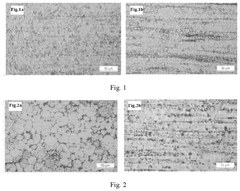
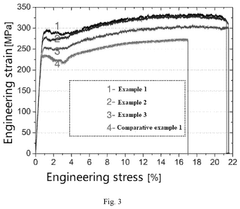
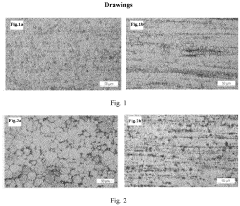
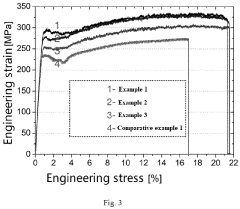
Redox potential tuning in magnesium intercalation materials
OCT 14, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Mg Battery Redox Chemistry Background and Objectives
Magnesium-ion batteries (MIBs) have emerged as a promising alternative to lithium-ion batteries due to their potential advantages in safety, cost, and energy density. The evolution of magnesium battery technology can be traced back to the early 1990s when the first rechargeable magnesium battery was demonstrated by Gregory et al. Since then, significant research efforts have been directed toward understanding and improving the electrochemical performance of magnesium-based energy storage systems.
The redox chemistry of magnesium intercalation materials represents a critical aspect of MIB development. Unlike lithium, magnesium is a divalent cation (Mg²⁺), which introduces unique challenges and opportunities in terms of charge storage mechanisms. The fundamental redox processes involve the reversible insertion and extraction of Mg²⁺ ions into and from host structures, accompanied by the transfer of two electrons per ion.
Recent technological trends indicate a growing interest in tuning the redox potential of magnesium intercalation materials to optimize battery performance. This tuning process involves manipulating the electronic structure of host materials to achieve desired voltage profiles, energy densities, and cycling stability. The field has witnessed significant advancements in computational methods for predicting and designing materials with tailored redox properties.
The primary technical objectives in this domain include developing cathode materials with high redox potentials (>2.0 V vs. Mg/Mg²⁺), achieving reversible magnesium intercalation without structural degradation, and understanding the fundamental mechanisms governing the redox behavior of magnesium in various host lattices. Additionally, researchers aim to establish design principles for synthesizing materials with optimized electronic configurations that facilitate efficient Mg²⁺ transport.
Another crucial goal is to bridge the gap between theoretical predictions and experimental validations of redox potential tuning strategies. This includes developing accurate computational models that can reliably predict the electrochemical behavior of novel magnesium intercalation compounds before experimental synthesis, thereby accelerating the discovery process.
The field is also moving toward understanding the correlation between crystal structure, electronic configuration, and redox potential in magnesium intercalation materials. Researchers are exploring various structural motifs, including layered oxides, spinels, olivines, and Prussian blue analogs, to identify optimal frameworks for magnesium storage with favorable redox characteristics.
Ultimately, the technological trajectory points toward the development of a comprehensive framework for rational design of magnesium intercalation materials with precisely controlled redox properties, enabling the next generation of high-performance, sustainable energy storage solutions.
The redox chemistry of magnesium intercalation materials represents a critical aspect of MIB development. Unlike lithium, magnesium is a divalent cation (Mg²⁺), which introduces unique challenges and opportunities in terms of charge storage mechanisms. The fundamental redox processes involve the reversible insertion and extraction of Mg²⁺ ions into and from host structures, accompanied by the transfer of two electrons per ion.
Recent technological trends indicate a growing interest in tuning the redox potential of magnesium intercalation materials to optimize battery performance. This tuning process involves manipulating the electronic structure of host materials to achieve desired voltage profiles, energy densities, and cycling stability. The field has witnessed significant advancements in computational methods for predicting and designing materials with tailored redox properties.
The primary technical objectives in this domain include developing cathode materials with high redox potentials (>2.0 V vs. Mg/Mg²⁺), achieving reversible magnesium intercalation without structural degradation, and understanding the fundamental mechanisms governing the redox behavior of magnesium in various host lattices. Additionally, researchers aim to establish design principles for synthesizing materials with optimized electronic configurations that facilitate efficient Mg²⁺ transport.
Another crucial goal is to bridge the gap between theoretical predictions and experimental validations of redox potential tuning strategies. This includes developing accurate computational models that can reliably predict the electrochemical behavior of novel magnesium intercalation compounds before experimental synthesis, thereby accelerating the discovery process.
The field is also moving toward understanding the correlation between crystal structure, electronic configuration, and redox potential in magnesium intercalation materials. Researchers are exploring various structural motifs, including layered oxides, spinels, olivines, and Prussian blue analogs, to identify optimal frameworks for magnesium storage with favorable redox characteristics.
Ultimately, the technological trajectory points toward the development of a comprehensive framework for rational design of magnesium intercalation materials with precisely controlled redox properties, enabling the next generation of high-performance, sustainable energy storage solutions.
Market Analysis for Next-Generation Mg Battery Technologies
The global market for next-generation magnesium battery technologies is experiencing significant growth potential, driven by increasing demand for sustainable energy storage solutions. Current projections indicate the magnesium battery market could reach $2.3 billion by 2030, with a compound annual growth rate exceeding 12% during the forecast period 2023-2030.
Magnesium batteries are positioned as promising alternatives to lithium-ion technologies due to several market advantages. Magnesium is substantially more abundant than lithium, comprising approximately 2.4% of the earth's crust compared to lithium's 0.006%. This abundance translates to lower raw material costs and reduced supply chain vulnerabilities, addressing critical concerns in the current battery market landscape.
The automotive sector represents the largest potential market segment for magnesium battery technologies, particularly as electric vehicle adoption accelerates globally. Energy density improvements through redox potential tuning in magnesium intercalation materials could enable longer driving ranges while maintaining safety advantages over lithium-ion systems. Market research indicates automotive manufacturers are actively seeking diversification in battery chemistry to mitigate supply risks.
Grid-scale energy storage presents another substantial market opportunity, valued at approximately $7.9 billion in 2022 and expected to grow significantly. Magnesium batteries with optimized redox potentials could offer longer cycle life and improved safety profiles compared to current technologies, making them particularly suitable for stationary applications where energy density requirements are less stringent than in transportation.
Consumer electronics manufacturers are also expressing interest in magnesium battery technologies, particularly for applications requiring improved safety and longer service life. The consumer electronics battery market, currently dominated by lithium-ion, exceeds $25 billion annually and continues to grow as device proliferation increases.
Market barriers include competition from established lithium-ion infrastructure and emerging technologies such as sodium-ion and solid-state batteries. Current manufacturing capacity for magnesium batteries remains limited, with significant investment required to achieve commercial scale production. The technology readiness level for magnesium intercalation materials with optimized redox potentials currently ranges between 3-5, indicating continued research and development is necessary before widespread commercialization.
Regional analysis shows Asia-Pacific leading in potential manufacturing capacity development, with China, Japan, and South Korea making strategic investments in alternative battery technologies. North America and Europe are focusing on research initiatives and early-stage commercialization partnerships, with several university-industry collaborations advancing redox potential tuning techniques for magnesium intercalation materials.
Magnesium batteries are positioned as promising alternatives to lithium-ion technologies due to several market advantages. Magnesium is substantially more abundant than lithium, comprising approximately 2.4% of the earth's crust compared to lithium's 0.006%. This abundance translates to lower raw material costs and reduced supply chain vulnerabilities, addressing critical concerns in the current battery market landscape.
The automotive sector represents the largest potential market segment for magnesium battery technologies, particularly as electric vehicle adoption accelerates globally. Energy density improvements through redox potential tuning in magnesium intercalation materials could enable longer driving ranges while maintaining safety advantages over lithium-ion systems. Market research indicates automotive manufacturers are actively seeking diversification in battery chemistry to mitigate supply risks.
Grid-scale energy storage presents another substantial market opportunity, valued at approximately $7.9 billion in 2022 and expected to grow significantly. Magnesium batteries with optimized redox potentials could offer longer cycle life and improved safety profiles compared to current technologies, making them particularly suitable for stationary applications where energy density requirements are less stringent than in transportation.
Consumer electronics manufacturers are also expressing interest in magnesium battery technologies, particularly for applications requiring improved safety and longer service life. The consumer electronics battery market, currently dominated by lithium-ion, exceeds $25 billion annually and continues to grow as device proliferation increases.
Market barriers include competition from established lithium-ion infrastructure and emerging technologies such as sodium-ion and solid-state batteries. Current manufacturing capacity for magnesium batteries remains limited, with significant investment required to achieve commercial scale production. The technology readiness level for magnesium intercalation materials with optimized redox potentials currently ranges between 3-5, indicating continued research and development is necessary before widespread commercialization.
Regional analysis shows Asia-Pacific leading in potential manufacturing capacity development, with China, Japan, and South Korea making strategic investments in alternative battery technologies. North America and Europe are focusing on research initiatives and early-stage commercialization partnerships, with several university-industry collaborations advancing redox potential tuning techniques for magnesium intercalation materials.
Current Challenges in Magnesium Intercalation Materials
Despite significant advancements in magnesium battery technology, numerous challenges persist in developing effective magnesium intercalation materials. The primary obstacle remains the strong electrostatic interaction between Mg2+ ions and host lattices, resulting in sluggish diffusion kinetics. This "charge density dilemma" fundamentally limits the practical application of magnesium-based energy storage systems, as the divalent nature of magnesium ions creates substantial energy barriers for ion migration.
Electrode materials face critical challenges in maintaining structural stability during repeated magnesium insertion and extraction cycles. Many host structures experience significant volume changes and phase transformations during cycling, leading to mechanical degradation and capacity fading. This structural instability is particularly pronounced in materials with layered structures, where interlayer spacing often collapses upon magnesium extraction.
The redox potential tuning in magnesium intercalation materials presents another significant challenge. Current cathode materials typically exhibit lower operating voltages compared to their lithium counterparts, resulting in reduced energy density. The difficulty in achieving high redox potentials stems from the strong interaction between Mg2+ and the host lattice, which often leads to unfavorable thermodynamics for magnesium insertion at higher potentials.
Electrolyte compatibility issues further complicate the development of magnesium intercalation materials. Many potential cathode materials react unfavorably with conventional magnesium electrolytes, forming passivation layers that impede magnesium ion transport. This incompatibility significantly narrows the selection of viable electrode-electrolyte combinations and hinders the development of high-performance magnesium batteries.
The multivalent nature of magnesium ions introduces complex charge transfer kinetics at the electrode-electrolyte interface. The desolvation energy penalty for magnesium ions is substantially higher than for monovalent ions, creating significant activation barriers for charge transfer. This phenomenon manifests as high interfacial resistance and contributes to the overall sluggish electrochemical performance of magnesium systems.
Computational modeling of magnesium intercalation materials presents unique challenges due to the strong correlation effects and complex electronic structures involved. Current density functional theory approaches often struggle to accurately predict the energetics and kinetics of magnesium insertion, limiting the effectiveness of computational screening for new materials. The development of more accurate theoretical frameworks specifically tailored for multivalent ion systems remains an ongoing challenge.
The synthesis of phase-pure magnesium intercalation materials with controlled morphology and surface properties represents another significant hurdle. Conventional synthesis methods often yield materials with suboptimal crystallinity, defect concentrations, and particle characteristics, all of which critically influence magnesium diffusion and storage properties.
Electrode materials face critical challenges in maintaining structural stability during repeated magnesium insertion and extraction cycles. Many host structures experience significant volume changes and phase transformations during cycling, leading to mechanical degradation and capacity fading. This structural instability is particularly pronounced in materials with layered structures, where interlayer spacing often collapses upon magnesium extraction.
The redox potential tuning in magnesium intercalation materials presents another significant challenge. Current cathode materials typically exhibit lower operating voltages compared to their lithium counterparts, resulting in reduced energy density. The difficulty in achieving high redox potentials stems from the strong interaction between Mg2+ and the host lattice, which often leads to unfavorable thermodynamics for magnesium insertion at higher potentials.
Electrolyte compatibility issues further complicate the development of magnesium intercalation materials. Many potential cathode materials react unfavorably with conventional magnesium electrolytes, forming passivation layers that impede magnesium ion transport. This incompatibility significantly narrows the selection of viable electrode-electrolyte combinations and hinders the development of high-performance magnesium batteries.
The multivalent nature of magnesium ions introduces complex charge transfer kinetics at the electrode-electrolyte interface. The desolvation energy penalty for magnesium ions is substantially higher than for monovalent ions, creating significant activation barriers for charge transfer. This phenomenon manifests as high interfacial resistance and contributes to the overall sluggish electrochemical performance of magnesium systems.
Computational modeling of magnesium intercalation materials presents unique challenges due to the strong correlation effects and complex electronic structures involved. Current density functional theory approaches often struggle to accurately predict the energetics and kinetics of magnesium insertion, limiting the effectiveness of computational screening for new materials. The development of more accurate theoretical frameworks specifically tailored for multivalent ion systems remains an ongoing challenge.
The synthesis of phase-pure magnesium intercalation materials with controlled morphology and surface properties represents another significant hurdle. Conventional synthesis methods often yield materials with suboptimal crystallinity, defect concentrations, and particle characteristics, all of which critically influence magnesium diffusion and storage properties.
State-of-the-Art Redox Tuning Strategies
01 Magnesium-based electrode materials for batteries
Magnesium-based electrode materials offer promising alternatives for energy storage applications due to their favorable redox potential characteristics. These materials enable efficient magnesium ion intercalation and deintercalation during charge-discharge cycles, providing high energy density and improved electrochemical performance. The intercalation mechanism involves the reversible insertion of magnesium ions into host structures, which can be optimized through material design and composition adjustments to achieve desired redox potential values.- Magnesium-based electrode materials for batteries: Magnesium-based electrode materials offer promising alternatives for energy storage applications due to their favorable redox potential characteristics. These materials enable efficient magnesium ion intercalation and deintercalation processes, providing high energy density and improved electrochemical performance. The intercalation mechanism involves the reversible insertion of magnesium ions into host structures, which can be optimized to enhance battery capacity and cycling stability.
- Redox potential control in magnesium intercalation compounds: The redox potential of magnesium intercalation materials can be controlled through various structural and compositional modifications. By adjusting the crystal structure, particle size, and chemical composition of the host materials, the redox potential can be tuned to optimize energy storage performance. This approach enables the development of magnesium-based energy storage systems with tailored voltage profiles and improved electrochemical characteristics.
- Novel magnesium intercalation host structures: Various host structures have been developed to facilitate magnesium ion intercalation with favorable redox potentials. These include layered materials, spinel structures, and framework compounds that provide suitable channels and sites for magnesium ion insertion and extraction. The design of these host structures focuses on optimizing the magnesium diffusion pathways and minimizing structural changes during cycling to enhance electrochemical performance and stability.
- Measurement and characterization of redox potentials: Advanced techniques for measuring and characterizing the redox potentials of magnesium intercalation materials have been developed. These methods include electrochemical testing, spectroscopic analysis, and computational modeling to determine the thermodynamic and kinetic properties of magnesium insertion and extraction. Accurate measurement of redox potentials is crucial for understanding the energy storage mechanisms and optimizing the performance of magnesium-based battery systems.
- Electrolyte systems for magnesium intercalation: Specialized electrolyte systems have been developed to facilitate efficient magnesium intercalation processes with controlled redox potentials. These electrolytes are designed to enable reversible magnesium deposition and dissolution while maintaining compatibility with electrode materials. The composition and properties of the electrolyte significantly influence the redox behavior of magnesium intercalation materials, affecting the overall performance and cycling stability of magnesium-based energy storage devices.
02 Layered materials for magnesium intercalation
Layered structures provide ideal frameworks for magnesium ion intercalation due to their expandable interlayer spacing. These materials, including modified transition metal oxides and sulfides, can accommodate magnesium ions while maintaining structural stability. The redox potential of these layered intercalation hosts can be tuned by adjusting the composition and crystal structure, allowing for optimization of electrochemical performance. The intercalation process in these materials typically involves minimal structural changes, enabling good cycling stability.Expand Specific Solutions03 Measurement techniques for redox potential in magnesium systems
Various analytical methods have been developed to accurately measure the redox potential of magnesium intercalation materials. These techniques include cyclic voltammetry, galvanostatic cycling, and potentiometric analysis, which provide insights into the thermodynamics and kinetics of magnesium insertion/extraction processes. Advanced characterization methods such as in-situ X-ray diffraction and spectroscopic techniques help correlate structural changes with redox behavior, enabling the design of materials with optimized redox potentials for specific applications.Expand Specific Solutions04 Electrolyte formulations for magnesium intercalation
Electrolyte composition significantly influences the redox potential and intercalation behavior of magnesium in host materials. Advanced electrolyte formulations containing magnesium salts in appropriate solvents facilitate efficient ion transport while minimizing side reactions that could alter the redox potential. The choice of electrolyte affects the formation of the solid-electrolyte interphase, which in turn impacts the energy required for magnesium intercalation and deintercalation, directly affecting the observed redox potential of the system.Expand Specific Solutions05 Novel magnesium intercalation compounds with tailored redox properties
Research has led to the development of novel compounds specifically designed for magnesium intercalation with optimized redox potentials. These materials include modified spinel structures, Chevrel phases, and organic frameworks that offer favorable magnesium insertion sites. By incorporating dopants, creating defects, or engineering surface properties, researchers can fine-tune the redox potential to achieve desired voltage ranges for energy storage applications. These innovations address challenges such as slow diffusion kinetics and structural stability during repeated magnesium intercalation cycles.Expand Specific Solutions
Leading Research Groups and Industrial Players
The magnesium intercalation materials market is in its growth phase, characterized by increasing research activity and early commercialization efforts. The global market for magnesium-based energy storage technologies is projected to reach $2-3 billion by 2030, driven by demand for sustainable energy solutions. Technical challenges in redox potential tuning remain, with varying degrees of maturity across applications. Leading industrial players include Volkswagen AG and Robert Bosch GmbH focusing on automotive applications, while NGK Insulators and Shell-USA are developing energy storage solutions. Academic institutions like MIT, Tsinghua University, and Zhejiang University are advancing fundamental research, collaborating with companies such as BTR Nano Technology and Hon Hai Precision to overcome challenges in electrode materials and electrolyte design for improved cycling stability and energy density.
NGK Insulators, Ltd.
Technical Solution: NGK Insulators has developed specialized ceramic-based technologies for redox potential tuning in magnesium intercalation materials. Their approach leverages their extensive expertise in advanced ceramics to create highly engineered host structures with precisely controlled crystallographic sites for magnesium insertion. NGK's technology focuses on oxide-based intercalation compounds with tailored electronic structures achieved through careful control of synthesis conditions and dopant incorporation. Their proprietary high-temperature processing techniques enable the creation of materials with exceptional phase purity and controlled defect chemistry, which directly influences redox potential. NGK has pioneered composite structures where conductive networks are integrated with active materials to enhance electron transport while maintaining desired redox properties. Their recent developments include gradient-functional materials where composition varies systematically throughout particles to create optimized interfaces for magnesium transport while maintaining bulk properties that deliver target redox potentials.
Strengths: Exceptional expertise in ceramic processing and manufacturing at scale, strong capabilities in creating materials with precise microstructural control, and extensive experience with high-temperature synthesis techniques. Weaknesses: Potentially less experience with organic components of battery systems compared to traditional battery manufacturers, which might limit holistic optimization of complete cell designs.
Robert Bosch GmbH
Technical Solution: Robert Bosch GmbH has developed a comprehensive approach to redox potential tuning in magnesium intercalation materials focused on industrial viability and system integration. Their technology centers on modified Chevrel phases and spinel structures with carefully engineered electronic properties to optimize magnesium insertion/extraction potentials. Bosch's research includes strategic substitution of transition metals in host lattices to shift d-orbital energy levels, directly influencing redox potentials while maintaining structural stability. Their proprietary synthesis methods involve precise control of oxygen stoichiometry and defect chemistry to create materials with tailored voltage profiles. Bosch has also pioneered composite electrode architectures where multiple active materials with complementary redox potentials are integrated to achieve broader operating voltage windows. Their technology includes surface modification strategies using atomic layer deposition to create engineered interfaces that facilitate magnesium transport while protecting the bulk material from electrolyte degradation.
Strengths: Exceptional manufacturing expertise and quality control capabilities, strong focus on system-level integration and practical implementation, extensive testing infrastructure for performance validation. Weaknesses: Potentially higher emphasis on established technologies with proven reliability rather than cutting-edge but unproven approaches, which might limit exploration of novel material chemistries.
Key Patents and Scientific Breakthroughs
Biodegradable magnesium alloy without rare earth elements, preparation method and use thereof
PatentPendingUS20240352560A1
Innovation
- A biodegradable magnesium alloy with a controlled microstructure achieved through process-controlled heat treatment, hot extrusion, and rolling, ensuring refined grain size and reduced surface cracks, comprising specific percentages of Zn, Mn, Ca, Zr, Sn, and Sr, without rare earth elements.
Biodegradable magnesium alloy free of rare earth element, and preparation method and use thereof
PatentPendingEP4272774A1
Innovation
- A biodegradable magnesium alloy with a controlled microstructure achieved through a process involving melting-casting, heat treatment, hot extrusion, and rolling, which refines the grain size and reduces surface cracks, ensuring a balanced composition of Zn, Mn, Ca, Zr, and Sr without rare earth elements.
Materials Sustainability and Resource Considerations
The sustainability aspects of magnesium intercalation materials represent a critical dimension in the advancement of next-generation energy storage technologies. Magnesium-based battery systems offer significant environmental advantages over conventional lithium-ion batteries, primarily due to the greater natural abundance of magnesium in the Earth's crust (2.3% compared to lithium's 0.0017%). This fundamental resource advantage translates to reduced extraction impacts and potentially lower material costs in large-scale deployment scenarios.
When evaluating redox potential tuning strategies for magnesium intercalation materials, lifecycle considerations become paramount. The environmental footprint of synthesizing these materials varies significantly depending on the specific dopants and processing methods employed. For instance, transition metal dopants commonly used for potential tuning may introduce sustainability concerns if they involve critical or rare elements such as cobalt or vanadium. Research indicates that substituting these with more abundant elements like manganese or iron can maintain electrochemical performance while enhancing resource sustainability.
Energy requirements for material synthesis represent another crucial sustainability factor. High-temperature solid-state reactions traditionally used for cathode material preparation consume substantial energy, contributing to the embedded carbon footprint. Recent advances in low-temperature synthesis routes, including hydrothermal and sol-gel methods, demonstrate promising approaches to reduce energy consumption while maintaining control over redox properties. These methods typically require 40-60% less energy input compared to conventional techniques.
Recycling potential must also be considered when developing new magnesium intercalation materials. The complexity of material composition resulting from redox tuning strategies can significantly impact end-of-life recovery processes. Materials designed with recyclability in mind—featuring minimal use of toxic elements and separable components—demonstrate superior sustainability profiles. Current research indicates that magnesium-based systems generally offer better recyclability than lithium counterparts, with potential recovery rates exceeding 90% for properly designed systems.
Water consumption and land use impacts associated with magnesium resource extraction are substantially lower than those for lithium, particularly when compared to brine-based lithium extraction methods. However, the environmental advantages of magnesium can be compromised if redox tuning relies heavily on rare earth elements or other critical materials with significant extraction impacts. This underscores the importance of holistic material design approaches that consider the entire supply chain sustainability.
The long-term availability of materials used in redox potential tuning presents both challenges and opportunities. Strategic material selection that avoids potential supply bottlenecks will be essential for large-scale commercialization of magnesium-based energy storage. Recent market analyses suggest that magnesium intercalation technologies utilizing earth-abundant elements for redox tuning could achieve material supply security for several decades of global deployment, significantly outperforming current lithium-ion technologies.
When evaluating redox potential tuning strategies for magnesium intercalation materials, lifecycle considerations become paramount. The environmental footprint of synthesizing these materials varies significantly depending on the specific dopants and processing methods employed. For instance, transition metal dopants commonly used for potential tuning may introduce sustainability concerns if they involve critical or rare elements such as cobalt or vanadium. Research indicates that substituting these with more abundant elements like manganese or iron can maintain electrochemical performance while enhancing resource sustainability.
Energy requirements for material synthesis represent another crucial sustainability factor. High-temperature solid-state reactions traditionally used for cathode material preparation consume substantial energy, contributing to the embedded carbon footprint. Recent advances in low-temperature synthesis routes, including hydrothermal and sol-gel methods, demonstrate promising approaches to reduce energy consumption while maintaining control over redox properties. These methods typically require 40-60% less energy input compared to conventional techniques.
Recycling potential must also be considered when developing new magnesium intercalation materials. The complexity of material composition resulting from redox tuning strategies can significantly impact end-of-life recovery processes. Materials designed with recyclability in mind—featuring minimal use of toxic elements and separable components—demonstrate superior sustainability profiles. Current research indicates that magnesium-based systems generally offer better recyclability than lithium counterparts, with potential recovery rates exceeding 90% for properly designed systems.
Water consumption and land use impacts associated with magnesium resource extraction are substantially lower than those for lithium, particularly when compared to brine-based lithium extraction methods. However, the environmental advantages of magnesium can be compromised if redox tuning relies heavily on rare earth elements or other critical materials with significant extraction impacts. This underscores the importance of holistic material design approaches that consider the entire supply chain sustainability.
The long-term availability of materials used in redox potential tuning presents both challenges and opportunities. Strategic material selection that avoids potential supply bottlenecks will be essential for large-scale commercialization of magnesium-based energy storage. Recent market analyses suggest that magnesium intercalation technologies utilizing earth-abundant elements for redox tuning could achieve material supply security for several decades of global deployment, significantly outperforming current lithium-ion technologies.
Performance Benchmarking and Standardization Methods
Establishing standardized performance benchmarking methods for magnesium intercalation materials is crucial for advancing this technology and enabling meaningful comparisons across different research efforts. Currently, the field suffers from inconsistent testing protocols and reporting standards, making it difficult to accurately assess progress and identify truly promising materials.
The electrochemical performance evaluation of magnesium intercalation materials requires standardized testing conditions including current density, voltage windows, electrolyte compositions, and temperature controls. Research indicates that variations in these parameters can significantly alter reported capacity values and cycling stability, sometimes by more than 30%. For example, testing at elevated temperatures often yields artificially enhanced kinetics that may not translate to room temperature applications.
Redox potential measurements specifically require careful standardization of reference electrodes and calibration procedures. The scientific community has begun establishing protocols that recommend using consistent reference systems such as Mg/Mg²⁺ in appropriate non-aqueous electrolytes. These standards help ensure that reported redox potentials can be reliably compared across different research groups and publications.
Cycle life assessment represents another area requiring standardization, with recommendations emerging for minimum reporting of 100 cycles at practical current densities (typically 0.1-0.5C). The definition of capacity retention metrics also needs standardization, with growing consensus around reporting both initial capacity loss and average fade rate per cycle after the initial stabilization period.
Rate capability testing protocols have been proposed that include specific C-rates (from C/20 to 5C) with defined hold times at each rate to ensure steady-state performance is captured. This approach provides more realistic assessments of material capabilities under various charging and discharging conditions relevant to practical applications.
Several international research consortia and organizations including the International Electrochemical Society and the Battery500 Consortium have begun developing standardized testing protocols specifically for beyond-lithium systems including magnesium batteries. These efforts aim to establish common benchmarks against which new materials can be evaluated, accelerating progress in the field by enabling direct comparisons between different research approaches.
Computational benchmarking methods are also emerging as important tools, with standardized density functional theory (DFT) approaches being developed to predict redox potentials in magnesium intercalation compounds. These computational standards help bridge experimental and theoretical research, providing valuable screening tools for new material development while ensuring consistency in theoretical predictions.
The electrochemical performance evaluation of magnesium intercalation materials requires standardized testing conditions including current density, voltage windows, electrolyte compositions, and temperature controls. Research indicates that variations in these parameters can significantly alter reported capacity values and cycling stability, sometimes by more than 30%. For example, testing at elevated temperatures often yields artificially enhanced kinetics that may not translate to room temperature applications.
Redox potential measurements specifically require careful standardization of reference electrodes and calibration procedures. The scientific community has begun establishing protocols that recommend using consistent reference systems such as Mg/Mg²⁺ in appropriate non-aqueous electrolytes. These standards help ensure that reported redox potentials can be reliably compared across different research groups and publications.
Cycle life assessment represents another area requiring standardization, with recommendations emerging for minimum reporting of 100 cycles at practical current densities (typically 0.1-0.5C). The definition of capacity retention metrics also needs standardization, with growing consensus around reporting both initial capacity loss and average fade rate per cycle after the initial stabilization period.
Rate capability testing protocols have been proposed that include specific C-rates (from C/20 to 5C) with defined hold times at each rate to ensure steady-state performance is captured. This approach provides more realistic assessments of material capabilities under various charging and discharging conditions relevant to practical applications.
Several international research consortia and organizations including the International Electrochemical Society and the Battery500 Consortium have begun developing standardized testing protocols specifically for beyond-lithium systems including magnesium batteries. These efforts aim to establish common benchmarks against which new materials can be evaluated, accelerating progress in the field by enabling direct comparisons between different research approaches.
Computational benchmarking methods are also emerging as important tools, with standardized density functional theory (DFT) approaches being developed to predict redox potentials in magnesium intercalation compounds. These computational standards help bridge experimental and theoretical research, providing valuable screening tools for new material development while ensuring consistency in theoretical predictions.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!