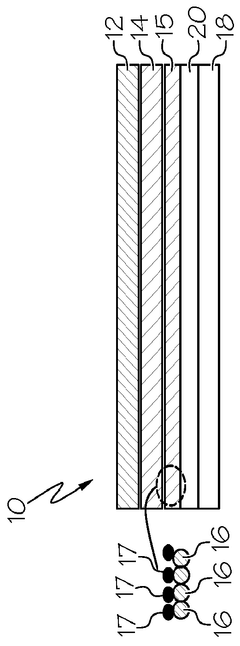
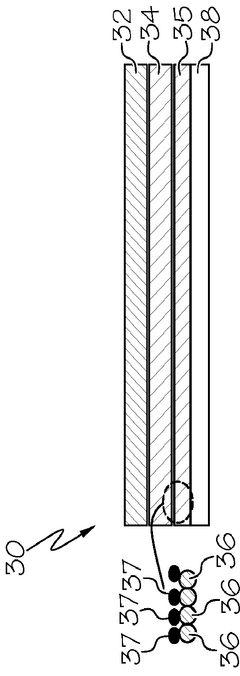
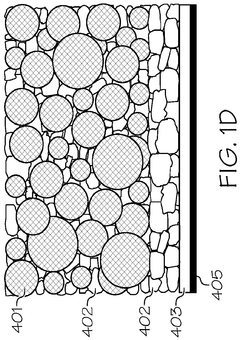
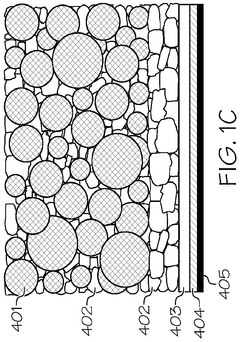
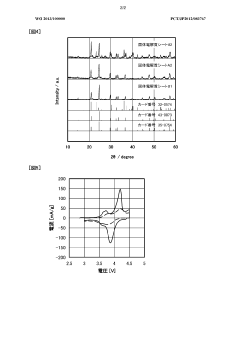
Interlayer design for high-conductivity magnesium solid electrolytes
OCT 14, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Mg Solid Electrolytes Background and Objectives
Magnesium-based batteries have emerged as promising alternatives to lithium-ion technology due to their potential for higher energy density, improved safety, and lower cost. Magnesium offers a theoretical volumetric capacity of 3833 mAh/cm³, significantly higher than lithium's 2062 mAh/cm³. Additionally, magnesium is the eighth most abundant element in Earth's crust, making it economically attractive for large-scale energy storage applications. The development trajectory of magnesium battery technology has accelerated over the past decade, with particular focus on overcoming the challenges associated with magnesium ion transport in solid-state systems.
Historically, research on magnesium batteries began in the 1990s but gained significant momentum in the 2000s when limitations of lithium-ion technology became increasingly apparent. The evolution of magnesium solid electrolytes has progressed from early polymer-based systems to more advanced ceramic and glass-ceramic compositions. A critical turning point occurred in 2015 when researchers demonstrated the feasibility of fast magnesium-ion conduction in certain crystalline frameworks, opening new pathways for solid electrolyte development.
The primary technical objective in this field is to develop solid electrolytes with magnesium ionic conductivity exceeding 10⁻⁴ S/cm at room temperature, while maintaining electrochemical stability against both magnesium metal anodes and high-voltage cathodes. Current state-of-the-art materials typically achieve conductivities in the range of 10⁻⁶ to 10⁻⁵ S/cm, highlighting the significant improvement required for practical applications.
Interlayer design has emerged as a promising strategy to enhance ionic conductivity in magnesium solid electrolytes. This approach involves the deliberate engineering of interfaces between different electrolyte components or phases to create pathways for enhanced magnesium ion transport. The concept draws inspiration from successful implementations in lithium solid electrolytes, where heterogeneous interfaces have demonstrated orders-of-magnitude improvements in ionic conductivity.
The technological trend is moving toward multi-component systems that leverage synergistic effects between different materials. Recent advances in computational modeling and high-throughput experimental techniques have accelerated the discovery of novel material combinations and interface designs. Machine learning approaches are increasingly being applied to predict promising interlayer compositions and structures, reducing the time and resources required for experimental validation.
Looking forward, the field aims to develop magnesium solid electrolytes that not only offer high ionic conductivity but also address challenges related to mechanical stability, interfacial resistance, and long-term cycling performance. The ultimate goal is to enable practical magnesium solid-state batteries that can outperform current lithium-ion technology in terms of energy density, safety, and cost-effectiveness, potentially revolutionizing both mobile and stationary energy storage applications.
Historically, research on magnesium batteries began in the 1990s but gained significant momentum in the 2000s when limitations of lithium-ion technology became increasingly apparent. The evolution of magnesium solid electrolytes has progressed from early polymer-based systems to more advanced ceramic and glass-ceramic compositions. A critical turning point occurred in 2015 when researchers demonstrated the feasibility of fast magnesium-ion conduction in certain crystalline frameworks, opening new pathways for solid electrolyte development.
The primary technical objective in this field is to develop solid electrolytes with magnesium ionic conductivity exceeding 10⁻⁴ S/cm at room temperature, while maintaining electrochemical stability against both magnesium metal anodes and high-voltage cathodes. Current state-of-the-art materials typically achieve conductivities in the range of 10⁻⁶ to 10⁻⁵ S/cm, highlighting the significant improvement required for practical applications.
Interlayer design has emerged as a promising strategy to enhance ionic conductivity in magnesium solid electrolytes. This approach involves the deliberate engineering of interfaces between different electrolyte components or phases to create pathways for enhanced magnesium ion transport. The concept draws inspiration from successful implementations in lithium solid electrolytes, where heterogeneous interfaces have demonstrated orders-of-magnitude improvements in ionic conductivity.
The technological trend is moving toward multi-component systems that leverage synergistic effects between different materials. Recent advances in computational modeling and high-throughput experimental techniques have accelerated the discovery of novel material combinations and interface designs. Machine learning approaches are increasingly being applied to predict promising interlayer compositions and structures, reducing the time and resources required for experimental validation.
Looking forward, the field aims to develop magnesium solid electrolytes that not only offer high ionic conductivity but also address challenges related to mechanical stability, interfacial resistance, and long-term cycling performance. The ultimate goal is to enable practical magnesium solid-state batteries that can outperform current lithium-ion technology in terms of energy density, safety, and cost-effectiveness, potentially revolutionizing both mobile and stationary energy storage applications.
Market Analysis for High-Conductivity Mg Batteries
The global market for magnesium batteries is experiencing significant growth potential, driven by increasing demand for high-energy density storage solutions across multiple sectors. Current projections indicate the magnesium battery market could reach substantial valuation by 2030, with a compound annual growth rate exceeding that of traditional lithium-ion technologies in specialized applications.
The primary market drivers for high-conductivity magnesium solid electrolytes stem from their theoretical advantages over lithium-based systems, including higher volumetric capacity, improved safety profiles, and the natural abundance of magnesium resources. These factors position magnesium battery technology as a promising alternative in the evolving energy storage landscape.
Industrial sectors showing the strongest interest in magnesium battery technology include electric vehicles, grid-scale energy storage, consumer electronics, and aerospace applications. Each sector presents unique requirements and adoption timelines, with automotive applications demonstrating particularly strong growth potential due to increasing electrification trends and safety concerns with current lithium technologies.
Regional market analysis reveals varying levels of investment and research focus. North America and Europe lead in research publications and patent filings related to magnesium solid electrolytes, while Asian markets, particularly Japan and China, demonstrate accelerating commercial development activities. This geographic distribution reflects both academic research strengths and industrial manufacturing capabilities.
Consumer demand patterns indicate growing interest in safer battery technologies with improved energy density. Market surveys show that safety concerns rank among the top three considerations for both industrial buyers and end consumers of battery-powered devices, creating a potential adoption pathway for magnesium-based systems despite their current performance limitations.
Competitive analysis reveals that magnesium battery technology remains predominantly in the pre-commercial research phase, with most market activity centered around research institutions and specialized startups rather than established battery manufacturers. This presents both opportunities for new market entrants and challenges in scaling production to commercial levels.
Market barriers include technical challenges in electrolyte conductivity, electrode compatibility issues, and manufacturing scalability. The interlayer design approach for high-conductivity magnesium solid electrolytes addresses several of these barriers, potentially accelerating market adoption timelines if successfully implemented.
Investment trends show increasing venture capital interest in advanced battery technologies, with funding for magnesium battery startups growing at approximately double the rate of general energy storage investments over the past three years. This suggests growing market confidence in the commercial viability of these technologies despite their early development stage.
The primary market drivers for high-conductivity magnesium solid electrolytes stem from their theoretical advantages over lithium-based systems, including higher volumetric capacity, improved safety profiles, and the natural abundance of magnesium resources. These factors position magnesium battery technology as a promising alternative in the evolving energy storage landscape.
Industrial sectors showing the strongest interest in magnesium battery technology include electric vehicles, grid-scale energy storage, consumer electronics, and aerospace applications. Each sector presents unique requirements and adoption timelines, with automotive applications demonstrating particularly strong growth potential due to increasing electrification trends and safety concerns with current lithium technologies.
Regional market analysis reveals varying levels of investment and research focus. North America and Europe lead in research publications and patent filings related to magnesium solid electrolytes, while Asian markets, particularly Japan and China, demonstrate accelerating commercial development activities. This geographic distribution reflects both academic research strengths and industrial manufacturing capabilities.
Consumer demand patterns indicate growing interest in safer battery technologies with improved energy density. Market surveys show that safety concerns rank among the top three considerations for both industrial buyers and end consumers of battery-powered devices, creating a potential adoption pathway for magnesium-based systems despite their current performance limitations.
Competitive analysis reveals that magnesium battery technology remains predominantly in the pre-commercial research phase, with most market activity centered around research institutions and specialized startups rather than established battery manufacturers. This presents both opportunities for new market entrants and challenges in scaling production to commercial levels.
Market barriers include technical challenges in electrolyte conductivity, electrode compatibility issues, and manufacturing scalability. The interlayer design approach for high-conductivity magnesium solid electrolytes addresses several of these barriers, potentially accelerating market adoption timelines if successfully implemented.
Investment trends show increasing venture capital interest in advanced battery technologies, with funding for magnesium battery startups growing at approximately double the rate of general energy storage investments over the past three years. This suggests growing market confidence in the commercial viability of these technologies despite their early development stage.
Technical Challenges in Mg Ion Conductivity
Despite significant advancements in solid-state battery technology, magnesium ion conductivity remains a critical bottleneck in the development of high-performance magnesium solid electrolytes. The divalent nature of Mg2+ ions creates strong electrostatic interactions with the host lattice, resulting in sluggish ion mobility compared to monovalent ions like Li+. Typical Mg2+ conductivity in solid electrolytes ranges from 10^-6 to 10^-4 S/cm at room temperature, significantly lower than the 10^-3 S/cm threshold required for practical applications.
The high charge density of magnesium ions leads to formation of strong coordination bonds with anions in the electrolyte framework, creating substantial energy barriers for ion hopping between sites. This fundamental challenge is compounded by the larger ionic radius of Mg2+ (0.72 Å) compared to Li+ (0.76 Å), which restricts ion transport through available migration pathways in most crystal structures.
Interface resistance presents another major hurdle, particularly at the electrode-electrolyte boundaries. The formation of passivation layers at these interfaces often blocks Mg2+ transport, leading to increased impedance and reduced overall conductivity. Unlike lithium systems where SEI formation can be beneficial, magnesium interfaces typically develop resistive layers that impede ion transfer.
Structural stability during cycling represents a significant challenge, as repeated magnesium insertion/extraction can cause lattice expansion/contraction, creating microcracks and degrading conductive pathways. This mechanical stress is particularly problematic at interlayer regions, where structural integrity is crucial for maintaining continuous ion transport channels.
Chemical compatibility between magnesium solid electrolytes and electrode materials presents additional complications. Many high-voltage cathode materials react with magnesium electrolytes, forming resistive interfacial phases that hinder ion transport. This reactivity often necessitates protective interlayers, adding complexity to cell design and manufacturing.
Temperature sensitivity further complicates magnesium solid electrolyte performance, with many systems showing adequate conductivity only at elevated temperatures (>60°C). Achieving room-temperature conductivity remains elusive due to the high activation energy required for Mg2+ migration, typically ranging from 0.5-1.2 eV compared to 0.2-0.4 eV for lithium systems.
The synthesis of phase-pure magnesium solid electrolytes with controlled stoichiometry and microstructure presents significant processing challenges. Impurities and grain boundaries often act as traps for magnesium ions, further reducing effective conductivity and necessitating precise control over interlayer composition and structure.
The high charge density of magnesium ions leads to formation of strong coordination bonds with anions in the electrolyte framework, creating substantial energy barriers for ion hopping between sites. This fundamental challenge is compounded by the larger ionic radius of Mg2+ (0.72 Å) compared to Li+ (0.76 Å), which restricts ion transport through available migration pathways in most crystal structures.
Interface resistance presents another major hurdle, particularly at the electrode-electrolyte boundaries. The formation of passivation layers at these interfaces often blocks Mg2+ transport, leading to increased impedance and reduced overall conductivity. Unlike lithium systems where SEI formation can be beneficial, magnesium interfaces typically develop resistive layers that impede ion transfer.
Structural stability during cycling represents a significant challenge, as repeated magnesium insertion/extraction can cause lattice expansion/contraction, creating microcracks and degrading conductive pathways. This mechanical stress is particularly problematic at interlayer regions, where structural integrity is crucial for maintaining continuous ion transport channels.
Chemical compatibility between magnesium solid electrolytes and electrode materials presents additional complications. Many high-voltage cathode materials react with magnesium electrolytes, forming resistive interfacial phases that hinder ion transport. This reactivity often necessitates protective interlayers, adding complexity to cell design and manufacturing.
Temperature sensitivity further complicates magnesium solid electrolyte performance, with many systems showing adequate conductivity only at elevated temperatures (>60°C). Achieving room-temperature conductivity remains elusive due to the high activation energy required for Mg2+ migration, typically ranging from 0.5-1.2 eV compared to 0.2-0.4 eV for lithium systems.
The synthesis of phase-pure magnesium solid electrolytes with controlled stoichiometry and microstructure presents significant processing challenges. Impurities and grain boundaries often act as traps for magnesium ions, further reducing effective conductivity and necessitating precise control over interlayer composition and structure.
Current Interlayer Design Approaches
01 Magnesium ion conducting solid electrolytes
Solid electrolytes that facilitate magnesium ion conduction are essential for developing high-performance magnesium batteries. These electrolytes typically consist of magnesium salts incorporated into solid matrices that allow for efficient ion transport while maintaining structural stability. The conductivity of these materials is a critical parameter that determines the overall performance of magnesium-based energy storage devices.- Magnesium ion conducting solid electrolytes: Solid electrolytes that facilitate magnesium ion conduction are essential for developing high-performance magnesium batteries. These electrolytes typically consist of magnesium salts embedded in various host materials to create pathways for magnesium ion transport. The conductivity of these electrolytes depends on their composition, crystal structure, and the mobility of magnesium ions within the material framework. Enhancing magnesium ion conductivity is crucial for improving the overall performance of magnesium-based energy storage systems.
- Polymer-based magnesium solid electrolytes: Polymer-based solid electrolytes for magnesium ion conduction incorporate magnesium salts into polymer matrices to create flexible and processable electrolyte materials. These electrolytes often use polymers such as polyethylene oxide (PEO) or polyvinylidene fluoride (PVDF) as hosts for magnesium salts. The conductivity of polymer-based magnesium electrolytes can be enhanced by adding plasticizers, ceramic fillers, or by modifying the polymer structure to increase free volume for ion transport. These materials offer advantages in terms of mechanical flexibility and ease of fabrication for battery applications.
- Ceramic and glass-ceramic magnesium electrolytes: Ceramic and glass-ceramic materials serve as robust hosts for magnesium ion conduction in solid-state electrolytes. These materials typically feature crystalline or partially crystalline structures with interconnected pathways that facilitate magnesium ion movement. Common ceramic systems include magnesium-doped aluminum oxides, magnesium phosphates, and magnesium borates. The conductivity of these electrolytes can be enhanced by controlling grain boundaries, introducing dopants, or creating composite structures. Ceramic-based magnesium electrolytes offer high thermal stability and excellent mechanical properties for battery applications.
- Composite magnesium solid electrolytes: Composite solid electrolytes combine different materials to enhance magnesium ion conductivity by leveraging the advantages of each component. These electrolytes typically consist of a combination of ceramic fillers dispersed in polymer matrices or multiple ceramic phases with complementary properties. The interfaces between different components often create additional pathways for ion transport, enhancing overall conductivity. Composite approaches can address limitations of single-component electrolytes by improving mechanical properties while maintaining high ionic conductivity. These materials represent a promising direction for developing practical magnesium battery systems.
- Novel synthesis methods for high-conductivity magnesium electrolytes: Advanced synthesis techniques are being developed to create magnesium solid electrolytes with enhanced ionic conductivity. These methods include sol-gel processing, mechanochemical synthesis, atomic layer deposition, and various nanofabrication approaches. By controlling the microstructure, crystallinity, and interfacial properties of the electrolyte materials, these techniques can significantly improve magnesium ion transport. Novel synthesis routes also enable the creation of hierarchical structures and precise control over dopant distribution, leading to electrolytes with optimized conduction pathways and minimized activation energies for ion movement.
02 Composite solid electrolytes with enhanced conductivity
Composite solid electrolytes combine different materials to achieve improved magnesium ion conductivity. These typically involve mixing magnesium-containing compounds with other materials such as polymers, ceramics, or glass to create heterogeneous structures that facilitate ion transport through multiple pathways. The interfaces between different components often play a crucial role in enhancing overall conductivity performance.Expand Specific Solutions03 Doping strategies for improving magnesium ion conductivity
Doping involves introducing small amounts of foreign elements into the crystal structure of magnesium-based solid electrolytes to create defects that enhance ionic conductivity. Common dopants include aluminum, zinc, and other metals that can modify the local environment around magnesium ions, reducing migration barriers and creating additional conduction pathways. This approach can significantly increase the conductivity of solid electrolytes at various operating temperatures.Expand Specific Solutions04 Novel synthesis methods for high-conductivity magnesium electrolytes
Advanced synthesis techniques are being developed to create magnesium solid electrolytes with superior conductivity properties. These methods include sol-gel processing, mechanochemical synthesis, solid-state reactions, and various nanofabrication approaches. The processing conditions significantly impact the microstructure, crystallinity, and defect concentration in the resulting materials, which directly affects their ionic conductivity performance.Expand Specific Solutions05 Interface engineering for enhanced magnesium ion transport
Interface engineering focuses on optimizing the boundaries between different components in magnesium solid electrolyte systems to improve overall conductivity. This includes surface modifications, creation of artificial interphases, and control of grain boundaries. By managing interfacial resistance and creating favorable pathways for magnesium ion migration across material interfaces, the overall conductivity of solid electrolyte systems can be significantly enhanced.Expand Specific Solutions
Leading Research Groups and Industrial Players
The magnesium solid electrolyte market is currently in an early growth phase, characterized by intensive R&D efforts to overcome conductivity challenges through interlayer design. The global market size remains relatively modest but is projected to expand significantly as electric vehicle adoption accelerates. From a technical maturity perspective, companies like Toyota Central R&D Labs and Samsung Electronics are leading fundamental research, while LG Chem, Murata Manufacturing, and Saft Groupe are advancing commercial applications. Chinese institutions including Shanghai Institute of Ceramics and Sichuan University are making notable contributions to material science innovations. The competitive landscape features both established battery manufacturers and specialized materials science companies, with collaboration between academic institutions and industry players driving technological breakthroughs in interface engineering and ionic conductivity enhancement.
Toyota Central R&D Labs, Inc.
Technical Solution: Toyota Central R&D Labs has developed innovative interlayer designs for high-conductivity magnesium solid electrolytes focusing on interface engineering between Mg metal anodes and solid electrolytes. Their approach involves creating buffer layers composed of MgF2-based composites that effectively suppress interfacial resistance while preventing dendrite formation. The company has pioneered a dual-layer electrolyte architecture where a thin protective coating is applied to the Mg metal surface, followed by a bulk solid electrolyte with optimized ionic conductivity. This design addresses the critical challenge of high interfacial resistance that typically plagues Mg-ion batteries. Toyota's research has demonstrated ionic conductivities exceeding 10^-4 S/cm at room temperature through careful control of grain boundaries and crystallographic orientation in their engineered interlayers. Their solid electrolytes incorporate magnesium borohydride (Mg(BH4)2) derivatives with modified interfaces to enhance Mg2+ transport while maintaining electrochemical stability.
Strengths: Superior interface stability between Mg metal and electrolyte, preventing dendrite formation while maintaining high ionic conductivity. The multi-layer approach effectively addresses both bulk and interfacial resistance issues simultaneously. Weaknesses: Manufacturing complexity of precisely engineered interlayers may increase production costs, and long-term stability under repeated cycling conditions remains challenging.
Nissan Motor Co., Ltd.
Technical Solution: Nissan Motor Co. has developed a proprietary interlayer design for magnesium solid electrolytes centered around a gradient composition approach. Their technology features strategically engineered interfaces between magnesium metal anodes and solid electrolyte materials, utilizing a series of transition layers with gradually changing chemical compositions. The outermost layer contacting the Mg anode contains MgO-doped structures that form a stable passivation film while still allowing Mg2+ transport. The middle transition layers incorporate magnesium halides (MgCl2, MgBr2) combined with organic frameworks to create flexible ion transport pathways. Nissan's research has demonstrated that this gradient approach can achieve ionic conductivities of approximately 5×10^-5 S/cm at room temperature while maintaining excellent interfacial stability. Their solid electrolyte system also incorporates nanostructured ceramic fillers to enhance mechanical properties and prevent crack propagation during cycling. The company has integrated this technology with their existing battery management systems to optimize performance in automotive applications.
Strengths: The gradient composition approach effectively balances ionic conductivity with interfacial stability, and the system shows good compatibility with existing battery manufacturing processes. Weaknesses: The complex multi-layer structure may present scaling challenges for mass production, and the overall ionic conductivity still lags behind liquid electrolyte alternatives for high-power applications.
Key Patents in Mg Electrolyte Interface Engineering
All-solid-state lithium secondary batteries and methods of preparing the same
PatentWO2025097059A1
Innovation
- The development of an interlayer for solid-state batteries comprising a percolating network of carbon nanomaterials and uniformly dispersed magnesium (Mg) nanoparticles, which regulates ion flux and enhances interfacial contact without the need for noble metals.
All-solid-state battery, and manufacturing method therefor
PatentWO2013100000A1
Innovation
- Incorporating a lithium-containing oxide as the first component and a compound containing magnesium, calcium, or strontium as the second component in the solid electrolyte layer, with a phosphoric acid compound, to enhance the sintering density and ionic conductivity of the solid electrolyte layer.
Materials Compatibility and Stability Issues
The compatibility and stability of materials in magnesium solid electrolyte systems present significant challenges for practical implementation. When designing interlayers for high-conductivity magnesium solid electrolytes, the chemical and electrochemical stability at interfaces becomes a critical concern. Magnesium metal anodes are highly reactive, forming passivation layers when in contact with most electrolytes, which impedes ion transport and increases interfacial resistance.
Interface reactions between magnesium metal and solid electrolytes often lead to the formation of interphases with poor ionic conductivity. These reactions can generate MgO, Mg(OH)2, or MgF2 layers that block ion transport pathways. The challenge is particularly pronounced with sulfide-based solid electrolytes, which tend to react with magnesium metal, forming insulating magnesium sulfides at the interface.
Cathode-electrolyte interfaces also present stability issues. High-voltage cathode materials can oxidize the solid electrolyte, creating resistive layers that hinder magnesium ion migration. This oxidative decomposition is especially problematic for sulfide and halide-based solid electrolytes, which typically have narrow electrochemical stability windows.
Environmental stability poses another significant challenge. Many magnesium solid electrolytes are hygroscopic or air-sensitive, degrading upon exposure to moisture or oxygen. For instance, chloride-based electrolytes readily absorb water, while borohydride-based systems can react with atmospheric oxygen, compromising their long-term performance and handling characteristics.
Thermal stability issues arise during battery operation, as temperature fluctuations can induce phase transitions or decomposition in solid electrolytes. Some promising magnesium conductors maintain stability only within narrow temperature ranges, limiting their practical application in diverse operating environments.
Mechanical stability at interfaces presents additional complications. Volume changes during magnesium insertion/extraction can create mechanical stress at interfaces, leading to contact loss and increased resistance. The difference in mechanical properties between rigid solid electrolytes and electrode materials can result in delamination or crack formation during cycling.
Addressing these compatibility and stability issues requires innovative interlayer design strategies. Protective coatings, buffer layers, and gradient interfaces are being explored to mitigate reactivity while maintaining high magnesium ion conductivity. Composite approaches incorporating stabilizing additives or elastomeric components show promise for enhancing both chemical and mechanical stability at critical interfaces.
Interface reactions between magnesium metal and solid electrolytes often lead to the formation of interphases with poor ionic conductivity. These reactions can generate MgO, Mg(OH)2, or MgF2 layers that block ion transport pathways. The challenge is particularly pronounced with sulfide-based solid electrolytes, which tend to react with magnesium metal, forming insulating magnesium sulfides at the interface.
Cathode-electrolyte interfaces also present stability issues. High-voltage cathode materials can oxidize the solid electrolyte, creating resistive layers that hinder magnesium ion migration. This oxidative decomposition is especially problematic for sulfide and halide-based solid electrolytes, which typically have narrow electrochemical stability windows.
Environmental stability poses another significant challenge. Many magnesium solid electrolytes are hygroscopic or air-sensitive, degrading upon exposure to moisture or oxygen. For instance, chloride-based electrolytes readily absorb water, while borohydride-based systems can react with atmospheric oxygen, compromising their long-term performance and handling characteristics.
Thermal stability issues arise during battery operation, as temperature fluctuations can induce phase transitions or decomposition in solid electrolytes. Some promising magnesium conductors maintain stability only within narrow temperature ranges, limiting their practical application in diverse operating environments.
Mechanical stability at interfaces presents additional complications. Volume changes during magnesium insertion/extraction can create mechanical stress at interfaces, leading to contact loss and increased resistance. The difference in mechanical properties between rigid solid electrolytes and electrode materials can result in delamination or crack formation during cycling.
Addressing these compatibility and stability issues requires innovative interlayer design strategies. Protective coatings, buffer layers, and gradient interfaces are being explored to mitigate reactivity while maintaining high magnesium ion conductivity. Composite approaches incorporating stabilizing additives or elastomeric components show promise for enhancing both chemical and mechanical stability at critical interfaces.
Scalability and Manufacturing Considerations
The scalability and manufacturing of interlayer designs for high-conductivity magnesium solid electrolytes present significant challenges that must be addressed for commercial viability. Current laboratory-scale fabrication methods often involve complex processes such as atomic layer deposition, physical vapor deposition, or solution-based coating techniques that are difficult to scale up for mass production. These methods typically yield excellent performance in controlled environments but face substantial hurdles when transitioning to industrial manufacturing scales.
Cost considerations represent a critical factor in the commercialization pathway. Many high-performance interlayer materials incorporate expensive elements or require specialized processing equipment, resulting in prohibitive production costs. For instance, ultrathin ceramic or polymer-based interlayers may require precision deposition techniques that are capital-intensive and have low throughput rates. Developing cost-effective alternatives that maintain performance metrics while utilizing more abundant materials and simpler processing methods is essential for market adoption.
Quality control and reproducibility emerge as paramount concerns in scaled manufacturing operations. The functional properties of interlayers for magnesium solid electrolytes are highly sensitive to thickness variations, compositional homogeneity, and interfacial characteristics. Establishing robust quality assurance protocols and in-line monitoring techniques becomes necessary to ensure consistent performance across production batches. Advanced characterization methods such as impedance spectroscopy and surface analysis must be adapted for high-throughput manufacturing environments.
Integration with existing battery production infrastructure represents another significant consideration. New interlayer technologies must be compatible with established manufacturing processes to minimize capital investment requirements. This includes considerations for processing temperatures, solvent compatibility, and mechanical handling during assembly. Ideally, interlayer deposition should be seamlessly incorporated into existing production lines with minimal modifications.
Environmental and safety aspects of manufacturing processes cannot be overlooked. Many current laboratory techniques utilize hazardous precursors or generate significant waste streams. Sustainable manufacturing approaches that minimize environmental impact through reduced energy consumption, decreased waste generation, and elimination of toxic materials will be increasingly important as production scales increase. This aligns with growing regulatory pressures and consumer demand for environmentally responsible products in the energy storage sector.
Cost considerations represent a critical factor in the commercialization pathway. Many high-performance interlayer materials incorporate expensive elements or require specialized processing equipment, resulting in prohibitive production costs. For instance, ultrathin ceramic or polymer-based interlayers may require precision deposition techniques that are capital-intensive and have low throughput rates. Developing cost-effective alternatives that maintain performance metrics while utilizing more abundant materials and simpler processing methods is essential for market adoption.
Quality control and reproducibility emerge as paramount concerns in scaled manufacturing operations. The functional properties of interlayers for magnesium solid electrolytes are highly sensitive to thickness variations, compositional homogeneity, and interfacial characteristics. Establishing robust quality assurance protocols and in-line monitoring techniques becomes necessary to ensure consistent performance across production batches. Advanced characterization methods such as impedance spectroscopy and surface analysis must be adapted for high-throughput manufacturing environments.
Integration with existing battery production infrastructure represents another significant consideration. New interlayer technologies must be compatible with established manufacturing processes to minimize capital investment requirements. This includes considerations for processing temperatures, solvent compatibility, and mechanical handling during assembly. Ideally, interlayer deposition should be seamlessly incorporated into existing production lines with minimal modifications.
Environmental and safety aspects of manufacturing processes cannot be overlooked. Many current laboratory techniques utilize hazardous precursors or generate significant waste streams. Sustainable manufacturing approaches that minimize environmental impact through reduced energy consumption, decreased waste generation, and elimination of toxic materials will be increasingly important as production scales increase. This aligns with growing regulatory pressures and consumer demand for environmentally responsible products in the energy storage sector.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!