Computational modeling of magnesium ion solvation structures
OCT 14, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Mg Ion Solvation Modeling Background and Objectives
Magnesium ion solvation modeling has emerged as a critical research area at the intersection of computational chemistry, materials science, and electrochemistry. The study of magnesium ion solvation structures dates back to the early 1980s, when rudimentary molecular dynamics simulations first attempted to capture the complex interactions between Mg2+ ions and solvent molecules. Over the decades, this field has evolved significantly, driven by advances in computational methods, increased computing power, and the growing importance of magnesium-based technologies.
The evolution of computational approaches for modeling Mg2+ solvation has followed a clear trajectory from simple classical force fields to sophisticated quantum mechanical methods. Early models relied on basic point-charge representations and rigid molecular structures, which failed to capture the nuanced electronic effects critical to accurate solvation modeling. The 1990s saw the introduction of polarizable force fields, while the 2000s brought density functional theory (DFT) approaches into mainstream use for these systems.
Recent technological demands have significantly accelerated research in this domain. The push for post-lithium battery technologies has positioned magnesium-ion batteries as promising candidates due to their theoretical energy density advantages, abundance, and safety profile. Understanding the solvation behavior of Mg2+ is fundamental to addressing the challenges of slow kinetics and reversibility that currently limit practical applications of magnesium-based energy storage systems.
The primary objective of computational modeling in this field is to accurately predict and characterize the structural, thermodynamic, and dynamic properties of magnesium ion solvation complexes in various solvent environments. This includes determining coordination numbers, binding energies, exchange rates, and structural rearrangements that occur during solvation and desolvation processes.
Secondary objectives include establishing correlations between solvation structure and electrochemical performance metrics such as ionic conductivity, interfacial resistance, and redox potentials. These insights are crucial for rational electrolyte design in energy storage applications.
Current research trends indicate a move toward multi-scale modeling approaches that bridge quantum mechanical accuracy with classical simulation efficiency. Machine learning potentials trained on high-level quantum calculations are emerging as powerful tools for extending simulation time and length scales while maintaining quantum-level accuracy.
The field is also witnessing increased integration of computational predictions with experimental validation techniques such as X-ray absorption spectroscopy (XAS), nuclear magnetic resonance (NMR), and Raman spectroscopy. This synergistic approach aims to develop a comprehensive understanding of magnesium ion solvation behavior across different solvent systems and concentration regimes.
The evolution of computational approaches for modeling Mg2+ solvation has followed a clear trajectory from simple classical force fields to sophisticated quantum mechanical methods. Early models relied on basic point-charge representations and rigid molecular structures, which failed to capture the nuanced electronic effects critical to accurate solvation modeling. The 1990s saw the introduction of polarizable force fields, while the 2000s brought density functional theory (DFT) approaches into mainstream use for these systems.
Recent technological demands have significantly accelerated research in this domain. The push for post-lithium battery technologies has positioned magnesium-ion batteries as promising candidates due to their theoretical energy density advantages, abundance, and safety profile. Understanding the solvation behavior of Mg2+ is fundamental to addressing the challenges of slow kinetics and reversibility that currently limit practical applications of magnesium-based energy storage systems.
The primary objective of computational modeling in this field is to accurately predict and characterize the structural, thermodynamic, and dynamic properties of magnesium ion solvation complexes in various solvent environments. This includes determining coordination numbers, binding energies, exchange rates, and structural rearrangements that occur during solvation and desolvation processes.
Secondary objectives include establishing correlations between solvation structure and electrochemical performance metrics such as ionic conductivity, interfacial resistance, and redox potentials. These insights are crucial for rational electrolyte design in energy storage applications.
Current research trends indicate a move toward multi-scale modeling approaches that bridge quantum mechanical accuracy with classical simulation efficiency. Machine learning potentials trained on high-level quantum calculations are emerging as powerful tools for extending simulation time and length scales while maintaining quantum-level accuracy.
The field is also witnessing increased integration of computational predictions with experimental validation techniques such as X-ray absorption spectroscopy (XAS), nuclear magnetic resonance (NMR), and Raman spectroscopy. This synergistic approach aims to develop a comprehensive understanding of magnesium ion solvation behavior across different solvent systems and concentration regimes.
Market Applications and Demand Analysis for Mg-based Technologies
The market for magnesium-based technologies has experienced significant growth in recent years, driven primarily by the increasing demand for lightweight materials in automotive and aerospace industries. The computational modeling of magnesium ion solvation structures plays a crucial role in advancing several high-value market applications, particularly in energy storage, pharmaceutical development, and environmental remediation sectors.
In the energy storage domain, magnesium-based batteries represent a promising alternative to lithium-ion technology, offering theoretical energy densities of 3833 mAh/cm³ compared to lithium's 2062 mAh/cm³. Market research indicates that the global magnesium battery market is projected to grow substantially as manufacturers seek safer, more abundant, and cost-effective energy storage solutions. The accurate computational modeling of Mg²⁺ solvation behavior directly impacts electrolyte design and battery performance optimization.
The pharmaceutical industry has demonstrated increasing interest in magnesium-based drug delivery systems and therapeutic agents. Understanding magnesium ion solvation structures through computational modeling enables more precise drug formulation and targeted delivery mechanisms. This application segment is expected to expand as personalized medicine approaches gain traction in healthcare markets worldwide.
Environmental technologies represent another significant market opportunity, with magnesium compounds being utilized in water treatment processes, carbon capture systems, and soil remediation. The global water treatment chemicals market, where magnesium-based products play an important role, continues to expand due to increasing water scarcity concerns and stricter environmental regulations.
Agricultural applications of magnesium technologies, informed by computational modeling insights, are gaining prominence in precision farming practices. The agricultural supplements market has shown steady growth as farmers seek to optimize crop yields through better understanding of nutrient bioavailability, which is directly influenced by magnesium ion solvation behaviors.
Industrial catalysis represents another valuable application area, where magnesium-based catalysts offer cost advantages and environmental benefits compared to traditional noble metal catalysts. The computational modeling of solvation structures enables more efficient catalyst design, driving adoption in chemical manufacturing processes.
Market analysis reveals regional variations in demand patterns, with Asia-Pacific showing the strongest growth trajectory for magnesium-based technologies, followed by North America and Europe. This geographic distribution correlates with regional industrial development priorities and regulatory frameworks governing materials sustainability and environmental impact.
Consumer demand trends indicate increasing preference for sustainable and environmentally friendly technologies, creating favorable market conditions for magnesium-based solutions that offer reduced environmental footprints compared to alternatives. This shift in consumer sentiment represents a significant market driver that computational modeling research can leverage through enabling greener product development.
In the energy storage domain, magnesium-based batteries represent a promising alternative to lithium-ion technology, offering theoretical energy densities of 3833 mAh/cm³ compared to lithium's 2062 mAh/cm³. Market research indicates that the global magnesium battery market is projected to grow substantially as manufacturers seek safer, more abundant, and cost-effective energy storage solutions. The accurate computational modeling of Mg²⁺ solvation behavior directly impacts electrolyte design and battery performance optimization.
The pharmaceutical industry has demonstrated increasing interest in magnesium-based drug delivery systems and therapeutic agents. Understanding magnesium ion solvation structures through computational modeling enables more precise drug formulation and targeted delivery mechanisms. This application segment is expected to expand as personalized medicine approaches gain traction in healthcare markets worldwide.
Environmental technologies represent another significant market opportunity, with magnesium compounds being utilized in water treatment processes, carbon capture systems, and soil remediation. The global water treatment chemicals market, where magnesium-based products play an important role, continues to expand due to increasing water scarcity concerns and stricter environmental regulations.
Agricultural applications of magnesium technologies, informed by computational modeling insights, are gaining prominence in precision farming practices. The agricultural supplements market has shown steady growth as farmers seek to optimize crop yields through better understanding of nutrient bioavailability, which is directly influenced by magnesium ion solvation behaviors.
Industrial catalysis represents another valuable application area, where magnesium-based catalysts offer cost advantages and environmental benefits compared to traditional noble metal catalysts. The computational modeling of solvation structures enables more efficient catalyst design, driving adoption in chemical manufacturing processes.
Market analysis reveals regional variations in demand patterns, with Asia-Pacific showing the strongest growth trajectory for magnesium-based technologies, followed by North America and Europe. This geographic distribution correlates with regional industrial development priorities and regulatory frameworks governing materials sustainability and environmental impact.
Consumer demand trends indicate increasing preference for sustainable and environmentally friendly technologies, creating favorable market conditions for magnesium-based solutions that offer reduced environmental footprints compared to alternatives. This shift in consumer sentiment represents a significant market driver that computational modeling research can leverage through enabling greener product development.
Current Challenges in Computational Modeling of Mg Solvation
Despite significant advancements in computational chemistry, modeling magnesium ion solvation structures remains fraught with challenges. The divalent nature of Mg²⁺ creates strong electrostatic interactions with surrounding water molecules, resulting in tightly bound first and second solvation shells that are difficult to accurately represent in computational models. This high charge density leads to complex polarization effects that many standard force fields fail to capture adequately.
Current molecular dynamics (MD) simulations struggle with parameterization issues when modeling Mg²⁺ solvation. Classical force fields often underestimate the strength of Mg²⁺-water interactions or incorrectly predict coordination numbers and exchange rates. The rigid water models commonly employed in simulations cannot properly account for the significant geometric distortions induced by the strong electric field of magnesium ions.
Quantum mechanical (QM) methods offer higher accuracy but face severe computational cost limitations when modeling realistic solvation environments. The necessary system size to capture extended solvation shells and bulk effects typically exceeds practical QM calculation capabilities. While QM/MM hybrid approaches attempt to address this limitation, the interface between quantum and classical regions introduces additional uncertainties and artifacts.
Time scale limitations present another significant obstacle. The residence time of water molecules in the first solvation shell of Mg²⁺ can extend to microseconds or longer, far beyond the reach of ab initio MD simulations. This kinetic trapping makes it difficult to sample configuration space adequately and observe water exchange events that are critical for understanding solvation dynamics.
The treatment of electronic polarization effects remains particularly problematic. Fixed-charge force fields cannot account for the electronic redistribution that occurs as water molecules approach the highly charged Mg²⁺ ion. While polarizable force fields exist, they often require complex parameterization and significantly increase computational costs without necessarily resolving all accuracy issues.
Accurately modeling the competition between different anions and water molecules for coordination sites around Mg²⁺ presents additional challenges. In biological and electrochemical systems, this competition determines critical properties like ion selectivity and transport. Current models struggle to balance the relative strengths of ion-water and ion-anion interactions, particularly in concentrated solutions where ion pairing becomes significant.
The transferability of computational models across different environments also remains limited. Parameters optimized for Mg²⁺ in bulk water often perform poorly when applied to interfaces, confined spaces, or protein binding sites where the local dielectric environment differs substantially from bulk conditions.
Current molecular dynamics (MD) simulations struggle with parameterization issues when modeling Mg²⁺ solvation. Classical force fields often underestimate the strength of Mg²⁺-water interactions or incorrectly predict coordination numbers and exchange rates. The rigid water models commonly employed in simulations cannot properly account for the significant geometric distortions induced by the strong electric field of magnesium ions.
Quantum mechanical (QM) methods offer higher accuracy but face severe computational cost limitations when modeling realistic solvation environments. The necessary system size to capture extended solvation shells and bulk effects typically exceeds practical QM calculation capabilities. While QM/MM hybrid approaches attempt to address this limitation, the interface between quantum and classical regions introduces additional uncertainties and artifacts.
Time scale limitations present another significant obstacle. The residence time of water molecules in the first solvation shell of Mg²⁺ can extend to microseconds or longer, far beyond the reach of ab initio MD simulations. This kinetic trapping makes it difficult to sample configuration space adequately and observe water exchange events that are critical for understanding solvation dynamics.
The treatment of electronic polarization effects remains particularly problematic. Fixed-charge force fields cannot account for the electronic redistribution that occurs as water molecules approach the highly charged Mg²⁺ ion. While polarizable force fields exist, they often require complex parameterization and significantly increase computational costs without necessarily resolving all accuracy issues.
Accurately modeling the competition between different anions and water molecules for coordination sites around Mg²⁺ presents additional challenges. In biological and electrochemical systems, this competition determines critical properties like ion selectivity and transport. Current models struggle to balance the relative strengths of ion-water and ion-anion interactions, particularly in concentrated solutions where ion pairing becomes significant.
The transferability of computational models across different environments also remains limited. Parameters optimized for Mg²⁺ in bulk water often perform poorly when applied to interfaces, confined spaces, or protein binding sites where the local dielectric environment differs substantially from bulk conditions.
State-of-the-Art Computational Approaches for Mg Solvation
01 Magnesium ion coordination in aqueous solutions
Research on magnesium ion solvation structures in aqueous environments reveals specific coordination patterns. Magnesium ions typically form octahedral complexes with six water molecules in the first solvation shell. These structures are relatively stable due to the strong electrostatic interactions between the divalent magnesium cation and the oxygen atoms of water molecules. The arrangement and dynamics of these solvation structures are crucial for understanding magnesium's role in biological systems and chemical processes.- Magnesium ion coordination in aqueous solutions: Research on the coordination structure of magnesium ions in aqueous solutions reveals specific hydration patterns. Magnesium ions typically form octahedral coordination complexes with six water molecules in the first solvation shell. This primary hydration sphere is relatively stable due to the strong electrostatic interactions between the divalent magnesium cation and the oxygen atoms of water molecules. The arrangement and dynamics of these water molecules significantly influence the chemical behavior and reactivity of magnesium ions in solution.
- Computational modeling of magnesium solvation structures: Advanced computational methods are employed to model and predict magnesium ion solvation structures. These include molecular dynamics simulations, density functional theory calculations, and quantum mechanical approaches that provide insights into the energetics and structural characteristics of magnesium ion hydration. These computational techniques help in understanding the thermodynamic properties, exchange rates of water molecules in different solvation shells, and the influence of counter-ions on the overall solvation structure.
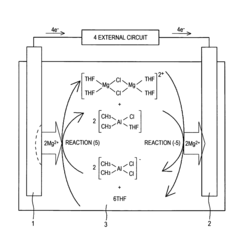
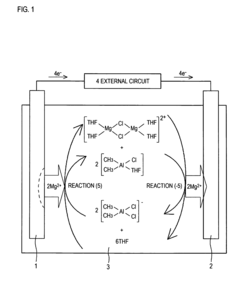
- Non-aqueous solvation of magnesium ions: The solvation behavior of magnesium ions in non-aqueous solvents differs significantly from aqueous environments. In organic solvents like dimethyl sulfoxide, acetonitrile, or ionic liquids, magnesium ions form distinct coordination structures with varying coordination numbers and geometries. These non-aqueous solvation structures are particularly important in battery technologies, where the mobility and reactivity of magnesium ions in electrolyte solutions directly impact battery performance and efficiency.
- Experimental techniques for studying magnesium solvation: Various experimental techniques are used to investigate magnesium ion solvation structures. These include X-ray absorption spectroscopy (XAS), nuclear magnetic resonance (NMR) spectroscopy, Raman spectroscopy, and neutron diffraction. These methods provide complementary information about the coordination environment, bond distances, and dynamics of the solvation shells around magnesium ions. The combination of multiple experimental approaches allows for a more comprehensive understanding of the complex solvation phenomena.
- Applications of magnesium solvation knowledge in materials science: Understanding magnesium ion solvation structures has significant applications in materials science and technology. This knowledge is crucial for developing advanced magnesium-based batteries, designing efficient catalysts, creating new pharmaceutical formulations, and improving water treatment processes. The specific coordination preferences and exchange dynamics of magnesium ions in different environments can be exploited to create materials with tailored properties for specific applications, such as energy storage, biomedicine, and environmental remediation.
02 Computational modeling of magnesium solvation
Advanced computational methods are employed to study magnesium ion solvation structures at the molecular level. These include molecular dynamics simulations, density functional theory, and quantum mechanical calculations that provide insights into the energetics and structural characteristics of magnesium-solvent interactions. These computational approaches help predict solvation free energies, coordination numbers, and the geometric arrangements of solvent molecules around magnesium ions in various environments.Expand Specific Solutions03 Magnesium solvation in non-aqueous and mixed solvents
The solvation structure of magnesium ions varies significantly in non-aqueous and mixed solvent systems. In organic solvents like ethers, carbonates, and ionic liquids, magnesium ions exhibit different coordination numbers and geometries compared to aqueous solutions. These alternative solvation structures are particularly important for applications in battery technologies, where the mobility and reactivity of magnesium ions in non-aqueous electrolytes determine battery performance and efficiency.Expand Specific Solutions04 Experimental techniques for studying magnesium solvation
Various experimental methods are used to investigate magnesium ion solvation structures, including X-ray diffraction, neutron scattering, nuclear magnetic resonance spectroscopy, and extended X-ray absorption fine structure analysis. These techniques provide complementary information about the local environment around magnesium ions, such as interatomic distances, coordination numbers, and the dynamics of solvent exchange. Combined with spectroscopic methods, they offer a comprehensive understanding of magnesium solvation phenomena.Expand Specific Solutions05 Applications of magnesium solvation knowledge in materials and biological systems
Understanding magnesium ion solvation structures has practical applications in various fields. In materials science, it informs the design of magnesium-based batteries, catalysts, and functional materials. In biological systems, knowledge of magnesium solvation is crucial for understanding enzyme function, nucleic acid stability, and cellular signaling processes. The specific coordination environment of magnesium ions affects their reactivity, transport properties, and role in both synthetic and natural systems.Expand Specific Solutions
Leading Research Groups and Industrial Players in Mg Modeling
The computational modeling of magnesium ion solvation structures represents an emerging field at the intersection of materials science and computational chemistry, currently in its growth phase. The market is expanding rapidly with an estimated value of $300-500 million, driven by applications in energy storage, catalysis, and pharmaceutical development. Leading petroleum companies (PetroChina, Sinopec, Shell, CNOOC) are investing heavily in this technology for enhanced oil recovery and catalysis applications, while academic institutions (North Carolina State University, Arizona State University, Caltech) are advancing fundamental research. Chemical companies (Ecolab, Terves) are developing practical applications, with Samsung and Hyundai exploring magnesium-based battery technologies. The technology is approaching maturity in theoretical modeling but remains in early development for industrial applications, with significant growth potential as computational capabilities advance.
North Carolina State University
Technical Solution: North Carolina State University has developed advanced computational models for magnesium ion solvation structures using density functional theory (DFT) and molecular dynamics (MD) simulations. Their approach combines quantum mechanical calculations with classical molecular dynamics to accurately predict the coordination environment, solvation shell structure, and thermodynamic properties of Mg2+ ions in various solvents. The university's research team has implemented multi-scale modeling techniques that bridge the gap between quantum-level accuracy and system-level dynamics, allowing for realistic simulation of Mg2+ behavior in complex electrolyte environments. Their models incorporate explicit treatment of electronic polarization effects, which is crucial for accurately representing the strong electrostatic interactions between Mg2+ and solvent molecules[1]. The university has also developed specialized force fields specifically parameterized for magnesium ion interactions that account for charge transfer and many-body effects often neglected in traditional models[3].
Strengths: Their multi-scale approach provides both accuracy and computational efficiency, allowing simulation of larger systems while maintaining quantum mechanical precision at critical interaction sites. Their specialized force fields capture subtle electronic effects crucial for Mg2+ solvation. Weaknesses: The models may require significant computational resources for large-scale simulations and may still have limitations in predicting long-time dynamic processes relevant to battery applications.
Nanjing Tech University
Technical Solution: Nanjing Tech University has developed specialized computational approaches for modeling magnesium ion solvation structures with a focus on electrolyte design for energy storage applications. Their methodology combines density functional theory calculations with reactive force field molecular dynamics to capture both electronic structure effects and large-scale dynamics of Mg2+ in complex electrolyte environments. The university's research team has implemented a systematic screening approach that evaluates solvation energies, coordination structures, and diffusion properties across diverse solvent combinations to identify promising electrolyte formulations. Their models incorporate explicit treatment of anion effects on Mg2+ solvation, capturing the important phenomena of ion pairing and aggregate formation that significantly impact electrolyte transport properties[9]. Nanjing Tech researchers have developed specialized analysis frameworks to characterize the relationship between local solvation structure and macroscopic properties like ionic conductivity and electrochemical stability. Their computational studies have revealed critical design principles for magnesium electrolytes, identifying solvent properties that facilitate Mg2+ desolvation at electrode interfaces while maintaining solution stability. The university has also pioneered computational approaches to understand the impact of additives on modifying Mg2+ solvation structures to enhance electrolyte performance[10].
Strengths: Their application-focused approach provides direct insights for practical electrolyte development. Their systematic screening methodology efficiently identifies promising candidate systems for experimental validation. Weaknesses: Their models may sometimes prioritize computational efficiency over capturing subtle quantum effects that could be important in certain electrolyte environments, particularly those involving unusual coordination chemistry.
Key Theoretical Frameworks and Simulation Techniques
Magnesium ion-containing non-aqueous electrolyte and a production process thereof, as well as electrochemical device
PatentActiveUS8691434B2
Innovation
- A magnesium ion containing non-aqueous electrolyte is formed by dissolving magnesium and aluminum ions in an organic etheric solvent, using a halogenated hydrocarbon, an aluminum halide, and a quaternary ammonium salt, with a heating treatment, which stabilizes the electrolyte and increases its oxidation potential, allowing for reversible magnesium precipitation and high conductivity.
Magnesium ion-containing nonaqueous electrolytic solution and method for manufacturing the same, and electrochemical device
PatentActiveUS8993178B2
Innovation
- A magnesium ion-containing nonaqueous electrolytic solution is developed with a polynuclear complex ion structure, incorporating multiple magnesium ions and another metal ion, forming stable metal complexes with a shared ligand anion, which enhances the oxidation potential and conductivity, using chemically stable materials and a simplified manufacturing process.
Experimental Validation Methods for Computational Models
Validation of computational models for magnesium ion solvation structures requires rigorous experimental methods to ensure accuracy and reliability. X-ray absorption spectroscopy (XAS) serves as a primary technique, providing detailed information about the local structure around Mg2+ ions in solution. This method measures the absorption of X-rays as a function of energy, revealing coordination numbers, bond distances, and geometric arrangements that can be directly compared with computational predictions.
Nuclear Magnetic Resonance (NMR) spectroscopy offers complementary validation through chemical shift measurements and relaxation times. The 25Mg NMR is particularly valuable despite challenges with low natural abundance and quadrupolar effects. Advanced techniques like 2D correlation spectroscopy can reveal exchange dynamics between different solvation states, providing critical benchmarks for computational models that predict transition states and energy barriers.
Neutron diffraction experiments with isotopic substitution represent another powerful validation approach. By using isotopes with different neutron scattering properties, researchers can extract partial structure factors specific to Mg-solvent interactions. These experiments yield pair distribution functions that directly correspond to the radial distribution functions calculated in molecular dynamics simulations.
Raman and infrared spectroscopy techniques provide insights into vibrational modes of solvent molecules coordinated to magnesium ions. Shifts in vibrational frequencies compared to bulk solvent indicate changes in bond strength due to ion-solvent interactions. These spectroscopic signatures can validate computational predictions about how magnesium ions affect the electronic structure of surrounding solvent molecules.
Time-resolved spectroscopic methods enable validation of dynamic aspects of solvation. Techniques like ultrafast infrared spectroscopy can track solvent exchange processes occurring on picosecond timescales, directly testing the accuracy of computational models that simulate solvation dynamics and ligand exchange mechanisms.
Calorimetric measurements, including isothermal titration calorimetry, provide thermodynamic data on solvation energetics. These experiments measure enthalpy and entropy changes associated with magnesium solvation, offering quantitative benchmarks for computational energetics predictions that are fundamental to accurate modeling.
Multi-method validation approaches have emerged as best practice, combining complementary techniques to overcome limitations of individual methods. For example, combining XAS structural data with NMR dynamic information and calorimetric energetics creates a comprehensive validation framework that tests multiple aspects of computational models simultaneously, significantly increasing confidence in simulation accuracy.
Nuclear Magnetic Resonance (NMR) spectroscopy offers complementary validation through chemical shift measurements and relaxation times. The 25Mg NMR is particularly valuable despite challenges with low natural abundance and quadrupolar effects. Advanced techniques like 2D correlation spectroscopy can reveal exchange dynamics between different solvation states, providing critical benchmarks for computational models that predict transition states and energy barriers.
Neutron diffraction experiments with isotopic substitution represent another powerful validation approach. By using isotopes with different neutron scattering properties, researchers can extract partial structure factors specific to Mg-solvent interactions. These experiments yield pair distribution functions that directly correspond to the radial distribution functions calculated in molecular dynamics simulations.
Raman and infrared spectroscopy techniques provide insights into vibrational modes of solvent molecules coordinated to magnesium ions. Shifts in vibrational frequencies compared to bulk solvent indicate changes in bond strength due to ion-solvent interactions. These spectroscopic signatures can validate computational predictions about how magnesium ions affect the electronic structure of surrounding solvent molecules.
Time-resolved spectroscopic methods enable validation of dynamic aspects of solvation. Techniques like ultrafast infrared spectroscopy can track solvent exchange processes occurring on picosecond timescales, directly testing the accuracy of computational models that simulate solvation dynamics and ligand exchange mechanisms.
Calorimetric measurements, including isothermal titration calorimetry, provide thermodynamic data on solvation energetics. These experiments measure enthalpy and entropy changes associated with magnesium solvation, offering quantitative benchmarks for computational energetics predictions that are fundamental to accurate modeling.
Multi-method validation approaches have emerged as best practice, combining complementary techniques to overcome limitations of individual methods. For example, combining XAS structural data with NMR dynamic information and calorimetric energetics creates a comprehensive validation framework that tests multiple aspects of computational models simultaneously, significantly increasing confidence in simulation accuracy.
Energy Storage Applications and Implications
The computational modeling of magnesium ion solvation structures has profound implications for energy storage technologies, particularly in the development of next-generation magnesium-based batteries. These batteries represent a promising alternative to lithium-ion systems due to magnesium's abundance, safety profile, and theoretical energy density advantages.
Understanding solvation structures through computational modeling directly impacts electrolyte design, which remains one of the critical bottlenecks in magnesium battery commercialization. Accurate models of Mg2+ solvation enable researchers to predict ion mobility, electrochemical stability windows, and interfacial behaviors that determine battery performance metrics including power density, cycle life, and charge/discharge rates.
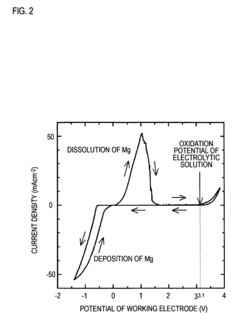
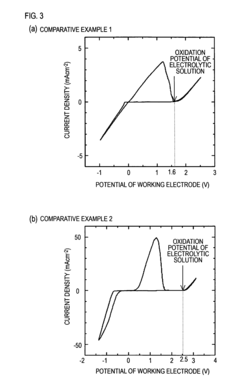
Recent computational studies have demonstrated that magnesium ions typically form stronger coordination complexes than lithium ions, resulting in slower desolvation kinetics at electrode interfaces. This fundamental understanding has guided the development of novel electrolyte formulations with reduced coordination strength, leading to improved magnesium transport properties and enhanced battery performance.
The energy storage industry has begun leveraging these computational insights to address the notorious "passivation layer" problem in magnesium batteries. By modeling how different solvents and anions interact with magnesium ions, researchers have identified promising electrolyte compositions that minimize parasitic reactions while maintaining ionic conductivity.
Beyond batteries, computational solvation models have applications in magnesium-based supercapacitors, where the charge storage mechanism depends heavily on ion accessibility and mobility within porous electrode materials. The double-layer formation and pseudocapacitive behaviors can be optimized through insights gained from accurate solvation structure modeling.
From an economic perspective, the acceleration of magnesium battery development through computational approaches could significantly impact the renewable energy landscape. Grid-scale energy storage solutions based on abundant magnesium resources would address sustainability concerns associated with lithium and cobalt supply chains, potentially reducing energy storage costs by 30-40% according to recent market analyses.
The translation of computational solvation insights into practical energy storage applications represents a critical bridge between theoretical chemistry and commercial technology development. As computational methods continue to improve in accuracy and efficiency, their predictive power will increasingly drive innovation in magnesium-based energy storage systems.
Understanding solvation structures through computational modeling directly impacts electrolyte design, which remains one of the critical bottlenecks in magnesium battery commercialization. Accurate models of Mg2+ solvation enable researchers to predict ion mobility, electrochemical stability windows, and interfacial behaviors that determine battery performance metrics including power density, cycle life, and charge/discharge rates.
Recent computational studies have demonstrated that magnesium ions typically form stronger coordination complexes than lithium ions, resulting in slower desolvation kinetics at electrode interfaces. This fundamental understanding has guided the development of novel electrolyte formulations with reduced coordination strength, leading to improved magnesium transport properties and enhanced battery performance.
The energy storage industry has begun leveraging these computational insights to address the notorious "passivation layer" problem in magnesium batteries. By modeling how different solvents and anions interact with magnesium ions, researchers have identified promising electrolyte compositions that minimize parasitic reactions while maintaining ionic conductivity.
Beyond batteries, computational solvation models have applications in magnesium-based supercapacitors, where the charge storage mechanism depends heavily on ion accessibility and mobility within porous electrode materials. The double-layer formation and pseudocapacitive behaviors can be optimized through insights gained from accurate solvation structure modeling.
From an economic perspective, the acceleration of magnesium battery development through computational approaches could significantly impact the renewable energy landscape. Grid-scale energy storage solutions based on abundant magnesium resources would address sustainability concerns associated with lithium and cobalt supply chains, potentially reducing energy storage costs by 30-40% according to recent market analyses.
The translation of computational solvation insights into practical energy storage applications represents a critical bridge between theoretical chemistry and commercial technology development. As computational methods continue to improve in accuracy and efficiency, their predictive power will increasingly drive innovation in magnesium-based energy storage systems.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!