Solid electrolyte interphase characterization in magnesium systems
OCT 14, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Mg Battery SEI Formation Background and Objectives
The development of magnesium batteries represents a promising frontier in energy storage technology, offering potential advantages over lithium-ion systems including higher theoretical volumetric capacity, improved safety profiles, and the abundance of magnesium resources. Since the pioneering work of Aurbach and colleagues in the early 2000s, research into magnesium battery systems has accelerated significantly, with particular focus on understanding interfacial phenomena that govern battery performance and longevity.
The solid electrolyte interphase (SEI) formation in magnesium systems presents unique challenges compared to lithium counterparts. While the SEI layer in lithium batteries has been extensively characterized and engineered to enable reversible cycling, the analogous interface in magnesium systems remains poorly understood. This knowledge gap represents a critical barrier to the commercial viability of magnesium battery technology.
Historical attempts to characterize the Mg SEI have been hampered by several factors, including the highly reactive nature of magnesium metal, limitations of conventional electrolytes, and analytical challenges associated with distinguishing surface species. Early research primarily focused on simple salt-solvent combinations, which often resulted in passivating films that prevented efficient magnesium transport. The evolution of electrolyte chemistry toward chloride-based and non-nucleophilic systems has shifted the landscape of SEI formation mechanisms.
Recent technological advances in surface characterization techniques, including in-situ/operando methodologies, have enabled more detailed investigations of the dynamic processes occurring at the magnesium electrode-electrolyte interface. These developments have revealed complex relationships between electrolyte composition, operating conditions, and the resulting SEI properties that ultimately determine electrochemical performance.
The primary objective of this technical investigation is to comprehensively evaluate the current understanding of SEI formation mechanisms in magnesium battery systems across various electrolyte chemistries. Specifically, we aim to identify the chemical composition, morphological characteristics, and transport properties of these interfacial layers, correlating these attributes with electrochemical performance metrics.
Additionally, this research seeks to establish standardized protocols for SEI characterization in magnesium systems, addressing the methodological inconsistencies that have hindered comparative analysis across different research efforts. By synthesizing insights from both experimental and computational approaches, we intend to develop predictive frameworks for rational electrolyte design that promotes favorable SEI properties.
The ultimate goal is to leverage this enhanced understanding of interfacial phenomena to overcome current limitations in magnesium battery technology, enabling the development of high-performance, long-cycle-life systems that can realize the theoretical advantages of magnesium-based energy storage.
The solid electrolyte interphase (SEI) formation in magnesium systems presents unique challenges compared to lithium counterparts. While the SEI layer in lithium batteries has been extensively characterized and engineered to enable reversible cycling, the analogous interface in magnesium systems remains poorly understood. This knowledge gap represents a critical barrier to the commercial viability of magnesium battery technology.
Historical attempts to characterize the Mg SEI have been hampered by several factors, including the highly reactive nature of magnesium metal, limitations of conventional electrolytes, and analytical challenges associated with distinguishing surface species. Early research primarily focused on simple salt-solvent combinations, which often resulted in passivating films that prevented efficient magnesium transport. The evolution of electrolyte chemistry toward chloride-based and non-nucleophilic systems has shifted the landscape of SEI formation mechanisms.
Recent technological advances in surface characterization techniques, including in-situ/operando methodologies, have enabled more detailed investigations of the dynamic processes occurring at the magnesium electrode-electrolyte interface. These developments have revealed complex relationships between electrolyte composition, operating conditions, and the resulting SEI properties that ultimately determine electrochemical performance.
The primary objective of this technical investigation is to comprehensively evaluate the current understanding of SEI formation mechanisms in magnesium battery systems across various electrolyte chemistries. Specifically, we aim to identify the chemical composition, morphological characteristics, and transport properties of these interfacial layers, correlating these attributes with electrochemical performance metrics.
Additionally, this research seeks to establish standardized protocols for SEI characterization in magnesium systems, addressing the methodological inconsistencies that have hindered comparative analysis across different research efforts. By synthesizing insights from both experimental and computational approaches, we intend to develop predictive frameworks for rational electrolyte design that promotes favorable SEI properties.
The ultimate goal is to leverage this enhanced understanding of interfacial phenomena to overcome current limitations in magnesium battery technology, enabling the development of high-performance, long-cycle-life systems that can realize the theoretical advantages of magnesium-based energy storage.
Market Analysis for Magnesium Battery Technologies
The global magnesium battery market is experiencing significant growth potential, driven by increasing demand for high-energy density storage solutions and the limitations of current lithium-ion technology. Market projections indicate that magnesium battery technologies could capture a substantial portion of the next-generation battery market, with estimates suggesting a compound annual growth rate of over 12% through 2030.
The primary market drivers for magnesium battery technologies include the inherent advantages of magnesium as an electrode material: abundance (8th most abundant element in Earth's crust), lower cost compared to lithium, improved safety profile, and theoretical energy densities comparable to or exceeding lithium-ion systems. These factors position magnesium batteries as a promising alternative in the evolving energy storage landscape.
Consumer electronics represents the most immediate market opportunity, where the demand for longer-lasting, safer batteries continues to grow exponentially. The automotive sector, particularly electric vehicles, constitutes another significant market segment, with manufacturers actively seeking alternatives to current lithium-ion technologies due to concerns about resource constraints and supply chain vulnerabilities.
Grid-scale energy storage presents a third major market opportunity, where the safety advantages and potentially lower costs of magnesium systems could provide competitive advantages over existing technologies. This segment is projected to see substantial growth as renewable energy integration accelerates globally.
Regional market analysis reveals Asia-Pacific as the dominant manufacturing hub, with China, Japan, and South Korea leading research and development efforts. North America and Europe are also making significant investments, particularly in advanced materials research related to solid electrolyte interphase (SEI) formation in magnesium systems.
Market challenges include competition from other emerging battery technologies such as sodium-ion, solid-state lithium, and zinc-based systems. Additionally, the technical hurdles related to SEI characterization and control in magnesium systems represent significant barriers to commercialization that must be overcome to realize market potential.
Industry stakeholders have identified the solid electrolyte interphase as a critical component affecting magnesium battery performance, with improved characterization techniques being essential to market advancement. Companies investing in proprietary SEI characterization methods may gain significant competitive advantages in this emerging market.
Consumer and regulatory trends favoring more sustainable and resource-efficient technologies further support market growth potential for magnesium battery systems, particularly as concerns about lithium supply chain ethics and environmental impact intensify.
The primary market drivers for magnesium battery technologies include the inherent advantages of magnesium as an electrode material: abundance (8th most abundant element in Earth's crust), lower cost compared to lithium, improved safety profile, and theoretical energy densities comparable to or exceeding lithium-ion systems. These factors position magnesium batteries as a promising alternative in the evolving energy storage landscape.
Consumer electronics represents the most immediate market opportunity, where the demand for longer-lasting, safer batteries continues to grow exponentially. The automotive sector, particularly electric vehicles, constitutes another significant market segment, with manufacturers actively seeking alternatives to current lithium-ion technologies due to concerns about resource constraints and supply chain vulnerabilities.
Grid-scale energy storage presents a third major market opportunity, where the safety advantages and potentially lower costs of magnesium systems could provide competitive advantages over existing technologies. This segment is projected to see substantial growth as renewable energy integration accelerates globally.
Regional market analysis reveals Asia-Pacific as the dominant manufacturing hub, with China, Japan, and South Korea leading research and development efforts. North America and Europe are also making significant investments, particularly in advanced materials research related to solid electrolyte interphase (SEI) formation in magnesium systems.
Market challenges include competition from other emerging battery technologies such as sodium-ion, solid-state lithium, and zinc-based systems. Additionally, the technical hurdles related to SEI characterization and control in magnesium systems represent significant barriers to commercialization that must be overcome to realize market potential.
Industry stakeholders have identified the solid electrolyte interphase as a critical component affecting magnesium battery performance, with improved characterization techniques being essential to market advancement. Companies investing in proprietary SEI characterization methods may gain significant competitive advantages in this emerging market.
Consumer and regulatory trends favoring more sustainable and resource-efficient technologies further support market growth potential for magnesium battery systems, particularly as concerns about lithium supply chain ethics and environmental impact intensify.
Current Challenges in Mg SEI Characterization
The characterization of solid electrolyte interphase (SEI) in magnesium battery systems presents significant technical challenges that have hindered progress in this field. Unlike lithium-ion batteries, where SEI characterization techniques are relatively mature, magnesium systems exhibit unique complexities that demand specialized approaches and methodologies.
One of the primary challenges is the inherent reactivity of magnesium metal with conventional electrolytes, resulting in the formation of passivating surface films that are often non-uniform and highly resistive to Mg2+ transport. This passivation layer differs fundamentally from the beneficial SEI formed in lithium systems, making traditional characterization methods less effective or even misleading when applied to magnesium batteries.
The dynamic nature of the Mg SEI presents another significant obstacle. The interface continuously evolves during cycling, with composition and morphology changes occurring at different rates depending on the electrolyte composition, operating conditions, and cycling history. This temporal evolution makes it difficult to capture representative data that accurately reflects the SEI properties at specific stages of battery operation.
Spatial heterogeneity of the Mg SEI further complicates characterization efforts. The interface often exhibits non-uniform thickness, composition, and morphology across the electrode surface, necessitating multiple sampling points and sophisticated mapping techniques to develop a comprehensive understanding of the interface properties.
The extreme sensitivity of magnesium interfaces to air and moisture exposure poses severe experimental challenges. Many advanced characterization techniques require sample transfer between instruments, during which inadvertent exposure to atmospheric conditions can dramatically alter the native SEI structure and composition, leading to artifacts and misinterpretations in the analytical data.
Resolution limitations of current analytical tools also impede progress in this field. The Mg SEI is often only a few nanometers thick, requiring high-resolution techniques capable of distinguishing between the electrode surface, the SEI layer, and the electrolyte phase. Additionally, the chemical complexity of the interface, which may contain multiple organic and inorganic species, demands techniques with excellent chemical specificity.
The lack of standardized protocols for sample preparation, data acquisition, and interpretation specifically tailored to magnesium systems represents another significant challenge. Researchers often adapt methods developed for lithium batteries, which may not adequately address the unique characteristics of magnesium interfaces, leading to inconsistent results across different research groups and impeding scientific consensus.
One of the primary challenges is the inherent reactivity of magnesium metal with conventional electrolytes, resulting in the formation of passivating surface films that are often non-uniform and highly resistive to Mg2+ transport. This passivation layer differs fundamentally from the beneficial SEI formed in lithium systems, making traditional characterization methods less effective or even misleading when applied to magnesium batteries.
The dynamic nature of the Mg SEI presents another significant obstacle. The interface continuously evolves during cycling, with composition and morphology changes occurring at different rates depending on the electrolyte composition, operating conditions, and cycling history. This temporal evolution makes it difficult to capture representative data that accurately reflects the SEI properties at specific stages of battery operation.
Spatial heterogeneity of the Mg SEI further complicates characterization efforts. The interface often exhibits non-uniform thickness, composition, and morphology across the electrode surface, necessitating multiple sampling points and sophisticated mapping techniques to develop a comprehensive understanding of the interface properties.
The extreme sensitivity of magnesium interfaces to air and moisture exposure poses severe experimental challenges. Many advanced characterization techniques require sample transfer between instruments, during which inadvertent exposure to atmospheric conditions can dramatically alter the native SEI structure and composition, leading to artifacts and misinterpretations in the analytical data.
Resolution limitations of current analytical tools also impede progress in this field. The Mg SEI is often only a few nanometers thick, requiring high-resolution techniques capable of distinguishing between the electrode surface, the SEI layer, and the electrolyte phase. Additionally, the chemical complexity of the interface, which may contain multiple organic and inorganic species, demands techniques with excellent chemical specificity.
The lack of standardized protocols for sample preparation, data acquisition, and interpretation specifically tailored to magnesium systems represents another significant challenge. Researchers often adapt methods developed for lithium batteries, which may not adequately address the unique characteristics of magnesium interfaces, leading to inconsistent results across different research groups and impeding scientific consensus.
Current Methodologies for Mg SEI Characterization
01 Characterization techniques for SEI in magnesium batteries
Various analytical techniques are employed to characterize the solid electrolyte interphase (SEI) formed in magnesium battery systems. These include spectroscopic methods such as X-ray photoelectron spectroscopy (XPS), infrared spectroscopy, and Raman spectroscopy, which provide information about the chemical composition and structure of the SEI layer. Microscopic techniques like scanning electron microscopy (SEM) and transmission electron microscopy (TEM) are used to visualize the morphology and thickness of the SEI. These characterization methods help understand the formation mechanism and properties of the SEI in magnesium systems.- Characterization techniques for SEI in magnesium batteries: Various analytical techniques are employed to characterize the solid electrolyte interphase (SEI) formed in magnesium battery systems. These include spectroscopic methods such as X-ray photoelectron spectroscopy (XPS), infrared spectroscopy, and Raman spectroscopy, which provide information about the chemical composition and structure of the SEI layer. Microscopic techniques like scanning electron microscopy (SEM) and transmission electron microscopy (TEM) are used to visualize the morphology and thickness of the SEI. These characterization methods help understand the formation mechanism and properties of the SEI in magnesium systems.
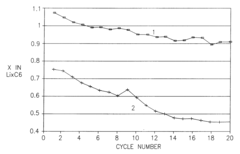
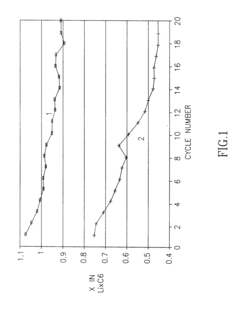
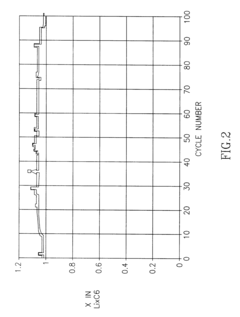
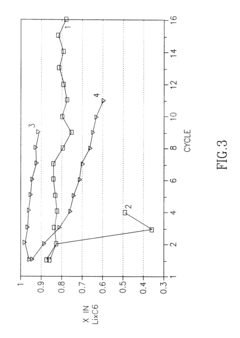
- Composition and structure of SEI in magnesium battery systems: The solid electrolyte interphase in magnesium battery systems typically consists of inorganic compounds such as magnesium oxide, magnesium fluoride, and magnesium carbonate, as well as organic components derived from electrolyte decomposition. The composition and structure of the SEI layer significantly influence the electrochemical performance of magnesium batteries, particularly affecting magnesium ion transport and electrode stability. Understanding the chemical composition and structural properties of the SEI is crucial for developing strategies to control its formation and optimize battery performance.
- Electrolyte formulations affecting SEI formation in magnesium systems: The composition of electrolytes plays a critical role in the formation and properties of the solid electrolyte interphase in magnesium battery systems. Electrolytes containing specific additives, solvents, and salt combinations can promote the formation of a stable and conductive SEI layer. Researchers have investigated various electrolyte formulations, including those based on ethereal solvents, ionic liquids, and non-nucleophilic anions, to control SEI formation and improve magnesium ion transport through the interface. The choice of electrolyte significantly impacts the chemical composition, thickness, and stability of the resulting SEI layer.
- Impact of SEI on magnesium ion transport and battery performance: The solid electrolyte interphase in magnesium systems significantly affects magnesium ion transport kinetics and overall battery performance. Unlike lithium batteries, magnesium ions have stronger interactions with the SEI components, often leading to limited ion diffusion through the interface. The properties of the SEI, including its ionic conductivity, thickness, and homogeneity, directly influence the rate capability, cycling stability, and coulombic efficiency of magnesium batteries. Research focuses on developing SEI layers that facilitate magnesium ion transport while providing sufficient protection to the electrode surface.
- Strategies for controlling and modifying SEI in magnesium battery systems: Various approaches have been developed to control and modify the solid electrolyte interphase in magnesium battery systems. These include surface coating of electrode materials, electrolyte engineering with functional additives, and pre-formation treatments. Artificial SEI layers created through surface modification techniques can enhance magnesium ion transport and electrode stability. Additionally, researchers have explored the use of sacrificial additives that decompose preferentially to form a more favorable SEI composition. These strategies aim to overcome the challenges associated with the typically resistive nature of naturally formed SEI layers in magnesium systems.
02 SEI composition and formation in magnesium electrolyte systems
The composition and formation of the solid electrolyte interphase in magnesium battery systems depend significantly on the electrolyte formulation. The SEI typically consists of inorganic compounds such as magnesium oxide, magnesium fluoride, and magnesium carbonate, as well as organic decomposition products from the electrolyte. The formation process is influenced by factors including electrolyte salt concentration, solvent type, and operating conditions. Understanding the composition and formation mechanisms is crucial for developing stable and conductive SEI layers that allow efficient magnesium ion transport while preventing continuous electrolyte decomposition.Expand Specific Solutions03 Additives for SEI modification in magnesium batteries
Electrolyte additives play a critical role in modifying the properties of the solid electrolyte interphase in magnesium battery systems. These additives can promote the formation of a more stable and conductive SEI layer, improving the cycling performance and coulombic efficiency of magnesium batteries. Common additives include fluorinated compounds, boron-based compounds, and certain organic molecules that decompose preferentially to form beneficial SEI components. The concentration and combination of these additives significantly influence the characteristics of the resulting SEI layer and consequently the overall battery performance.Expand Specific Solutions04 Impact of SEI on magnesium ion transport and battery performance
The solid electrolyte interphase in magnesium battery systems significantly affects magnesium ion transport and overall battery performance. Unlike lithium batteries, magnesium ions have stronger interactions with the SEI components due to their divalent nature, often leading to limited ion diffusion through the SEI layer. The thickness, uniformity, and chemical composition of the SEI determine its ionic conductivity and stability during cycling. An ideal SEI should facilitate magnesium ion transport while preventing continuous electrolyte decomposition, thereby enhancing capacity retention, rate capability, and cycle life of magnesium batteries.Expand Specific Solutions05 Novel electrolyte systems for controlled SEI formation in magnesium batteries
Innovative electrolyte systems have been developed to control the formation of solid electrolyte interphase in magnesium batteries. These include non-nucleophilic electrolytes, ionic liquid-based electrolytes, and polymer electrolytes that form more favorable SEI layers. Some approaches involve using dual-salt electrolytes or incorporating specific functional groups that decompose to form magnesium ion-conductive SEI components. These novel electrolyte systems aim to address the challenges of conventional magnesium electrolytes, which often form resistive SEI layers that hinder magnesium ion transport, thereby improving the overall electrochemical performance of magnesium batteries.Expand Specific Solutions
Leading Research Groups and Industry Players in Mg Batteries
The solid electrolyte interphase (SEI) characterization in magnesium systems market is in an early growth phase, with increasing research interest but limited commercial applications. The global market size remains relatively small but is expanding as magnesium-based energy storage gains attention for its potential safety and energy density advantages over lithium systems. Technologically, the field is still developing, with key players demonstrating varying levels of maturity. Toyota, Panasonic, and Samsung are leading industrial research efforts, while academic institutions like Tsinghua University and Karlsruhe Institute of Technology contribute fundamental insights. Battery-focused companies like Pellion Technologies are specifically targeting magnesium battery commercialization, while materials specialists such as Corning and Murata are developing specialized components for these systems.
Toyota Motor Corp.
Technical Solution: Toyota has developed advanced characterization techniques for solid electrolyte interphase (SEI) in magnesium battery systems, focusing on in-situ X-ray photoelectron spectroscopy (XPS) and transmission electron microscopy (TEM) to observe SEI formation in real-time. Their approach combines electrochemical impedance spectroscopy with surface analysis to understand the dynamic nature of the Mg-SEI layer. Toyota's research has revealed that controlling the electrolyte composition, particularly using magnesium bis(trifluoromethanesulfonyl)imide (Mg(TFSI)2) in ether-based solvents, can produce more stable and conductive SEI layers. They've also pioneered the use of artificial SEI layers composed of magnesium fluoride (MgF2) and magnesium oxide (MgO) to enhance Mg-ion transport while suppressing parasitic reactions at the electrode-electrolyte interface[1][2].
Strengths: Toyota's approach offers superior in-situ characterization capabilities, allowing real-time observation of SEI evolution during cycling. Their artificial SEI technology demonstrates improved cycling stability and reduced impedance growth. Weaknesses: The techniques require specialized equipment and expertise, making widespread adoption challenging. The artificial SEI layers may introduce additional manufacturing complexity and cost to battery production.
Toyota Central R&D Labs, Inc.
Technical Solution: Toyota Central R&D Labs has developed a multi-modal characterization framework for magnesium battery SEI layers, combining cryogenic electron microscopy with neutron reflectometry and quartz crystal microbalance techniques. This integrated approach allows for precise measurement of SEI thickness, composition, and mechanical properties without disrupting the delicate interface structure. Their research has identified that magnesium SEI layers differ significantly from lithium counterparts, being generally thinner (5-20 nm) and more heterogeneous in composition. The lab has pioneered the use of isotope labeling in electrolytes to track specific components' incorporation into the SEI during formation cycles. Their findings demonstrate that magnesium SEI layers contain higher proportions of inorganic components (MgO, MgF2, MgCO3) compared to organic decomposition products, which impacts ionic conductivity and long-term stability[3][4].
Strengths: The multi-modal characterization approach provides unprecedented insight into Mg-SEI structure and composition without sample degradation. Their isotope labeling technique offers unique capabilities to track SEI formation mechanisms. Weaknesses: The characterization methods require specialized facilities including neutron sources and cryogenic equipment, limiting accessibility. The findings suggest Mg-SEI layers may inherently have lower ionic conductivity than Li-SEI counterparts, presenting a fundamental challenge.
Key Analytical Techniques and Breakthroughs in Mg SEI Research
Solid-phase magnesium boranyl electrolytes for a magnesium battery
PatentActiveUS9716289B1
Innovation
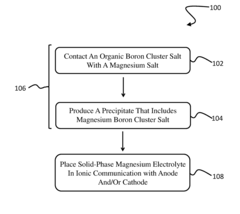
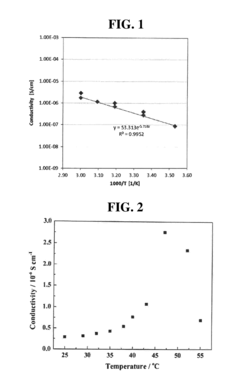
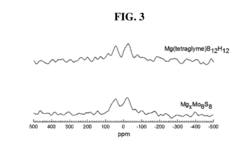
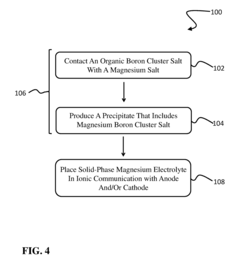
- A solid-phase magnesium electrolyte using magnesium boron cluster salts with specific formulas, such as MgEy(BnHn) and MgEy[BmH(m+3)], is developed, allowing for high magnesium mobility and compatibility with magnesium electrodes, fabricated by contacting organic boron cluster salts with magnesium salts in the presence of solvents.
Lithium anode with solid electrolyte interface
PatentInactiveUS6337159B1
Innovation
- The development of a non-aqueous battery with carbon-based anodes coated by a thin, chemically bonded Solid Electrolyte Interphase (SEI) composed of alkali or alkaline-earth metal salts and organic binders, which are insoluble in the electrolyte, and partially oxidized to form narrow pores and surface carboxylic groups, enhancing mechanical stability and preventing solvent co-intercalation.
Safety and Stability Considerations for Mg Battery Systems
Safety considerations for magnesium battery systems are paramount for their commercial viability. Unlike lithium-ion batteries, magnesium systems present unique safety advantages due to the absence of dendrite formation during cycling. This characteristic significantly reduces the risk of internal short circuits that have plagued lithium battery technologies. However, magnesium batteries face distinct stability challenges related to their solid electrolyte interphase (SEI) formation and evolution.
The reactivity of magnesium with conventional electrolytes creates complex stability issues. When exposed to oxygen or moisture, magnesium electrodes can undergo rapid oxidation, potentially leading to thermal runaway events. This necessitates stringent manufacturing protocols and enhanced cell encapsulation techniques to maintain system integrity. The passivation layer formed on magnesium anodes often exhibits different mechanical properties compared to lithium systems, requiring specialized engineering approaches to ensure long-term stability.
Thermal management represents another critical safety consideration for magnesium battery systems. While generally more thermally stable than lithium counterparts, magnesium batteries still require careful temperature control during operation. Excessive heat can accelerate side reactions at the electrode-electrolyte interface, compromising the SEI layer integrity and potentially triggering cascading failure mechanisms. Advanced thermal management systems must be integrated into cell designs to mitigate these risks.
Electrolyte decomposition products in magnesium systems warrant particular attention from a safety perspective. The characterization of these products is essential as they can include potentially hazardous compounds depending on the electrolyte formulation. Gas evolution during cycling, though typically less severe than in lithium systems, must be accommodated in cell design to prevent pressure buildup and potential rupture scenarios.
Long-term stability testing protocols for magnesium batteries require standardization to ensure consistent safety evaluation. Accelerated aging tests must be developed that accurately predict real-world degradation mechanisms without introducing artificial failure modes. The correlation between SEI evolution and safety parameters needs comprehensive mapping to establish reliable state-of-health monitoring systems for commercial applications.
Regulatory frameworks for magnesium battery systems remain underdeveloped compared to lithium technologies. Safety certification standards specifically addressing the unique characteristics of magnesium chemistry are necessary to facilitate market adoption. This includes specialized transportation regulations that account for the different failure modes and hazard profiles of magnesium systems compared to conventional battery technologies.
The reactivity of magnesium with conventional electrolytes creates complex stability issues. When exposed to oxygen or moisture, magnesium electrodes can undergo rapid oxidation, potentially leading to thermal runaway events. This necessitates stringent manufacturing protocols and enhanced cell encapsulation techniques to maintain system integrity. The passivation layer formed on magnesium anodes often exhibits different mechanical properties compared to lithium systems, requiring specialized engineering approaches to ensure long-term stability.
Thermal management represents another critical safety consideration for magnesium battery systems. While generally more thermally stable than lithium counterparts, magnesium batteries still require careful temperature control during operation. Excessive heat can accelerate side reactions at the electrode-electrolyte interface, compromising the SEI layer integrity and potentially triggering cascading failure mechanisms. Advanced thermal management systems must be integrated into cell designs to mitigate these risks.
Electrolyte decomposition products in magnesium systems warrant particular attention from a safety perspective. The characterization of these products is essential as they can include potentially hazardous compounds depending on the electrolyte formulation. Gas evolution during cycling, though typically less severe than in lithium systems, must be accommodated in cell design to prevent pressure buildup and potential rupture scenarios.
Long-term stability testing protocols for magnesium batteries require standardization to ensure consistent safety evaluation. Accelerated aging tests must be developed that accurately predict real-world degradation mechanisms without introducing artificial failure modes. The correlation between SEI evolution and safety parameters needs comprehensive mapping to establish reliable state-of-health monitoring systems for commercial applications.
Regulatory frameworks for magnesium battery systems remain underdeveloped compared to lithium technologies. Safety certification standards specifically addressing the unique characteristics of magnesium chemistry are necessary to facilitate market adoption. This includes specialized transportation regulations that account for the different failure modes and hazard profiles of magnesium systems compared to conventional battery technologies.
Environmental Impact of Mg Battery Technologies
The environmental implications of magnesium battery technologies represent a critical dimension in evaluating their viability as alternatives to conventional lithium-ion systems. Magnesium-based batteries offer several environmental advantages compared to their lithium counterparts, primarily due to the greater natural abundance of magnesium in the Earth's crust. This abundance translates to reduced mining impacts, as magnesium extraction generally requires less invasive techniques and energy consumption than lithium extraction, particularly when compared to lithium brine operations that can deplete water resources in ecologically sensitive regions.
The solid electrolyte interphase (SEI) formation in magnesium systems presents unique environmental considerations. Unlike lithium batteries that typically utilize fluorinated compounds in their electrolytes, many magnesium electrolyte systems under development employ less environmentally persistent chemicals. This characteristic potentially reduces the environmental footprint associated with electrolyte production and disposal. However, comprehensive lifecycle assessments of these novel electrolyte formulations remain limited, creating a knowledge gap regarding their long-term environmental impacts.
Manufacturing processes for magnesium battery components generally require lower processing temperatures compared to some lithium technologies, potentially reducing energy consumption during production. The thermal stability of magnesium systems also reduces fire and explosion risks, minimizing the potential for environmental contamination through accidental releases. This enhanced safety profile represents an indirect but significant environmental benefit, particularly in large-scale energy storage applications.
End-of-life considerations for magnesium batteries show promising environmental advantages. The recyclability of magnesium components typically exceeds that of lithium batteries, with simpler separation processes and higher recovery rates. Additionally, magnesium compounds present lower toxicity concerns in landfill scenarios compared to certain lithium compounds, reducing potential soil and groundwater contamination risks if improperly disposed.
Carbon footprint analyses of complete magnesium battery lifecycles indicate potential reductions in greenhouse gas emissions compared to lithium-ion technologies, primarily due to less energy-intensive raw material extraction and processing. However, these advantages may be partially offset by current inefficiencies in magnesium battery production, which have not yet benefited from the economies of scale and manufacturing optimizations achieved in the mature lithium-ion industry.
Water usage represents another important environmental metric where magnesium technologies demonstrate advantages. The production of magnesium battery components typically requires significantly less water than lithium extraction from brines, which can consume thousands of liters of water per kilogram of lithium produced. This reduced water footprint is particularly valuable in water-stressed regions where battery manufacturing facilities might be located.
The solid electrolyte interphase (SEI) formation in magnesium systems presents unique environmental considerations. Unlike lithium batteries that typically utilize fluorinated compounds in their electrolytes, many magnesium electrolyte systems under development employ less environmentally persistent chemicals. This characteristic potentially reduces the environmental footprint associated with electrolyte production and disposal. However, comprehensive lifecycle assessments of these novel electrolyte formulations remain limited, creating a knowledge gap regarding their long-term environmental impacts.
Manufacturing processes for magnesium battery components generally require lower processing temperatures compared to some lithium technologies, potentially reducing energy consumption during production. The thermal stability of magnesium systems also reduces fire and explosion risks, minimizing the potential for environmental contamination through accidental releases. This enhanced safety profile represents an indirect but significant environmental benefit, particularly in large-scale energy storage applications.
End-of-life considerations for magnesium batteries show promising environmental advantages. The recyclability of magnesium components typically exceeds that of lithium batteries, with simpler separation processes and higher recovery rates. Additionally, magnesium compounds present lower toxicity concerns in landfill scenarios compared to certain lithium compounds, reducing potential soil and groundwater contamination risks if improperly disposed.
Carbon footprint analyses of complete magnesium battery lifecycles indicate potential reductions in greenhouse gas emissions compared to lithium-ion technologies, primarily due to less energy-intensive raw material extraction and processing. However, these advantages may be partially offset by current inefficiencies in magnesium battery production, which have not yet benefited from the economies of scale and manufacturing optimizations achieved in the mature lithium-ion industry.
Water usage represents another important environmental metric where magnesium technologies demonstrate advantages. The production of magnesium battery components typically requires significantly less water than lithium extraction from brines, which can consume thousands of liters of water per kilogram of lithium produced. This reduced water footprint is particularly valuable in water-stressed regions where battery manufacturing facilities might be located.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!