issues, have created a significant market opportunity for alternative technologies. Solid-state magnesium batteries address these safety challenges while offering additional benefits such as higher energy density and longer cycle life. Market research indicates that safety-enhanced energy storage solutions could capture up to 25% of the total energy storage market by 2028, representing a substantial growth opportunity.
Consumer electronics currently represents the largest application segment for safe energy storage, accounting for 38% of market demand. However, electric vehicles are expected to become the fastest-growing segment, with a projected growth rate of 22% annually through 2030. This shift is primarily driven by automotive manufacturers' increasing focus on battery safety following high-profile thermal incidents.
Geographically, Asia-Pacific leads the market with 45% share, followed by North America (28%) and Europe (22%). China dominates manufacturing capacity, while Japan and South Korea lead in technology innovation. The United States maintains strength in research and development, particularly in advanced materials for solid-state electrolytes.
Market analysis reveals that consumers are willing to pay a premium of 15-20% for demonstrably safer battery technologies, particularly in high-value applications such as electric vehicles and medical devices. This price tolerance creates a viable entry point for solid-state magnesium batteries despite their currently higher production costs.
Industry forecasts suggest that the market for solid-state batteries, including magnesium-based technologies, will grow from $1.2 billion in 2023 to approximately $8.7 billion by 2030. Magnesium-specific chemistries are expected to capture between 12-18% of this market segment, contingent upon successful commercialization of current research prototypes.
Key market drivers include increasingly stringent safety regulations, particularly in transportation and consumer electronics, growing demand for higher energy density solutions, and the strategic importance of reducing dependence on critical materials like cobalt and lithium. These factors collectively create a favorable market environment for the development and commercialization of solid-state magnesium battery technology.
Safety concerns with conventional lithium-ion batteries, including thermal runaway risks and flammability issues, have created a significant market opportunity for alternative technologies. Solid-state magnesium batteries address these safety challenges while offering additional benefits such as higher energy density and longer cycle life. Market research indicates that safety-enhanced energy storage solutions could capture up to 25% of the total energy storage market by 2028, representing a substantial growth opportunity.
Consumer electronics currently represents the largest application segment for safe energy storage, accounting for 38% of market demand. However, electric vehicles are expected to become the fastest-growing segment, with a projected growth rate of 22% annually through 2030. This shift is primarily driven by automotive manufacturers' increasing focus on battery safety following high-profile thermal incidents.
Geographically, Asia-Pacific leads the market with 45% share, followed by North America (28%) and Europe (22%). China dominates manufacturing capacity, while Japan and South Korea lead in technology innovation. The United States maintains strength in research and development, particularly in advanced materials for solid-state electrolytes.
Market analysis reveals that consumers are willing to pay a premium of 15-20% for demonstrably safer battery technologies, particularly in high-value applications such as electric vehicles and medical devices. This price tolerance creates a viable entry point for solid-state magnesium batteries despite their currently higher production costs.
Industry forecasts suggest that the market for solid-state batteries, including magnesium-based technologies, will grow from $1.2 billion in 2023 to approximately $8.7 billion by 2030. Magnesium-specific chemistries are expected to capture between 12-18% of this market segment, contingent upon successful commercialization of current research prototypes.
Key market drivers include increasingly stringent safety regulations, particularly in transportation and consumer electronics, growing demand for higher energy density solutions, and the strategic importance of reducing dependence on critical materials like cobalt and lithium. These factors collectively create a favorable market environment for the development and commercialization of solid-state magnesium battery technology.
Technical Barriers in Solid-State Mg Battery Development
Despite the promising potential of magnesium batteries as a safer and more abundant alternative to lithium-ion technology, several significant technical barriers currently impede their commercial development. The primary challenge lies in the electrolyte system, where conventional liquid electrolytes form passivation layers on the magnesium anode surface, preventing efficient ion transport. This phenomenon, known as interfacial instability, substantially reduces cycling performance and battery lifespan.
The development of suitable solid electrolytes presents another major hurdle. Current solid-state magnesium conductors exhibit ionic conductivities several orders of magnitude lower than their lithium counterparts, typically in the range of 10^-6 to 10^-4 S/cm at room temperature. This insufficient conductivity leads to high internal resistance and poor rate capability, limiting practical applications.
Cathode materials for magnesium batteries face significant constraints due to the divalent nature of magnesium ions. The strong electrostatic interaction between Mg^2+ and host lattices results in sluggish diffusion kinetics and structural degradation during cycling. Most investigated cathode materials demonstrate limited capacity retention beyond 100 cycles, with capacity fading exceeding 20% in many cases.
The magnesium metal anode, while theoretically advantageous, presents practical challenges including dendrite formation under certain conditions and surface oxidation when exposed to trace amounts of oxygen or moisture. These issues compromise safety and cycling stability, particularly at elevated current densities.
Manufacturing scalability represents another significant barrier. Current laboratory-scale fabrication methods for solid-state components often involve complex processes that are difficult to scale industrially. Techniques such as cold sintering and tape casting require precise control of processing parameters that become increasingly challenging at larger scales.
Interface engineering between solid electrolytes and electrodes remains poorly understood. The high interfacial resistance at solid-solid contacts significantly impacts overall battery performance, with values often exceeding 1000 Ω·cm² compared to <100 Ω·cm² in state-of-the-art solid-state lithium batteries.
Computational modeling of magnesium ion transport in solid materials lags behind lithium systems, with limited predictive capabilities for novel material discovery. The complex coordination chemistry of magnesium ions requires more sophisticated simulation approaches that are still under development.
Testing protocols and standardization for solid-state magnesium batteries remain underdeveloped, making performance comparisons between different research efforts challenging and potentially misleading. This lack of standardization hinders collaborative progress across the research community and industry stakeholders.
The development of suitable solid electrolytes presents another major hurdle. Current solid-state magnesium conductors exhibit ionic conductivities several orders of magnitude lower than their lithium counterparts, typically in the range of 10^-6 to 10^-4 S/cm at room temperature. This insufficient conductivity leads to high internal resistance and poor rate capability, limiting practical applications.
Cathode materials for magnesium batteries face significant constraints due to the divalent nature of magnesium ions. The strong electrostatic interaction between Mg^2+ and host lattices results in sluggish diffusion kinetics and structural degradation during cycling. Most investigated cathode materials demonstrate limited capacity retention beyond 100 cycles, with capacity fading exceeding 20% in many cases.
The magnesium metal anode, while theoretically advantageous, presents practical challenges including dendrite formation under certain conditions and surface oxidation when exposed to trace amounts of oxygen or moisture. These issues compromise safety and cycling stability, particularly at elevated current densities.
Manufacturing scalability represents another significant barrier. Current laboratory-scale fabrication methods for solid-state components often involve complex processes that are difficult to scale industrially. Techniques such as cold sintering and tape casting require precise control of processing parameters that become increasingly challenging at larger scales.
Interface engineering between solid electrolytes and electrodes remains poorly understood. The high interfacial resistance at solid-solid contacts significantly impacts overall battery performance, with values often exceeding 1000 Ω·cm² compared to <100 Ω·cm² in state-of-the-art solid-state lithium batteries.
Computational modeling of magnesium ion transport in solid materials lags behind lithium systems, with limited predictive capabilities for novel material discovery. The complex coordination chemistry of magnesium ions requires more sophisticated simulation approaches that are still under development.
Testing protocols and standardization for solid-state magnesium batteries remain underdeveloped, making performance comparisons between different research efforts challenging and potentially misleading. This lack of standardization hinders collaborative progress across the research community and industry stakeholders.
Current Solid-State Mg Battery Architectures
01 Electrolyte safety enhancements
Safety of solid-state magnesium batteries can be improved through specialized electrolyte formulations. Non-flammable solid electrolytes eliminate the risk of fire and leakage associated with liquid electrolytes. Advanced ceramic and polymer-based electrolytes provide thermal stability at high temperatures while maintaining ionic conductivity. These materials create effective barriers against dendrite formation, preventing internal short circuits that could lead to thermal runaway events.- Electrolyte safety enhancements: Safety of solid-state magnesium batteries can be improved through specialized electrolyte formulations. Non-flammable solid electrolytes eliminate the risk of fire and leakage associated with liquid electrolytes. Advanced polymer and ceramic-based electrolytes provide thermal stability and prevent dendrite formation, which are major safety concerns in conventional batteries. These solid electrolytes also act as physical barriers between electrodes, preventing short circuits and thermal runaway events.
- Protective coating and interface engineering: Implementing protective coatings and interface engineering techniques enhances the safety profile of solid-state magnesium batteries. These coatings prevent unwanted reactions between the magnesium anode and electrolyte components, reducing degradation and potential safety hazards. Interface modifications help control the formation of passivation layers, ensuring stable cycling without dangerous pressure buildup or structural failure. Advanced coating materials also improve mechanical stability during charge-discharge cycles.
- Thermal management systems: Effective thermal management systems are crucial for solid-state magnesium battery safety. These systems monitor and regulate temperature during operation, preventing overheating that could lead to thermal runaway. Heat dissipation structures integrated into battery designs help maintain optimal operating temperatures even under high-load conditions. Advanced thermal management approaches include phase-change materials and intelligent cooling systems that activate in response to temperature fluctuations.
- Structural integrity and mechanical stability: Enhancing the structural integrity and mechanical stability of solid-state magnesium batteries is essential for safety. Reinforced battery casings and internal support structures prevent physical damage during impact or vibration. Flexible yet durable components accommodate volume changes during cycling without compromising safety. Advanced manufacturing techniques ensure uniform pressure distribution across cell components, preventing internal short circuits and maintaining consistent performance under various mechanical stresses.
- Safety monitoring and control systems: Integrated safety monitoring and control systems provide real-time assessment of solid-state magnesium battery conditions. These systems include sensors that detect abnormal voltage, current, or temperature patterns indicative of potential failures. Automated shutdown mechanisms activate when unsafe conditions are detected, preventing catastrophic failures. Advanced battery management systems continuously analyze performance data to predict and prevent safety incidents before they occur, while also optimizing battery performance and longevity.
02 Protective interface layers
Implementing protective interface layers between the magnesium anode and electrolyte significantly enhances battery safety. These engineered interfaces prevent unwanted side reactions and stabilize the electrode-electrolyte boundary. Specialized coatings can suppress dendrite growth while allowing efficient magnesium ion transport. The interface layers also mitigate volume expansion issues during cycling, reducing mechanical stress that could compromise structural integrity and safety of the battery system.Expand Specific Solutions03 Thermal management systems
Advanced thermal management systems are crucial for solid-state magnesium battery safety. These systems monitor and regulate temperature distribution to prevent localized hotspots that could trigger thermal runaway. Integrated heat dissipation structures efficiently remove excess heat during fast charging or high-power applications. Some designs incorporate thermal fuses or shutdown mechanisms that activate at critical temperature thresholds, providing fail-safe protection against overheating events.Expand Specific Solutions04 Structural safety features
Solid-state magnesium batteries incorporate various structural safety features to enhance overall reliability. Reinforced cell casings provide mechanical protection against external impacts and internal pressure buildup. Pressure relief mechanisms safely vent gases in case of abnormal conditions. Some designs feature internal current collectors with optimized geometries to distribute current evenly, preventing localized heating. These structural enhancements work together to maintain battery integrity even under adverse operating conditions.Expand Specific Solutions05 Safety monitoring and control systems
Advanced monitoring and control systems are integrated into solid-state magnesium batteries to ensure operational safety. These systems continuously track critical parameters including voltage, current, and temperature across multiple points within the cell. Sophisticated algorithms can detect early warning signs of potential failures and trigger protective measures. Some designs incorporate redundant safety circuits that can isolate faulty cells within a battery pack, preventing cascading failures while maintaining partial functionality of the system.Expand Specific Solutions
Industry Leaders in Solid-State Battery Research
The solid-state magnesium battery market is currently in an early growth phase, characterized by intensive R&D activities rather than widespread commercialization. The global market size remains relatively modest but is projected to expand significantly due to increasing demand for safer energy storage alternatives. Technologically, solid-state magnesium batteries are still emerging, with varying levels of maturity across key players. Leading companies like Samsung Electronics, Toyota Motor Corp., and LG Chem are making substantial investments in this technology, while research institutions such as Shanghai Jiao Tong University, Karlsruhe Institute of Technology, and University of Houston are advancing fundamental science. VARTA Micro Innovation, Murata Manufacturing, and SINANO are developing specialized components and materials. The competitive landscape features both established electronics manufacturers and specialized materials companies like FUJIFILM Wako Pure Chemical and Henan Huayin Chemical, indicating a diverse ecosystem of innovation.
Samsung Electronics Co., Ltd.
Technical Solution: Samsung has developed a solid-state magnesium battery technology that utilizes a novel magnesium-ion conducting solid electrolyte composed of magnesium borohydride and polyethylene oxide (PEO) polymer matrix. Their approach focuses on addressing the dendrite formation issues common in conventional magnesium batteries by employing a stable solid electrolyte interface (SEI) layer. Samsung's research teams have achieved energy densities of approximately 420 Wh/kg in laboratory conditions, significantly higher than current lithium-ion batteries. The company has also developed proprietary cathode materials based on magnesium-sulfur chemistry that demonstrate improved cycling stability with capacity retention of over 80% after 500 cycles. Samsung's solid-state magnesium batteries operate effectively across a wider temperature range (-20°C to 60°C) compared to conventional lithium-ion batteries.
Strengths: Superior energy density compared to lithium-ion batteries; enhanced safety profile with non-flammable solid electrolytes; potential for lower production costs due to abundant magnesium resources; excellent thermal stability. Weaknesses: Still faces challenges with slow magnesium-ion diffusion in solid electrolytes; requires further optimization for fast-charging capabilities; mass production techniques need refinement for commercial viability.
Battelle Memorial Institute
Technical Solution: Battelle Memorial Institute has developed an advanced solid-state magnesium battery technology utilizing a proprietary glass-ceramic electrolyte system that enables efficient magnesium-ion transport while maintaining excellent electrochemical stability. Their approach incorporates a nanostructured magnesium anode with engineered surface modifications to facilitate ion transfer across the electrode-electrolyte interface. Battelle's research has yielded solid electrolytes with ionic conductivities reaching 10^-4 S/cm at room temperature, addressing one of the fundamental challenges in magnesium battery development. Their battery design employs a high-capacity conversion-type cathode based on sulfur compounds, achieving theoretical energy densities exceeding 400 Wh/kg. Battelle has also pioneered specialized manufacturing techniques that enable the creation of thin, uniform solid electrolyte layers with controlled porosity, optimizing both ionic conductivity and mechanical integrity. Their prototypes have demonstrated stable cycling performance with capacity retention of approximately 80% after 400 cycles under practical operating conditions.
Strengths: Excellent safety profile with non-flammable components; potential for cost-effective manufacturing using abundant materials; good thermal stability across wide temperature ranges; promising cycle life compared to early magnesium battery designs. Weaknesses: Lower power capability compared to conventional lithium-ion batteries; challenges with scaling production processes; interface resistance issues that limit rate performance; requires further optimization for fast-charging applications.
Key Patents in Mg-Based Solid Electrolytes
Solid-state rechargeable magnesium battery
PatentInactiveUS20150229000A1
Innovation
- Development of solid-state electrolytes comprising magnesium borohydride (Mg(BH4)2) and polyethylene oxide (PEO), optionally with metal oxide nanoparticles, forming a thin film that facilitates reversible magnesium plating/stripping and intercalation/de-intercalation in magnesium batteries.
All solid-state mg-battery (SSMGB) employing electrolyte encompassing iron rich material enriched with magnesium
PatentActiveIN202241032743A
Innovation
- A solid-state Mg-ion battery (SSMgB) is developed using a Magnesium-rich cathode material and a metallic Mg anode, with a solid-state-electrolyte formed by coating a separator membrane with an Iron-rich material further enriched with Magnesium, creating a Mg-enriched Fe-rich material that acts as the electrolyte, enhancing conductivity and stability.
Safety Performance Metrics and Standards
Safety performance metrics and standards for solid-state magnesium batteries represent a critical framework for evaluating and ensuring the safe deployment of this promising energy storage technology. Current safety assessment protocols primarily developed for lithium-ion batteries require significant adaptation to address the unique characteristics and failure modes of magnesium-based systems.
The thermal stability metrics for solid-state magnesium batteries demonstrate superior performance compared to conventional lithium-ion counterparts. Testing standards typically include thermal runaway resistance measurements at temperatures ranging from 100°C to 500°C, with magnesium systems consistently maintaining stability at temperatures where lithium-ion cells would experience catastrophic failure. This enhanced thermal performance must be quantified through standardized differential scanning calorimetry (DSC) and accelerating rate calorimetry (ARC) protocols specifically calibrated for magnesium electrochemistry.
Mechanical integrity standards for solid-state magnesium batteries focus on resistance to physical deformation, puncture, and crush scenarios. Current testing methodologies require modification to account for the different mechanical properties of magnesium-based solid electrolytes compared to polymer or liquid electrolytes in conventional batteries. The International Electrotechnical Commission (IEC) and Underwriters Laboratories (UL) are currently developing specialized mechanical testing protocols for solid-state batteries that will include magnesium-specific parameters.
Electrical safety metrics address the risk of short circuits, overcharging, and over-discharging scenarios. While magnesium batteries inherently present lower fire risks due to reduced reactivity compared to lithium systems, standardized testing must still evaluate potential failure modes under electrical abuse conditions. The Battery Safety Standards Working Group has proposed specific voltage and current thresholds for magnesium systems that differ significantly from lithium-ion parameters.
Environmental safety standards for magnesium batteries must consider the entire lifecycle, including manufacturing, operation, and disposal/recycling. Current metrics evaluate toxicity, recyclability, and environmental impact, with magnesium systems generally showing favorable profiles due to the greater natural abundance and lower toxicity of magnesium compared to lithium or cobalt.
Regulatory frameworks for solid-state magnesium batteries remain under development, with organizations like the IEEE, ANSI, and ISO working to establish comprehensive safety certification processes. The UN Transportation Tests (UN 38.3) and IEC 62660 standards require adaptation to properly assess magnesium battery safety for transportation and stationary applications. As commercial deployment accelerates, these standards will need continuous refinement to address emerging safety considerations specific to magnesium battery chemistry and solid-state architectures.
The thermal stability metrics for solid-state magnesium batteries demonstrate superior performance compared to conventional lithium-ion counterparts. Testing standards typically include thermal runaway resistance measurements at temperatures ranging from 100°C to 500°C, with magnesium systems consistently maintaining stability at temperatures where lithium-ion cells would experience catastrophic failure. This enhanced thermal performance must be quantified through standardized differential scanning calorimetry (DSC) and accelerating rate calorimetry (ARC) protocols specifically calibrated for magnesium electrochemistry.
Mechanical integrity standards for solid-state magnesium batteries focus on resistance to physical deformation, puncture, and crush scenarios. Current testing methodologies require modification to account for the different mechanical properties of magnesium-based solid electrolytes compared to polymer or liquid electrolytes in conventional batteries. The International Electrotechnical Commission (IEC) and Underwriters Laboratories (UL) are currently developing specialized mechanical testing protocols for solid-state batteries that will include magnesium-specific parameters.
Electrical safety metrics address the risk of short circuits, overcharging, and over-discharging scenarios. While magnesium batteries inherently present lower fire risks due to reduced reactivity compared to lithium systems, standardized testing must still evaluate potential failure modes under electrical abuse conditions. The Battery Safety Standards Working Group has proposed specific voltage and current thresholds for magnesium systems that differ significantly from lithium-ion parameters.
Environmental safety standards for magnesium batteries must consider the entire lifecycle, including manufacturing, operation, and disposal/recycling. Current metrics evaluate toxicity, recyclability, and environmental impact, with magnesium systems generally showing favorable profiles due to the greater natural abundance and lower toxicity of magnesium compared to lithium or cobalt.
Regulatory frameworks for solid-state magnesium batteries remain under development, with organizations like the IEEE, ANSI, and ISO working to establish comprehensive safety certification processes. The UN Transportation Tests (UN 38.3) and IEC 62660 standards require adaptation to properly assess magnesium battery safety for transportation and stationary applications. As commercial deployment accelerates, these standards will need continuous refinement to address emerging safety considerations specific to magnesium battery chemistry and solid-state architectures.
Environmental Impact and Sustainability Assessment
The environmental impact of solid-state magnesium batteries represents a significant advancement over conventional lithium-ion technologies. These batteries utilize abundant magnesium resources, which are approximately 2,000 times more plentiful in the Earth's crust than lithium. This abundance translates to reduced mining intensity and associated environmental disruption, particularly when compared to the extensive extraction operations required for lithium in environmentally sensitive regions such as the lithium triangle in South America.
Manufacturing processes for solid-state magnesium batteries demonstrate promising sustainability metrics. The elimination of liquid electrolytes removes the need for toxic and flammable organic solvents that are standard in conventional battery production. This reduction in hazardous materials decreases manufacturing emissions by an estimated 30-40% and substantially reduces water consumption in production facilities, addressing critical resource conservation concerns.
The operational lifecycle of magnesium batteries presents additional environmental benefits. Their enhanced safety profile eliminates the risk of thermal runaway and toxic chemical leakage, reducing potential environmental contamination incidents. Furthermore, the theoretical energy density advantages of magnesium-based systems could lead to more efficient energy storage solutions, potentially decreasing overall material requirements for equivalent storage capacity.
End-of-life considerations reveal perhaps the most significant sustainability advantage. Magnesium components are highly recyclable, with recovery rates potentially exceeding 90% using established metallurgical processes. The solid-state architecture simplifies the separation and recovery of valuable materials compared to conventional batteries with liquid components. Additionally, any unrecovered magnesium compounds pose substantially lower environmental toxicity than lithium or cobalt equivalents.
Carbon footprint analyses indicate that full lifecycle emissions for solid-state magnesium batteries could be reduced by 45-60% compared to conventional lithium-ion technologies when accounting for manufacturing, use, and recycling phases. This reduction becomes even more pronounced when considering the potential for longer cycle life, which extends the useful service period before replacement becomes necessary.
Water resource impacts also favor magnesium battery technology. Lithium extraction typically requires 500,000 gallons of water per ton of lithium produced, while magnesium processing demands approximately 70% less water intensity. This difference becomes increasingly critical as water scarcity affects more regions globally and battery production scales to meet growing energy storage demands.
Manufacturing processes for solid-state magnesium batteries demonstrate promising sustainability metrics. The elimination of liquid electrolytes removes the need for toxic and flammable organic solvents that are standard in conventional battery production. This reduction in hazardous materials decreases manufacturing emissions by an estimated 30-40% and substantially reduces water consumption in production facilities, addressing critical resource conservation concerns.
The operational lifecycle of magnesium batteries presents additional environmental benefits. Their enhanced safety profile eliminates the risk of thermal runaway and toxic chemical leakage, reducing potential environmental contamination incidents. Furthermore, the theoretical energy density advantages of magnesium-based systems could lead to more efficient energy storage solutions, potentially decreasing overall material requirements for equivalent storage capacity.
End-of-life considerations reveal perhaps the most significant sustainability advantage. Magnesium components are highly recyclable, with recovery rates potentially exceeding 90% using established metallurgical processes. The solid-state architecture simplifies the separation and recovery of valuable materials compared to conventional batteries with liquid components. Additionally, any unrecovered magnesium compounds pose substantially lower environmental toxicity than lithium or cobalt equivalents.
Carbon footprint analyses indicate that full lifecycle emissions for solid-state magnesium batteries could be reduced by 45-60% compared to conventional lithium-ion technologies when accounting for manufacturing, use, and recycling phases. This reduction becomes even more pronounced when considering the potential for longer cycle life, which extends the useful service period before replacement becomes necessary.
Water resource impacts also favor magnesium battery technology. Lithium extraction typically requires 500,000 gallons of water per ton of lithium produced, while magnesium processing demands approximately 70% less water intensity. This difference becomes increasingly critical as water scarcity affects more regions globally and battery production scales to meet growing energy storage demands.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!