Electrolyte engineering for reversible magnesium deposition
OCT 14, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Mg Battery Electrolyte Development Background & Objectives
Magnesium batteries have emerged as a promising alternative to lithium-ion batteries due to their potential advantages in energy density, safety, and cost. The development of magnesium batteries can be traced back to the early 1990s when the first rechargeable magnesium battery was demonstrated. However, despite the theoretical advantages, the practical implementation of magnesium batteries has been hindered by several challenges, with electrolyte limitations being the most significant barrier.
The evolution of magnesium battery electrolytes has progressed through several generations. Initial research focused on simple magnesium salts in conventional solvents, which proved ineffective due to passivation layers forming on the magnesium anode. A breakthrough came in 2000 with the development of Grignard-based electrolytes, followed by non-nucleophilic electrolytes that demonstrated improved electrochemical stability and compatibility with various cathode materials.
Recent trends in magnesium battery electrolyte development include the exploration of ionic liquids, polymer-based systems, and dual-salt strategies that combine magnesium salts with other metal salts to enhance performance. Additionally, there is growing interest in chloride-free electrolytes to address corrosion issues and improve compatibility with cell components.
The primary technical objective in magnesium electrolyte engineering is to develop formulations that enable reversible magnesium deposition and stripping with high Coulombic efficiency. This requires electrolytes that can dissolve magnesium salts effectively, maintain stability across wide voltage windows, and facilitate rapid magnesium-ion transport while preventing unwanted side reactions at electrode interfaces.
Secondary objectives include enhancing the electrolyte's thermal stability, reducing its environmental impact, and ensuring compatibility with a variety of cathode materials to expand the application scope of magnesium batteries. Researchers are also focusing on understanding the fundamental mechanisms of magnesium deposition and dissolution to guide rational electrolyte design.
The development of advanced magnesium electrolytes aims to address the energy storage needs of various sectors, including grid storage, electric vehicles, and portable electronics. The ultimate goal is to create magnesium battery systems that can outperform current lithium-ion technologies in terms of energy density, cycle life, safety, and cost, while utilizing more abundant and geographically distributed raw materials.
As the field progresses, interdisciplinary approaches combining computational modeling, advanced characterization techniques, and high-throughput experimental methods are becoming increasingly important for accelerating the discovery and optimization of novel magnesium electrolyte systems.
The evolution of magnesium battery electrolytes has progressed through several generations. Initial research focused on simple magnesium salts in conventional solvents, which proved ineffective due to passivation layers forming on the magnesium anode. A breakthrough came in 2000 with the development of Grignard-based electrolytes, followed by non-nucleophilic electrolytes that demonstrated improved electrochemical stability and compatibility with various cathode materials.
Recent trends in magnesium battery electrolyte development include the exploration of ionic liquids, polymer-based systems, and dual-salt strategies that combine magnesium salts with other metal salts to enhance performance. Additionally, there is growing interest in chloride-free electrolytes to address corrosion issues and improve compatibility with cell components.
The primary technical objective in magnesium electrolyte engineering is to develop formulations that enable reversible magnesium deposition and stripping with high Coulombic efficiency. This requires electrolytes that can dissolve magnesium salts effectively, maintain stability across wide voltage windows, and facilitate rapid magnesium-ion transport while preventing unwanted side reactions at electrode interfaces.
Secondary objectives include enhancing the electrolyte's thermal stability, reducing its environmental impact, and ensuring compatibility with a variety of cathode materials to expand the application scope of magnesium batteries. Researchers are also focusing on understanding the fundamental mechanisms of magnesium deposition and dissolution to guide rational electrolyte design.
The development of advanced magnesium electrolytes aims to address the energy storage needs of various sectors, including grid storage, electric vehicles, and portable electronics. The ultimate goal is to create magnesium battery systems that can outperform current lithium-ion technologies in terms of energy density, cycle life, safety, and cost, while utilizing more abundant and geographically distributed raw materials.
As the field progresses, interdisciplinary approaches combining computational modeling, advanced characterization techniques, and high-throughput experimental methods are becoming increasingly important for accelerating the discovery and optimization of novel magnesium electrolyte systems.
Market Analysis for Rechargeable Mg Battery Systems
The global rechargeable battery market is experiencing significant growth, with lithium-ion batteries currently dominating the landscape. However, magnesium-based battery systems are emerging as a promising alternative due to their potential advantages in safety, energy density, and cost-effectiveness. The market for rechargeable magnesium battery systems is still in its nascent stage but shows considerable promise for future expansion.
Current market estimates suggest that the global advanced battery market will reach approximately $90 billion by 2025, with alternative battery chemistries including magnesium-based systems projected to capture an increasing share. While lithium-ion batteries currently hold over 90% of the rechargeable battery market, concerns about lithium supply constraints and price volatility are driving interest in alternative technologies like magnesium batteries.
The automotive sector represents the most significant potential market for magnesium battery systems, particularly as electric vehicle adoption accelerates globally. The EV market is growing at a compound annual growth rate of 21%, creating substantial demand for high-performance, safe, and cost-effective energy storage solutions. Magnesium batteries, with their theoretical energy density advantages and improved safety profile, could address key consumer concerns regarding range anxiety and battery safety.
Grid-scale energy storage represents another substantial market opportunity, projected to grow to $30 billion by 2030. The intermittent nature of renewable energy sources necessitates efficient and economical storage solutions, where magnesium batteries could offer advantages in terms of cycle life and cost per kilowatt-hour compared to current technologies.
Consumer electronics constitutes a third significant market segment, with demand for longer-lasting, safer batteries continuing to rise. This sector values energy density and form factor flexibility, areas where advanced magnesium battery systems could potentially excel once the technology matures.
Market barriers for magnesium battery commercialization include the current performance limitations related to electrolyte engineering challenges, manufacturing scalability issues, and the established infrastructure supporting competing technologies. The technology readiness level remains relatively low compared to commercial lithium-ion systems, requiring significant additional R&D investment before widespread market adoption becomes feasible.
Regional market analysis indicates that Asia-Pacific, particularly China, Japan, and South Korea, leads in battery manufacturing capacity and R&D investment. North America and Europe are also making substantial investments in next-generation battery technologies, with several research institutions and companies focusing specifically on magnesium battery development.
Current market estimates suggest that the global advanced battery market will reach approximately $90 billion by 2025, with alternative battery chemistries including magnesium-based systems projected to capture an increasing share. While lithium-ion batteries currently hold over 90% of the rechargeable battery market, concerns about lithium supply constraints and price volatility are driving interest in alternative technologies like magnesium batteries.
The automotive sector represents the most significant potential market for magnesium battery systems, particularly as electric vehicle adoption accelerates globally. The EV market is growing at a compound annual growth rate of 21%, creating substantial demand for high-performance, safe, and cost-effective energy storage solutions. Magnesium batteries, with their theoretical energy density advantages and improved safety profile, could address key consumer concerns regarding range anxiety and battery safety.
Grid-scale energy storage represents another substantial market opportunity, projected to grow to $30 billion by 2030. The intermittent nature of renewable energy sources necessitates efficient and economical storage solutions, where magnesium batteries could offer advantages in terms of cycle life and cost per kilowatt-hour compared to current technologies.
Consumer electronics constitutes a third significant market segment, with demand for longer-lasting, safer batteries continuing to rise. This sector values energy density and form factor flexibility, areas where advanced magnesium battery systems could potentially excel once the technology matures.
Market barriers for magnesium battery commercialization include the current performance limitations related to electrolyte engineering challenges, manufacturing scalability issues, and the established infrastructure supporting competing technologies. The technology readiness level remains relatively low compared to commercial lithium-ion systems, requiring significant additional R&D investment before widespread market adoption becomes feasible.
Regional market analysis indicates that Asia-Pacific, particularly China, Japan, and South Korea, leads in battery manufacturing capacity and R&D investment. North America and Europe are also making substantial investments in next-generation battery technologies, with several research institutions and companies focusing specifically on magnesium battery development.
Current Challenges in Mg Electrolyte Technology
Despite significant advancements in magnesium battery technology, several critical challenges persist in the development of effective electrolytes for reversible magnesium deposition. The primary obstacle remains the formation of a passivation layer on the magnesium anode surface, which significantly impedes ion transport and leads to high overpotentials. Unlike lithium-ion systems, where the solid electrolyte interphase (SEI) facilitates ion transport, magnesium ions encounter severe kinetic limitations due to their divalent nature and stronger coordination with solvent molecules.
Conventional electrolytes based on simple magnesium salts (e.g., Mg(ClO4)2, Mg(TFSI)2) in ethereal solvents demonstrate poor electrochemical performance, with limited reversibility and rapid capacity fading. The strong coordination between Mg2+ ions and solvent molecules creates high desolvation energies at the electrode-electrolyte interface, resulting in sluggish deposition kinetics and dendrite formation during cycling.
Nucleophilicity presents another significant challenge, as many potential electrolyte systems exhibit incompatibility with high-voltage cathode materials. This severely restricts the practical energy density achievable in full-cell configurations. Additionally, the narrow electrochemical stability window of current electrolyte formulations (typically below 3.0V vs. Mg/Mg2+) limits the selection of viable cathode materials and overall cell voltage.
The corrosive nature of chloride-containing electrolytes, which historically have shown better reversibility, poses serious engineering challenges for practical cell design. These electrolytes often attack current collectors and cell components, necessitating specialized materials that increase manufacturing complexity and cost. Furthermore, the high sensitivity of magnesium electrolytes to moisture and oxygen contamination demands rigorous handling protocols and advanced packaging solutions.
Scalability remains a persistent issue, as many laboratory-demonstrated electrolyte systems rely on complex synthesis procedures or expensive precursors that are impractical for large-scale production. The lack of standardized testing protocols and performance metrics also hinders meaningful comparison between different electrolyte formulations reported in literature.
Temperature sensitivity further complicates electrolyte engineering, with most current systems showing dramatic performance degradation outside narrow operating windows (typically 20-40°C). This severely limits practical applications in real-world environments where temperature fluctuations are common. The poor ionic conductivity at lower temperatures particularly remains a significant barrier to commercial viability.
Recent efforts have focused on developing non-corrosive, high-performance electrolytes through molecular engineering approaches, including the design of weakly coordinating anions and magnesium-conducting ionic liquids. However, achieving the optimal balance between reversibility, conductivity, electrochemical stability, and practical manufacturability continues to challenge researchers in this field.
Conventional electrolytes based on simple magnesium salts (e.g., Mg(ClO4)2, Mg(TFSI)2) in ethereal solvents demonstrate poor electrochemical performance, with limited reversibility and rapid capacity fading. The strong coordination between Mg2+ ions and solvent molecules creates high desolvation energies at the electrode-electrolyte interface, resulting in sluggish deposition kinetics and dendrite formation during cycling.
Nucleophilicity presents another significant challenge, as many potential electrolyte systems exhibit incompatibility with high-voltage cathode materials. This severely restricts the practical energy density achievable in full-cell configurations. Additionally, the narrow electrochemical stability window of current electrolyte formulations (typically below 3.0V vs. Mg/Mg2+) limits the selection of viable cathode materials and overall cell voltage.
The corrosive nature of chloride-containing electrolytes, which historically have shown better reversibility, poses serious engineering challenges for practical cell design. These electrolytes often attack current collectors and cell components, necessitating specialized materials that increase manufacturing complexity and cost. Furthermore, the high sensitivity of magnesium electrolytes to moisture and oxygen contamination demands rigorous handling protocols and advanced packaging solutions.
Scalability remains a persistent issue, as many laboratory-demonstrated electrolyte systems rely on complex synthesis procedures or expensive precursors that are impractical for large-scale production. The lack of standardized testing protocols and performance metrics also hinders meaningful comparison between different electrolyte formulations reported in literature.
Temperature sensitivity further complicates electrolyte engineering, with most current systems showing dramatic performance degradation outside narrow operating windows (typically 20-40°C). This severely limits practical applications in real-world environments where temperature fluctuations are common. The poor ionic conductivity at lower temperatures particularly remains a significant barrier to commercial viability.
Recent efforts have focused on developing non-corrosive, high-performance electrolytes through molecular engineering approaches, including the design of weakly coordinating anions and magnesium-conducting ionic liquids. However, achieving the optimal balance between reversibility, conductivity, electrochemical stability, and practical manufacturability continues to challenge researchers in this field.
Current Electrolyte Solutions for Reversible Mg Deposition
01 Electrolyte compositions for magnesium batteries
Various electrolyte compositions have been developed specifically for magnesium batteries to enable efficient and reversible magnesium deposition. These compositions typically include magnesium salts dissolved in appropriate solvents to create stable electrolyte solutions that facilitate magnesium ion transport while minimizing side reactions. The electrolyte composition plays a crucial role in determining the efficiency of magnesium deposition and stripping processes, which directly impacts battery performance and cycle life.- Magnesium electrolyte compositions for rechargeable batteries: Various electrolyte compositions have been developed specifically for magnesium-based rechargeable batteries. These compositions typically include magnesium salts dissolved in appropriate solvents to facilitate reversible deposition and dissolution of magnesium during charge-discharge cycles. The electrolyte formulations are designed to overcome challenges such as passivation layers and to enhance the electrochemical performance of magnesium batteries.
- Additives for improving magnesium deposition efficiency: Certain additives can be incorporated into magnesium electrolytes to improve the efficiency and reversibility of magnesium deposition. These additives help to modify the solid-electrolyte interphase, prevent dendrite formation, and enhance the coulombic efficiency of the electrochemical process. Examples include specific organic compounds, ionic liquids, and inorganic salts that can be used in combination with the primary magnesium electrolyte.
- Novel solvent systems for magnesium electrolytes: Research has focused on developing novel solvent systems that can effectively dissolve magnesium salts while allowing for reversible magnesium deposition. These solvent systems include ethereal solvents, ionic liquids, and mixed solvent systems that provide high ionic conductivity and wide electrochemical stability windows. The choice of solvent significantly impacts the electrochemical behavior of magnesium, including its deposition morphology and cycling stability.
- Surface treatments and interfaces for magnesium electrodes: Various surface treatment methods have been developed to improve the interface between magnesium electrodes and electrolytes. These treatments modify the electrode surface to facilitate magnesium ion transport and prevent the formation of passivation layers that hinder reversible deposition. Techniques include coating the electrodes with specific materials, plasma treatment, and chemical modification of the electrode surface.
- Dual-salt and hybrid electrolyte systems: Dual-salt and hybrid electrolyte systems have been developed to overcome limitations of traditional magnesium electrolytes. These systems combine multiple salts or different types of electrolytes to achieve improved performance characteristics. The synergistic effects between different components can enhance conductivity, stability, and the reversibility of magnesium deposition. These systems often show reduced overpotential for magnesium deposition and improved cycling performance.
02 Additives for enhancing magnesium deposition
Specific additives can be incorporated into magnesium electrolytes to improve the reversibility of magnesium deposition. These additives help to modify the solid electrolyte interphase (SEI) formation, reduce dendrite growth, and enhance the uniformity of magnesium plating. Common additives include certain organic compounds, metal salts, and ionic liquids that can significantly improve coulombic efficiency and cycling stability of magnesium-based energy storage systems.Expand Specific Solutions03 Novel solvent systems for magnesium electrolytes
Research has focused on developing novel solvent systems that can effectively dissolve magnesium salts while promoting reversible magnesium deposition. These solvent systems include ethereal solvents, ionic liquids, and mixed solvent approaches that aim to balance conductivity, electrochemical stability, and compatibility with electrode materials. The choice of solvent significantly affects the coordination environment of magnesium ions and their transport properties, which in turn influences the deposition behavior at the electrode surface.Expand Specific Solutions04 Electrode surface modifications for improved magnesium deposition
Surface modifications of electrodes have been developed to enhance the reversibility of magnesium deposition. These modifications include surface coatings, nanostructuring, and chemical treatments that can alter the interfacial properties between the electrode and the electrolyte. By optimizing the electrode surface, the energy barrier for magnesium nucleation can be reduced, leading to more uniform deposition and improved cycling performance in magnesium-based energy storage systems.Expand Specific Solutions05 Non-corrosive magnesium electrolyte systems
Non-corrosive magnesium electrolyte systems have been developed to address the challenges of corrosion in conventional magnesium battery components. These systems utilize specially designed magnesium salts and solvent combinations that minimize reactivity with current collectors and cell components while maintaining good ionic conductivity and electrochemical performance. Non-corrosive electrolytes are essential for practical magnesium battery applications, as they enable the use of conventional battery materials and manufacturing processes.Expand Specific Solutions
Leading Research Groups and Industrial Players in Mg Battery Field
The magnesium battery electrolyte engineering market is currently in an early growth phase, characterized by intensive R&D efforts rather than mass commercialization. The global market size remains relatively small but is expanding rapidly due to increasing demand for high-energy density storage solutions. From a technological maturity perspective, significant challenges persist in developing stable electrolytes for reversible magnesium deposition. Key players include established automotive manufacturers like Toyota Motor Corp. pursuing next-generation battery technologies, specialized startups such as Pellion Technologies focusing exclusively on magnesium battery development, and research-intensive organizations including Tsinghua University, NIMS, and Arizona State University. Academic-industrial partnerships, particularly involving materials companies like Mitsubishi Materials and FUJIFILM Wako Pure Chemical, are accelerating innovation in this promising but technically challenging field.
Toyota Motor Corp.
Technical Solution: Toyota Motor Corporation has developed innovative electrolyte engineering solutions for reversible magnesium deposition through their advanced materials research division. Their approach centers on non-corrosive magnesium electrolytes based on magnesium bis(hexamethyldisilazide) (Mg(HMDS)2) combined with aluminum chloride in tetrahydrofuran (THF) solvent systems. This formulation creates highly active magnesium chloride complex cations that facilitate efficient electrodeposition. Toyota's research has demonstrated that controlling the coordination environment around the magnesium ion is crucial for reversible deposition, achieving this through precise molecular design of the electrolyte components. Their electrolytes show enhanced stability against oxidation at the cathode interface and reduced passivation at the magnesium anode, enabling higher voltage operation and improved cycling performance in prototype magnesium battery cells.
Strengths: Excellent compatibility with automotive battery requirements; superior electrochemical stability window; reduced corrosivity against standard battery components. Weaknesses: Complex synthesis procedures increase production costs; sensitivity to moisture contamination requires stringent manufacturing controls; limited ionic conductivity at lower temperatures.
VARTA Micro Innovation GmbH
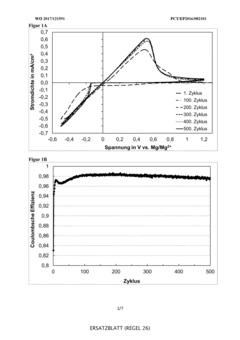
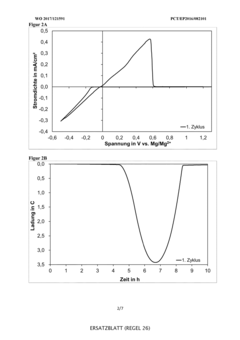
Technical Solution: VARTA Micro Innovation has developed specialized electrolyte systems for reversible magnesium deposition focusing on practical commercial applications. Their approach utilizes magnesium borohydride Mg(BH4)2 combined with lithium bis(trifluoromethanesulfonyl)imide (LiTFSI) in diglyme solvent matrices. This unique formulation creates a synergistic effect where the borohydride provides high magnesium mobility while the TFSI anion stabilizes the electrolyte against decomposition. VARTA's innovation includes proprietary additives that form a favorable solid electrolyte interphase (SEI) on the magnesium anode, facilitating reversible deposition without significant overpotentials. Their electrolyte technology has demonstrated stable cycling for over 500 cycles with coulombic efficiency consistently above 98%, addressing one of the key challenges in magnesium battery commercialization.
Strengths: Excellent long-term cycling stability; compatible with existing manufacturing infrastructure; good rate capability for faster charging applications. Weaknesses: Higher cost compared to lithium-ion electrolytes; sensitivity to oxygen exposure requires special handling; limited shelf life without stabilizing additives.
Key Innovations in Mg Electrolyte Chemistry
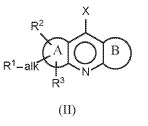
MG-coordination compounds for use in an electrolyte for an electrochemical cell
PatentWO2017121591A1
Innovation
- The use of magnesocene, specifically bis(cyclopentadienyl)magnesium, and its derivatives as conductive salts in an electrolyte with an aprotic solvent, allowing reversible magnesium deposition and dissolution on magnesium and inert metal electrodes, while providing high anodic and cathodic stability and being non-toxic and non-corrosive.
Safety and Stability Considerations for Mg Battery Electrolytes
Safety considerations for magnesium battery electrolytes are paramount due to the reactive nature of magnesium metal and the complex chemistry of electrolyte solutions. Unlike lithium-ion batteries, magnesium systems often employ electrolytes with different chemical compositions that present unique safety challenges. The flammability of organic solvents commonly used in Mg battery electrolytes, such as tetrahydrofuran (THF) and diglyme, poses significant fire risks, especially under thermal runaway conditions.
Thermal stability remains a critical concern for Mg battery electrolytes. Many Grignard-based electrolytes decompose at temperatures above 100°C, potentially releasing flammable gases and causing pressure build-up within cells. Recent research has focused on developing electrolytes with higher thermal decomposition thresholds, with some ether-based formulations showing stability up to 180°C when combined with specific magnesium salts.
Chemical stability presents another challenge, particularly regarding the compatibility between electrolytes and other battery components. Nucleophilic electrolytes can attack polymer separators and current collectors, leading to degradation over time. The corrosive nature of chloride-containing electrolytes toward conventional current collectors necessitates the use of corrosion-resistant materials, adding complexity and cost to battery design.
Environmental and health considerations must also be addressed when engineering Mg battery electrolytes. Several common solvents like THF and dimethoxyethane present toxicity concerns, while the long-term environmental impact of novel magnesium salts remains inadequately studied. Developing green electrolyte formulations with reduced toxicity and environmental footprint represents an emerging research direction.
Stability against atmospheric contaminants poses a significant challenge for practical implementation. Many high-performance Mg electrolytes are extremely sensitive to moisture and oxygen, requiring stringent handling protocols and hermetically sealed battery designs. Recent advances in electrolyte engineering have yielded formulations with improved air stability, though complete tolerance to atmospheric exposure remains elusive.
Electrochemical stability windows of Mg electrolytes typically range from 2.0-3.5V vs. Mg/Mg², limiting the selection of compatible cathode materials. Expanding this stability window through innovative electrolyte design represents a critical path toward higher energy density Mg batteries. Recent research utilizing boron-based anion receptors and non-nucleophilic anions has demonstrated promising improvements in oxidative stability.
Long-term cycling stability presents perhaps the most significant challenge, as many electrolytes that enable reversible Mg deposition initially show performance degradation over extended cycling. This degradation often stems from gradual electrolyte decomposition and the formation of passivation layers that impede Mg²⁺ transport. Engineering electrolytes with enhanced cycling durability remains a key focus for transitioning Mg batteries from laboratory curiosities to commercial reality.
Thermal stability remains a critical concern for Mg battery electrolytes. Many Grignard-based electrolytes decompose at temperatures above 100°C, potentially releasing flammable gases and causing pressure build-up within cells. Recent research has focused on developing electrolytes with higher thermal decomposition thresholds, with some ether-based formulations showing stability up to 180°C when combined with specific magnesium salts.
Chemical stability presents another challenge, particularly regarding the compatibility between electrolytes and other battery components. Nucleophilic electrolytes can attack polymer separators and current collectors, leading to degradation over time. The corrosive nature of chloride-containing electrolytes toward conventional current collectors necessitates the use of corrosion-resistant materials, adding complexity and cost to battery design.
Environmental and health considerations must also be addressed when engineering Mg battery electrolytes. Several common solvents like THF and dimethoxyethane present toxicity concerns, while the long-term environmental impact of novel magnesium salts remains inadequately studied. Developing green electrolyte formulations with reduced toxicity and environmental footprint represents an emerging research direction.
Stability against atmospheric contaminants poses a significant challenge for practical implementation. Many high-performance Mg electrolytes are extremely sensitive to moisture and oxygen, requiring stringent handling protocols and hermetically sealed battery designs. Recent advances in electrolyte engineering have yielded formulations with improved air stability, though complete tolerance to atmospheric exposure remains elusive.
Electrochemical stability windows of Mg electrolytes typically range from 2.0-3.5V vs. Mg/Mg², limiting the selection of compatible cathode materials. Expanding this stability window through innovative electrolyte design represents a critical path toward higher energy density Mg batteries. Recent research utilizing boron-based anion receptors and non-nucleophilic anions has demonstrated promising improvements in oxidative stability.
Long-term cycling stability presents perhaps the most significant challenge, as many electrolytes that enable reversible Mg deposition initially show performance degradation over extended cycling. This degradation often stems from gradual electrolyte decomposition and the formation of passivation layers that impede Mg²⁺ transport. Engineering electrolytes with enhanced cycling durability remains a key focus for transitioning Mg batteries from laboratory curiosities to commercial reality.
Environmental Impact and Sustainability of Mg Battery Technologies
Magnesium battery technologies offer significant environmental advantages over conventional lithium-ion batteries, primarily due to the abundance and widespread distribution of magnesium resources. Unlike lithium, which is concentrated in specific regions, magnesium is the eighth most abundant element in the Earth's crust and can be sourced from seawater, reducing geopolitical supply risks and environmental impacts associated with mining operations.
The environmental footprint of magnesium battery production is potentially lower than lithium-ion batteries when considering the entire lifecycle. The electrolyte engineering processes for reversible magnesium deposition typically involve less toxic materials compared to conventional lithium battery electrolytes. Many research directions focus on developing electrolytes based on more environmentally benign components, moving away from highly fluorinated compounds that pose environmental persistence concerns.
Energy consumption during manufacturing represents another critical sustainability factor. Current magnesium battery production processes require less energy-intensive electrode preparation compared to lithium technologies. However, the overall manufacturing energy requirements remain dependent on specific electrolyte formulations and processing techniques. Ongoing research aims to optimize these processes to further reduce the carbon footprint associated with production.
End-of-life considerations strongly favor magnesium battery technologies. The recyclability of magnesium-based systems presents a significant sustainability advantage, as magnesium can be more easily recovered and reprocessed compared to lithium. This circular economy potential reduces waste and decreases the need for primary resource extraction, aligning with global sustainability goals and extended producer responsibility frameworks.
Safety aspects of magnesium batteries also contribute to their environmental profile. The non-dendritic deposition behavior of magnesium, which is directly influenced by electrolyte engineering, reduces fire and explosion risks compared to lithium systems. This inherent safety characteristic minimizes the potential for environmental contamination from battery failures and accidents, particularly important as energy storage deployment scales globally.
Water consumption and pollution impacts must also be considered in sustainability assessments. Current electrolyte engineering approaches for magnesium batteries generally require less water-intensive processes than those used in lithium extraction. Additionally, properly designed magnesium electrolytes pose reduced risks of groundwater contamination compared to conventional battery technologies that rely on more toxic components.
The path toward commercialization of magnesium battery technologies must prioritize sustainability metrics alongside performance parameters. Life cycle assessment (LCA) studies indicate that optimizing electrolyte compositions not only for electrochemical performance but also for environmental impact will be crucial for ensuring these technologies deliver on their sustainability promise as they move from laboratory to market.
The environmental footprint of magnesium battery production is potentially lower than lithium-ion batteries when considering the entire lifecycle. The electrolyte engineering processes for reversible magnesium deposition typically involve less toxic materials compared to conventional lithium battery electrolytes. Many research directions focus on developing electrolytes based on more environmentally benign components, moving away from highly fluorinated compounds that pose environmental persistence concerns.
Energy consumption during manufacturing represents another critical sustainability factor. Current magnesium battery production processes require less energy-intensive electrode preparation compared to lithium technologies. However, the overall manufacturing energy requirements remain dependent on specific electrolyte formulations and processing techniques. Ongoing research aims to optimize these processes to further reduce the carbon footprint associated with production.
End-of-life considerations strongly favor magnesium battery technologies. The recyclability of magnesium-based systems presents a significant sustainability advantage, as magnesium can be more easily recovered and reprocessed compared to lithium. This circular economy potential reduces waste and decreases the need for primary resource extraction, aligning with global sustainability goals and extended producer responsibility frameworks.
Safety aspects of magnesium batteries also contribute to their environmental profile. The non-dendritic deposition behavior of magnesium, which is directly influenced by electrolyte engineering, reduces fire and explosion risks compared to lithium systems. This inherent safety characteristic minimizes the potential for environmental contamination from battery failures and accidents, particularly important as energy storage deployment scales globally.
Water consumption and pollution impacts must also be considered in sustainability assessments. Current electrolyte engineering approaches for magnesium batteries generally require less water-intensive processes than those used in lithium extraction. Additionally, properly designed magnesium electrolytes pose reduced risks of groundwater contamination compared to conventional battery technologies that rely on more toxic components.
The path toward commercialization of magnesium battery technologies must prioritize sustainability metrics alongside performance parameters. Life cycle assessment (LCA) studies indicate that optimizing electrolyte compositions not only for electrochemical performance but also for environmental impact will be crucial for ensuring these technologies deliver on their sustainability promise as they move from laboratory to market.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!