Electrodeposition morphology control in magnesium batteries
OCT 14, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Mg Battery Electrodeposition Background and Objectives
Magnesium batteries have emerged as a promising alternative to lithium-ion batteries due to their potential advantages in safety, cost, and energy density. The electrodeposition process, which involves the deposition of magnesium ions onto an electrode surface during charging, plays a crucial role in determining the performance and lifespan of these batteries. The morphology of these deposits significantly impacts battery efficiency, safety, and cycle life, making morphology control a critical area of research.
The evolution of magnesium battery technology can be traced back to the early 1990s, but significant progress has been made only in the past decade. Initially, researchers focused primarily on electrolyte development to enable reversible magnesium deposition. However, attention has gradually shifted toward understanding and controlling the electrodeposition process itself, as irregular deposition patterns can lead to dendrite formation, short circuits, and premature battery failure.
Recent technological trends indicate a growing interest in advanced characterization techniques to observe magnesium deposition in real-time, including in-situ transmission electron microscopy and atomic force microscopy. These tools have revealed that magnesium tends to deposit in different morphologies depending on electrolyte composition, current density, and electrode surface properties, ranging from smooth films to dendritic structures.
The primary technical objective in this field is to achieve uniform, dendrite-free magnesium electrodeposition that maintains consistency over multiple charge-discharge cycles. This involves developing strategies to control nucleation sites, growth rates, and crystal orientation during the deposition process. Secondary objectives include improving the reversibility of the magnesium deposition/dissolution process and reducing the overpotential required for deposition.
Understanding the fundamental mechanisms governing magnesium electrodeposition represents another critical goal. This includes elucidating the role of the solid electrolyte interphase (SEI) layer, solvation structures, and surface chemistry in determining deposit morphology. By gaining insights into these mechanisms, researchers aim to develop rational design principles for electrolytes and electrode materials that promote favorable deposition patterns.
The long-term vision for this technology involves enabling high-energy-density, safe, and cost-effective magnesium batteries that can compete with or surpass lithium-ion technology in specific applications. Achieving controlled electrodeposition is widely recognized as one of the key hurdles that must be overcome to realize this vision, making it a focal point for research efforts worldwide.
The evolution of magnesium battery technology can be traced back to the early 1990s, but significant progress has been made only in the past decade. Initially, researchers focused primarily on electrolyte development to enable reversible magnesium deposition. However, attention has gradually shifted toward understanding and controlling the electrodeposition process itself, as irregular deposition patterns can lead to dendrite formation, short circuits, and premature battery failure.
Recent technological trends indicate a growing interest in advanced characterization techniques to observe magnesium deposition in real-time, including in-situ transmission electron microscopy and atomic force microscopy. These tools have revealed that magnesium tends to deposit in different morphologies depending on electrolyte composition, current density, and electrode surface properties, ranging from smooth films to dendritic structures.
The primary technical objective in this field is to achieve uniform, dendrite-free magnesium electrodeposition that maintains consistency over multiple charge-discharge cycles. This involves developing strategies to control nucleation sites, growth rates, and crystal orientation during the deposition process. Secondary objectives include improving the reversibility of the magnesium deposition/dissolution process and reducing the overpotential required for deposition.
Understanding the fundamental mechanisms governing magnesium electrodeposition represents another critical goal. This includes elucidating the role of the solid electrolyte interphase (SEI) layer, solvation structures, and surface chemistry in determining deposit morphology. By gaining insights into these mechanisms, researchers aim to develop rational design principles for electrolytes and electrode materials that promote favorable deposition patterns.
The long-term vision for this technology involves enabling high-energy-density, safe, and cost-effective magnesium batteries that can compete with or surpass lithium-ion technology in specific applications. Achieving controlled electrodeposition is widely recognized as one of the key hurdles that must be overcome to realize this vision, making it a focal point for research efforts worldwide.
Market Analysis for Next-Generation Mg Battery Technologies
The global magnesium battery market is experiencing significant growth potential, driven by increasing demand for sustainable energy storage solutions. Current market valuations indicate that while lithium-ion batteries dominate the energy storage landscape with a market size exceeding $50 billion, magnesium battery technologies are emerging as a promising alternative, with projections suggesting a compound annual growth rate of 12-15% over the next decade.
The primary market drivers for magnesium battery technologies include the abundant availability of magnesium resources, which are approximately 1000 times more plentiful in the earth's crust than lithium. This abundance translates to potentially lower raw material costs and reduced supply chain vulnerabilities. Additionally, magnesium's theoretical energy density of 3.8 Ah/g surpasses that of lithium (3.86 Ah/g) when considering volumetric capacity, making it attractive for applications where space constraints are critical.
Market segmentation for next-generation magnesium batteries reveals several key application areas. The electric vehicle sector represents the largest potential market, with automotive manufacturers actively seeking alternatives to lithium-ion batteries due to concerns about resource limitations and cost fluctuations. The stationary energy storage sector follows closely, particularly for grid-scale applications where cost-effectiveness over long lifecycles is prioritized over energy density.
Consumer electronics constitutes another significant market segment, where the safety advantages of magnesium batteries—including non-flammability and stability—could provide competitive differentiation. Military and aerospace applications represent a smaller but premium market segment, where the robustness and theoretical performance capabilities of magnesium systems align with demanding operational requirements.
Regional market analysis indicates that Asia-Pacific, particularly China, Japan, and South Korea, leads in research investment and potential manufacturing capacity for magnesium battery technologies. North America and Europe follow with substantial research initiatives but less immediate commercialization infrastructure.
Market barriers include the technical challenges of electrodeposition morphology control, which directly impacts cycle life and performance consistency. The current performance gap between prototype magnesium batteries and commercial lithium-ion systems represents a significant market entry barrier, with investors requiring clear technology roadmaps demonstrating pathways to performance parity or superiority.
The competitive landscape features both established battery manufacturers exploring magnesium technologies as portfolio diversification and specialized startups focused exclusively on overcoming specific technical challenges like dendrite formation during electrodeposition. Strategic partnerships between material science companies, battery manufacturers, and end-users are increasingly common, accelerating the transition from laboratory research to commercial viability.
The primary market drivers for magnesium battery technologies include the abundant availability of magnesium resources, which are approximately 1000 times more plentiful in the earth's crust than lithium. This abundance translates to potentially lower raw material costs and reduced supply chain vulnerabilities. Additionally, magnesium's theoretical energy density of 3.8 Ah/g surpasses that of lithium (3.86 Ah/g) when considering volumetric capacity, making it attractive for applications where space constraints are critical.
Market segmentation for next-generation magnesium batteries reveals several key application areas. The electric vehicle sector represents the largest potential market, with automotive manufacturers actively seeking alternatives to lithium-ion batteries due to concerns about resource limitations and cost fluctuations. The stationary energy storage sector follows closely, particularly for grid-scale applications where cost-effectiveness over long lifecycles is prioritized over energy density.
Consumer electronics constitutes another significant market segment, where the safety advantages of magnesium batteries—including non-flammability and stability—could provide competitive differentiation. Military and aerospace applications represent a smaller but premium market segment, where the robustness and theoretical performance capabilities of magnesium systems align with demanding operational requirements.
Regional market analysis indicates that Asia-Pacific, particularly China, Japan, and South Korea, leads in research investment and potential manufacturing capacity for magnesium battery technologies. North America and Europe follow with substantial research initiatives but less immediate commercialization infrastructure.
Market barriers include the technical challenges of electrodeposition morphology control, which directly impacts cycle life and performance consistency. The current performance gap between prototype magnesium batteries and commercial lithium-ion systems represents a significant market entry barrier, with investors requiring clear technology roadmaps demonstrating pathways to performance parity or superiority.
The competitive landscape features both established battery manufacturers exploring magnesium technologies as portfolio diversification and specialized startups focused exclusively on overcoming specific technical challenges like dendrite formation during electrodeposition. Strategic partnerships between material science companies, battery manufacturers, and end-users are increasingly common, accelerating the transition from laboratory research to commercial viability.
Current Challenges in Mg Electrodeposition Morphology Control
Despite significant advancements in magnesium battery technology, electrodeposition morphology control remains one of the most critical challenges hindering commercial viability. The fundamental issue stems from magnesium's tendency to form dendritic structures during plating, which can lead to short circuits, reduced cycling efficiency, and safety hazards. Unlike lithium-ion batteries, where dendrite formation mechanisms are well understood, magnesium electrodeposition follows different pathways that are not fully characterized.
The electrolyte composition presents a significant challenge in controlling deposition morphology. Conventional magnesium electrolytes, particularly those containing chloride-based compounds, often promote uneven deposition due to their complex coordination chemistry with magnesium ions. These electrolytes frequently suffer from narrow electrochemical windows and high sensitivity to moisture and oxygen, further complicating morphology control efforts.
Surface chemistry at the electrode-electrolyte interface represents another major hurdle. The formation of passivation layers on magnesium anodes can lead to inhomogeneous current distribution, resulting in preferential deposition sites and irregular morphology. Unlike the beneficial SEI layer in lithium-ion batteries, these passivation films in magnesium systems often increase impedance and promote non-uniform deposition.
Current density distribution poses additional challenges, as high local current densities can accelerate dendritic growth. The lack of advanced in-situ characterization techniques specifically designed for magnesium systems limits researchers' ability to observe and understand real-time morphological evolution during cycling, making it difficult to develop effective control strategies.
Temperature management during electrodeposition represents another significant challenge. Magnesium plating kinetics are highly temperature-dependent, with lower temperatures often exacerbating irregular deposition patterns. However, elevated temperatures can accelerate side reactions with electrolytes, creating a narrow operational window for optimal morphology control.
The mechanical properties of magnesium deposits further complicate control efforts. The relatively high hardness and brittleness of magnesium compared to lithium can lead to stress accumulation during cycling, promoting crack formation and irregular growth patterns that compromise battery performance and safety.
Scaling laboratory solutions to commercial applications presents perhaps the most significant practical challenge. Techniques that successfully control morphology in small-scale experimental cells often fail when implemented in larger format batteries due to differences in current distribution, heat management, and pressure conditions. This scaling gap has prevented many promising laboratory advances from reaching commercial viability.
The electrolyte composition presents a significant challenge in controlling deposition morphology. Conventional magnesium electrolytes, particularly those containing chloride-based compounds, often promote uneven deposition due to their complex coordination chemistry with magnesium ions. These electrolytes frequently suffer from narrow electrochemical windows and high sensitivity to moisture and oxygen, further complicating morphology control efforts.
Surface chemistry at the electrode-electrolyte interface represents another major hurdle. The formation of passivation layers on magnesium anodes can lead to inhomogeneous current distribution, resulting in preferential deposition sites and irregular morphology. Unlike the beneficial SEI layer in lithium-ion batteries, these passivation films in magnesium systems often increase impedance and promote non-uniform deposition.
Current density distribution poses additional challenges, as high local current densities can accelerate dendritic growth. The lack of advanced in-situ characterization techniques specifically designed for magnesium systems limits researchers' ability to observe and understand real-time morphological evolution during cycling, making it difficult to develop effective control strategies.
Temperature management during electrodeposition represents another significant challenge. Magnesium plating kinetics are highly temperature-dependent, with lower temperatures often exacerbating irregular deposition patterns. However, elevated temperatures can accelerate side reactions with electrolytes, creating a narrow operational window for optimal morphology control.
The mechanical properties of magnesium deposits further complicate control efforts. The relatively high hardness and brittleness of magnesium compared to lithium can lead to stress accumulation during cycling, promoting crack formation and irregular growth patterns that compromise battery performance and safety.
Scaling laboratory solutions to commercial applications presents perhaps the most significant practical challenge. Techniques that successfully control morphology in small-scale experimental cells often fail when implemented in larger format batteries due to differences in current distribution, heat management, and pressure conditions. This scaling gap has prevented many promising laboratory advances from reaching commercial viability.
Current Approaches to Mg Electrodeposition Morphology Control
01 Electrolyte composition effects on Mg electrodeposition morphology
The composition of electrolytes significantly influences the morphology of magnesium electrodeposition in batteries. Different electrolyte formulations can lead to varied deposition patterns, from dendritic to smooth uniform layers. Specific additives and solvent systems can be optimized to control nucleation and growth processes, resulting in more stable and efficient magnesium anodes with improved cycling performance.- Electrolyte composition effects on Mg electrodeposition morphology: The composition of electrolytes significantly influences the morphology of magnesium electrodeposition in batteries. Different electrolyte formulations can lead to varied deposition patterns, from dendritic to smooth uniform layers. Specific additives and solvent systems can promote more homogeneous deposition, reducing the formation of dendrites that could cause short circuits. Optimized electrolyte compositions enable better control over the electrodeposition process, improving the cycling stability and safety of magnesium batteries.
- Surface modification techniques for controlled Mg deposition: Surface modification of electrodes plays a crucial role in controlling magnesium electrodeposition morphology. Various techniques including coating with protective layers, surface functionalization, and nanostructuring can be employed to guide the deposition process. These modifications can create preferential nucleation sites, reduce interfacial resistance, and promote uniform magnesium deposition. By engineering the electrode surface properties, dendrite formation can be suppressed while enhancing the electrochemical performance of magnesium batteries.
- Current density and pulse electrodeposition strategies: The applied current density and electrodeposition protocols significantly impact the morphology of deposited magnesium. Pulse electrodeposition techniques, where current is applied intermittently rather than continuously, can produce more uniform and dense magnesium deposits. Controlling parameters such as pulse duration, frequency, and amplitude allows for precise management of nucleation and growth processes. These strategies help minimize dendritic growth and improve the quality of the electrodeposited magnesium layer, enhancing battery performance and safety.
- Temperature and pressure effects on deposition morphology: Temperature and pressure conditions during electrodeposition significantly influence the crystallization and growth patterns of magnesium deposits. Operating at optimized temperature ranges can affect the kinetics of ion transport and reduction reactions, leading to more favorable deposition morphologies. Similarly, controlled pressure environments can impact the solubility of reaction species and the stability of intermediates formed during electrodeposition. These parameters can be adjusted to achieve smoother, more compact magnesium deposits with improved electrochemical properties.
- Novel electrode materials for improved Mg deposition: Innovative electrode materials can fundamentally alter magnesium electrodeposition behavior. Advanced materials including nanostructured carbon, metal alloys, and composite electrodes offer unique surface properties that can guide magnesium deposition. These materials may provide enhanced conductivity, increased active surface area, or specific crystal orientation that promotes uniform magnesium growth. The development of such electrode materials represents a promising approach to overcome challenges related to irregular deposition morphologies in magnesium batteries, potentially enabling higher energy density and longer cycle life.
02 Surface modification techniques for controlled Mg deposition
Surface modification of electrodes plays a crucial role in controlling magnesium electrodeposition morphology. Techniques include coating substrates with specific materials, surface functionalization, and creating engineered interfaces that guide deposition patterns. These modifications can suppress dendritic growth, improve adhesion of deposited magnesium, and enhance the uniformity of the deposition layer, leading to better battery performance and safety.Expand Specific Solutions03 Current density and pulse electrodeposition strategies
The application of controlled current densities and pulse electrodeposition techniques significantly impacts magnesium deposition morphology. Lower current densities typically produce more uniform deposits, while pulsed electrodeposition can disrupt dendritic growth patterns. These approaches allow for precise control over nucleation and growth kinetics, resulting in more compact and homogeneous magnesium layers with improved electrochemical performance.Expand Specific Solutions04 Temperature and pressure effects on deposition characteristics
Temperature and pressure conditions during electrodeposition significantly influence the morphology of magnesium deposits. Higher temperatures can increase ion mobility and reaction kinetics, while controlled pressure environments can affect the solubility of intermediates and reaction pathways. Optimizing these parameters helps achieve desired crystal structures and surface characteristics, leading to improved battery performance and cycle life.Expand Specific Solutions05 Novel electrode materials and structures for improved Mg deposition
Innovative electrode materials and architectures are being developed to enhance magnesium electrodeposition morphology. These include three-dimensional electrode structures, composite materials with specific surface properties, and hierarchical porous frameworks. Such designs provide controlled nucleation sites, facilitate ion transport, and accommodate volume changes during cycling, resulting in more uniform magnesium deposition and superior battery performance.Expand Specific Solutions
Leading Research Groups and Industry Players in Mg Batteries
The magnesium battery electrodeposition morphology control market is currently in an early growth phase, with increasing research activity but limited commercial deployment. The global market size remains relatively small but is expanding as magnesium batteries emerge as promising alternatives to lithium-ion technology. Leading players include established automotive manufacturers (Toyota Motor Corp., Honda Motor, Renault SA) investing in next-generation battery technologies, specialized battery companies (Furukawa Battery, VARTA Micro Innovation, Saft Groupe), and research-focused institutions. Technical maturity varies significantly across organizations, with Toyota, Samsung Electronics, and Pellion Technologies demonstrating more advanced capabilities in controlling dendritic growth and improving cycling stability. Academic institutions like Tsinghua University and Shanghai Jiao Tong University are making significant contributions to fundamental understanding of electrodeposition mechanisms.
Toyota Motor Corp.
Technical Solution: Toyota has developed advanced electrolyte formulations containing magnesium bis(trifluoromethanesulfonyl)imide (Mg(TFSI)2) with additives to control dendrite formation during magnesium electrodeposition. Their approach focuses on creating uniform deposition morphology through the use of electrolyte additives like magnesium fluoride (MgF2) that modify the solid electrolyte interphase (SEI) layer. Toyota's research demonstrates that controlling the composition of the electrolyte solution significantly impacts the nucleation and growth processes during magnesium deposition, resulting in smoother, more homogeneous deposits with reduced dendrite formation. They have also explored the use of ionic liquids as co-solvents to improve the electrochemical stability window and enhance the reversibility of magnesium deposition/dissolution processes, which is crucial for practical magnesium battery applications.
Strengths: Toyota's approach effectively reduces dendrite formation without sacrificing electrochemical performance, and their electrolyte formulations are compatible with existing battery manufacturing processes. Weaknesses: The additives may increase overall battery cost, and some formulations may have limited stability over extended cycling, particularly at elevated temperatures.
VARTA Micro Innovation GmbH
Technical Solution: VARTA has pioneered a surface modification technique for magnesium electrodeposition that utilizes nanoscale surface engineering of current collectors. Their approach involves creating precisely controlled surface roughness patterns on copper and stainless steel substrates through electrochemical etching and subsequent coating with thin layers of noble metals like gold or platinum. This creates preferential nucleation sites that guide magnesium deposition into uniform patterns. VARTA's research shows that these engineered surfaces can reduce the overpotential required for magnesium deposition by up to 40% while simultaneously improving the morphological uniformity of the deposits. The company has also developed proprietary electrolyte formulations containing chelating agents that coordinate with magnesium ions in solution, altering their solvation structure and deposition behavior to further enhance morphology control.
Strengths: VARTA's surface modification approach provides excellent control over deposition morphology without requiring complex electrolyte formulations, and the reduced overpotential improves energy efficiency. Weaknesses: The surface preparation techniques add manufacturing complexity and cost, and the long-term stability of the modified surfaces under repeated cycling needs further validation.
Key Patents and Scientific Breakthroughs in Mg Electrodeposition
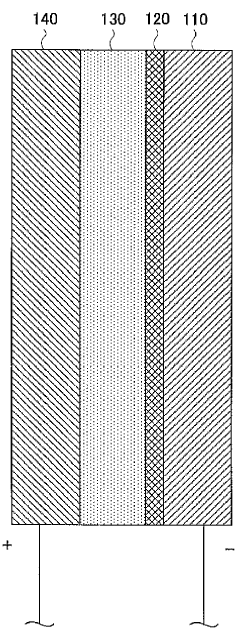
Magnesium battery
PatentWO2012020791A1
Innovation
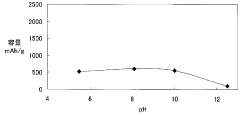
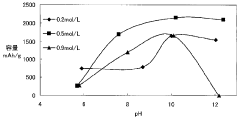
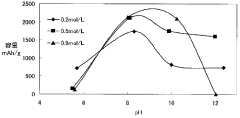
- A magnesium battery design utilizing a citrate aqueous solution as the electrolyte, which complexes magnesium ions to increase solubility, suppress magnesium oxide deposition, and maintain pH within a specific range to prevent hydrogen generation and film formation, along with an aluminum hydroxide complex to enhance hydrogenation voltage and self-discharge suppression.
Materials Science Innovations for Mg Battery Interfaces
The interface between magnesium metal anodes and electrolytes represents a critical frontier in advancing magnesium battery technology. Recent materials science innovations have focused on engineering these interfaces to overcome persistent challenges in electrochemical performance. Electrolyte formulations incorporating specific additives have demonstrated remarkable improvements in interfacial stability, with fluorinated compounds and crown ethers emerging as particularly effective in modifying the solid electrolyte interphase (SEI) properties.
Novel coating technologies have revolutionized magnesium anode protection strategies. Atomic layer deposition (ALD) techniques now enable the creation of ultrathin protective layers that simultaneously prevent parasitic reactions while facilitating magnesium ion transport. These nanoscale engineered interfaces have shown up to 300% improvement in cycling stability compared to unprotected anodes in recent studies.
Cathode-electrolyte interfaces have similarly benefited from materials science breakthroughs. Surface modification of sulfur cathodes with conductive polymers has addressed the notorious "shuttle effect" while maintaining high theoretical capacity. Additionally, innovative composite structures incorporating graphene and metal-organic frameworks have created hierarchical interfaces that optimize ion diffusion pathways.
Advanced characterization techniques have been instrumental in these developments. In-situ transmission electron microscopy now allows researchers to directly observe interfacial evolution during cycling, while synchrotron-based X-ray absorption spectroscopy provides atomic-level insights into chemical transformations at these critical boundaries. These analytical approaches have revealed previously undetected intermediate phases that form during magnesium deposition and stripping.
Computational modeling has accelerated interface engineering through density functional theory calculations that predict interfacial stability and reaction mechanisms. Machine learning algorithms have begun identifying optimal material combinations for specific interface requirements, significantly reducing experimental trial-and-error approaches.
Biomimetic interface designs represent an emerging frontier, with structures inspired by biological membranes showing promise for selective ion transport. These bio-inspired interfaces incorporate precisely positioned functional groups that facilitate magnesium ion desolvation while blocking larger solvent molecules, addressing a fundamental challenge in magnesium electrochemistry.
The integration of these materials science innovations has led to prototype cells demonstrating dramatically improved rate capability and cycle life. Commercial development remains challenged by scalability concerns, but recent manufacturing advances in controlled atmosphere processing suggest pathways toward practical implementation of these interface technologies.
Novel coating technologies have revolutionized magnesium anode protection strategies. Atomic layer deposition (ALD) techniques now enable the creation of ultrathin protective layers that simultaneously prevent parasitic reactions while facilitating magnesium ion transport. These nanoscale engineered interfaces have shown up to 300% improvement in cycling stability compared to unprotected anodes in recent studies.
Cathode-electrolyte interfaces have similarly benefited from materials science breakthroughs. Surface modification of sulfur cathodes with conductive polymers has addressed the notorious "shuttle effect" while maintaining high theoretical capacity. Additionally, innovative composite structures incorporating graphene and metal-organic frameworks have created hierarchical interfaces that optimize ion diffusion pathways.
Advanced characterization techniques have been instrumental in these developments. In-situ transmission electron microscopy now allows researchers to directly observe interfacial evolution during cycling, while synchrotron-based X-ray absorption spectroscopy provides atomic-level insights into chemical transformations at these critical boundaries. These analytical approaches have revealed previously undetected intermediate phases that form during magnesium deposition and stripping.
Computational modeling has accelerated interface engineering through density functional theory calculations that predict interfacial stability and reaction mechanisms. Machine learning algorithms have begun identifying optimal material combinations for specific interface requirements, significantly reducing experimental trial-and-error approaches.
Biomimetic interface designs represent an emerging frontier, with structures inspired by biological membranes showing promise for selective ion transport. These bio-inspired interfaces incorporate precisely positioned functional groups that facilitate magnesium ion desolvation while blocking larger solvent molecules, addressing a fundamental challenge in magnesium electrochemistry.
The integration of these materials science innovations has led to prototype cells demonstrating dramatically improved rate capability and cycle life. Commercial development remains challenged by scalability concerns, but recent manufacturing advances in controlled atmosphere processing suggest pathways toward practical implementation of these interface technologies.
Safety and Performance Metrics for Mg Battery Commercialization
The commercialization of magnesium batteries requires rigorous safety and performance standards to ensure market viability. Unlike lithium-ion batteries, magnesium systems present unique safety advantages, including reduced flammability and lower risk of thermal runaway. However, these benefits must be quantified through standardized testing protocols specifically designed for magnesium electrochemistry.
Performance metrics for commercial viability include energy density targets of >200 Wh/kg and >400 Wh/L at the cell level, which remain challenging due to current limitations in electrodeposition morphology control. Cycle life requirements for automotive applications (>1000 cycles with 80% capacity retention) and consumer electronics (>500 cycles) necessitate stable, uniform magnesium deposition to prevent capacity fade and internal short circuits.
Rate capability represents another critical metric, with commercial applications requiring charge/discharge rates of at least 1C, while current magnesium systems often struggle at rates beyond 0.5C due to sluggish kinetics and uneven deposition. Temperature performance standards (-20°C to 60°C operating range) present additional challenges, as magnesium electrodeposition morphology is highly temperature-dependent, often becoming more dendritic at lower temperatures.
Cost considerations establish targets of <$100/kWh for automotive applications and <$150/kWh for stationary storage. Current electrodeposition challenges, requiring specialized electrolytes and controlled deposition conditions, significantly impact manufacturing costs and scalability. The development of cost-effective deposition control strategies remains essential for commercial viability.
Safety certification standards must be adapted for magnesium battery chemistry, including modified nail penetration, crush, and thermal abuse tests that account for magnesium's unique properties. While magnesium presents lower fire risks than lithium, its reaction with water produces hydrogen gas, necessitating specific safety protocols and containment strategies.
Environmental and regulatory considerations include recyclability metrics (>50% recoverable materials) and compliance with regulations like RoHS and REACH. The environmental impact of electrolytes used for morphology control must be assessed, with preference given to non-toxic, biodegradable formulations that maintain deposition quality.
Manufacturing scalability metrics require demonstration of consistent electrodeposition quality across increasing electrode dimensions, from coin cells to pouch and prismatic formats. Current laboratory-scale morphology control techniques must be adapted for high-throughput production environments while maintaining uniform deposition characteristics.
Performance metrics for commercial viability include energy density targets of >200 Wh/kg and >400 Wh/L at the cell level, which remain challenging due to current limitations in electrodeposition morphology control. Cycle life requirements for automotive applications (>1000 cycles with 80% capacity retention) and consumer electronics (>500 cycles) necessitate stable, uniform magnesium deposition to prevent capacity fade and internal short circuits.
Rate capability represents another critical metric, with commercial applications requiring charge/discharge rates of at least 1C, while current magnesium systems often struggle at rates beyond 0.5C due to sluggish kinetics and uneven deposition. Temperature performance standards (-20°C to 60°C operating range) present additional challenges, as magnesium electrodeposition morphology is highly temperature-dependent, often becoming more dendritic at lower temperatures.
Cost considerations establish targets of <$100/kWh for automotive applications and <$150/kWh for stationary storage. Current electrodeposition challenges, requiring specialized electrolytes and controlled deposition conditions, significantly impact manufacturing costs and scalability. The development of cost-effective deposition control strategies remains essential for commercial viability.
Safety certification standards must be adapted for magnesium battery chemistry, including modified nail penetration, crush, and thermal abuse tests that account for magnesium's unique properties. While magnesium presents lower fire risks than lithium, its reaction with water produces hydrogen gas, necessitating specific safety protocols and containment strategies.
Environmental and regulatory considerations include recyclability metrics (>50% recoverable materials) and compliance with regulations like RoHS and REACH. The environmental impact of electrolytes used for morphology control must be assessed, with preference given to non-toxic, biodegradable formulations that maintain deposition quality.
Manufacturing scalability metrics require demonstration of consistent electrodeposition quality across increasing electrode dimensions, from coin cells to pouch and prismatic formats. Current laboratory-scale morphology control techniques must be adapted for high-throughput production environments while maintaining uniform deposition characteristics.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!