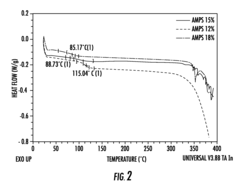
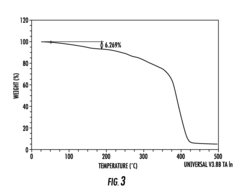
Comparative Study of Alkaline vs. Proton Exchange Membrane Electrolytic Cells
AUG 1, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Electrolysis Tech Evolution
The evolution of electrolysis technology has been marked by significant advancements over the past century, with a particular focus on improving efficiency, reducing costs, and enhancing sustainability. The journey began with traditional alkaline electrolysis, which has been the dominant technology for hydrogen production since the early 20th century. This method, while robust and well-established, faced limitations in terms of energy efficiency and production rates.
A major breakthrough came in the 1960s with the development of proton exchange membrane (PEM) electrolysis. This technology offered several advantages over alkaline systems, including higher current densities, greater efficiency, and the ability to operate at higher pressures. The introduction of PEM electrolysis marked a significant shift in the field, opening up new possibilities for hydrogen production, particularly in applications requiring high purity hydrogen.
Throughout the 1970s and 1980s, research efforts focused on improving both alkaline and PEM technologies. For alkaline systems, this involved developing more stable and efficient electrodes, as well as optimizing the electrolyte composition. PEM technology saw advancements in membrane materials, catalyst development, and cell design, leading to increased durability and reduced costs.
The 1990s and early 2000s witnessed a renewed interest in electrolysis, driven by growing concerns about climate change and the need for clean energy solutions. This period saw significant improvements in both alkaline and PEM technologies, with a particular emphasis on scaling up systems for industrial applications. Alkaline electrolysis benefited from the development of zero-gap cell designs and advanced diaphragm materials, while PEM systems saw further enhancements in catalyst performance and membrane durability.
In recent years, the focus has shifted towards developing high-temperature electrolysis technologies, such as solid oxide electrolysis cells (SOEC). These systems offer the potential for even higher efficiencies by utilizing waste heat from industrial processes. Additionally, there has been growing interest in anion exchange membrane (AEM) electrolysis, which aims to combine the benefits of both alkaline and PEM technologies.
The comparative study of alkaline and PEM electrolytic cells has been a central theme throughout this evolution. Each technology has its strengths and weaknesses, with alkaline systems generally being more cost-effective and suitable for large-scale applications, while PEM systems offer higher efficiency and flexibility. The ongoing research and development in this field continue to narrow the gap between these technologies, with both seeing improvements in performance, durability, and cost-effectiveness.
Looking ahead, the evolution of electrolysis technology is likely to focus on further increasing efficiency, reducing costs, and exploring new materials and designs. The integration of renewable energy sources with electrolysis systems is also expected to play a crucial role in the future development of this technology, particularly in the context of green hydrogen production and energy storage solutions.
A major breakthrough came in the 1960s with the development of proton exchange membrane (PEM) electrolysis. This technology offered several advantages over alkaline systems, including higher current densities, greater efficiency, and the ability to operate at higher pressures. The introduction of PEM electrolysis marked a significant shift in the field, opening up new possibilities for hydrogen production, particularly in applications requiring high purity hydrogen.
Throughout the 1970s and 1980s, research efforts focused on improving both alkaline and PEM technologies. For alkaline systems, this involved developing more stable and efficient electrodes, as well as optimizing the electrolyte composition. PEM technology saw advancements in membrane materials, catalyst development, and cell design, leading to increased durability and reduced costs.
The 1990s and early 2000s witnessed a renewed interest in electrolysis, driven by growing concerns about climate change and the need for clean energy solutions. This period saw significant improvements in both alkaline and PEM technologies, with a particular emphasis on scaling up systems for industrial applications. Alkaline electrolysis benefited from the development of zero-gap cell designs and advanced diaphragm materials, while PEM systems saw further enhancements in catalyst performance and membrane durability.
In recent years, the focus has shifted towards developing high-temperature electrolysis technologies, such as solid oxide electrolysis cells (SOEC). These systems offer the potential for even higher efficiencies by utilizing waste heat from industrial processes. Additionally, there has been growing interest in anion exchange membrane (AEM) electrolysis, which aims to combine the benefits of both alkaline and PEM technologies.
The comparative study of alkaline and PEM electrolytic cells has been a central theme throughout this evolution. Each technology has its strengths and weaknesses, with alkaline systems generally being more cost-effective and suitable for large-scale applications, while PEM systems offer higher efficiency and flexibility. The ongoing research and development in this field continue to narrow the gap between these technologies, with both seeing improvements in performance, durability, and cost-effectiveness.
Looking ahead, the evolution of electrolysis technology is likely to focus on further increasing efficiency, reducing costs, and exploring new materials and designs. The integration of renewable energy sources with electrolysis systems is also expected to play a crucial role in the future development of this technology, particularly in the context of green hydrogen production and energy storage solutions.
Market Demand Analysis
The market demand for electrolytic cells, particularly alkaline and proton exchange membrane (PEM) technologies, has been experiencing significant growth driven by the increasing focus on clean energy solutions and hydrogen production. This surge in demand is primarily fueled by the global push towards decarbonization and the adoption of renewable energy sources.
In the industrial sector, there is a growing need for large-scale hydrogen production for various applications, including ammonia synthesis, methanol production, and steel manufacturing. Alkaline electrolyzers have traditionally dominated this market due to their lower capital costs and proven reliability in industrial settings. However, PEM electrolyzers are gaining traction in this segment, especially for applications requiring high-purity hydrogen or rapid response times.
The transportation sector represents another key driver of market demand for electrolytic cells. As hydrogen fuel cell vehicles gain popularity, there is an increasing need for efficient and scalable hydrogen production methods. PEM electrolyzers are particularly well-suited for this application due to their compact size, high efficiency, and ability to produce high-purity hydrogen.
The power-to-gas sector is emerging as a significant market for electrolytic cells, particularly in regions with high renewable energy penetration. Both alkaline and PEM technologies are being deployed to convert excess renewable electricity into hydrogen, which can be stored or injected into existing natural gas networks. This application is expected to see substantial growth as countries seek to balance their grids and maximize the utilization of intermittent renewable energy sources.
In terms of regional demand, Europe is currently leading the market for electrolytic cells, driven by ambitious hydrogen strategies and supportive policies. Countries like Germany, France, and the Netherlands have announced significant investments in hydrogen infrastructure, creating a robust market for both alkaline and PEM technologies. Asia-Pacific is also showing strong growth potential, with countries like Japan, South Korea, and China investing heavily in hydrogen technologies to support their energy transition goals.
The market for small-scale and distributed electrolysis systems is also expanding, particularly for applications in remote areas, backup power systems, and small industrial processes. This segment is creating new opportunities for both alkaline and PEM technologies, with each offering unique advantages depending on the specific application requirements.
Overall, the market demand for electrolytic cells is expected to continue its upward trajectory, with both alkaline and PEM technologies playing crucial roles in the evolving hydrogen economy. The choice between these technologies will largely depend on specific application requirements, scale of production, and regional factors, driving innovation and competition in the electrolysis market.
In the industrial sector, there is a growing need for large-scale hydrogen production for various applications, including ammonia synthesis, methanol production, and steel manufacturing. Alkaline electrolyzers have traditionally dominated this market due to their lower capital costs and proven reliability in industrial settings. However, PEM electrolyzers are gaining traction in this segment, especially for applications requiring high-purity hydrogen or rapid response times.
The transportation sector represents another key driver of market demand for electrolytic cells. As hydrogen fuel cell vehicles gain popularity, there is an increasing need for efficient and scalable hydrogen production methods. PEM electrolyzers are particularly well-suited for this application due to their compact size, high efficiency, and ability to produce high-purity hydrogen.
The power-to-gas sector is emerging as a significant market for electrolytic cells, particularly in regions with high renewable energy penetration. Both alkaline and PEM technologies are being deployed to convert excess renewable electricity into hydrogen, which can be stored or injected into existing natural gas networks. This application is expected to see substantial growth as countries seek to balance their grids and maximize the utilization of intermittent renewable energy sources.
In terms of regional demand, Europe is currently leading the market for electrolytic cells, driven by ambitious hydrogen strategies and supportive policies. Countries like Germany, France, and the Netherlands have announced significant investments in hydrogen infrastructure, creating a robust market for both alkaline and PEM technologies. Asia-Pacific is also showing strong growth potential, with countries like Japan, South Korea, and China investing heavily in hydrogen technologies to support their energy transition goals.
The market for small-scale and distributed electrolysis systems is also expanding, particularly for applications in remote areas, backup power systems, and small industrial processes. This segment is creating new opportunities for both alkaline and PEM technologies, with each offering unique advantages depending on the specific application requirements.
Overall, the market demand for electrolytic cells is expected to continue its upward trajectory, with both alkaline and PEM technologies playing crucial roles in the evolving hydrogen economy. The choice between these technologies will largely depend on specific application requirements, scale of production, and regional factors, driving innovation and competition in the electrolysis market.
Current Challenges
The comparative study of alkaline and proton exchange membrane (PEM) electrolytic cells reveals several significant challenges that currently hinder their widespread adoption and optimal performance. These challenges span across technical, economic, and environmental domains, necessitating a multifaceted approach to overcome them.
One of the primary technical challenges for alkaline electrolyzers is the limited current density achievable, which impacts overall system efficiency. This limitation is primarily due to the formation of gas bubbles on electrode surfaces, impeding the electrolyte's contact with the electrodes. Additionally, the corrosive nature of the alkaline environment poses material degradation issues, particularly for the diaphragm and electrode materials, leading to reduced longevity and increased maintenance requirements.
PEM electrolyzers, while offering higher current densities and efficiencies, face their own set of challenges. The most significant hurdle is the high cost of materials, particularly the platinum-group metal catalysts and the perfluorosulfonic acid membranes. These expensive components contribute substantially to the overall system cost, making PEM electrolyzers less economically viable for large-scale hydrogen production.
Both technologies struggle with durability issues, albeit for different reasons. Alkaline systems suffer from carbonate formation when exposed to atmospheric CO2, which can degrade the electrolyte and reduce efficiency over time. PEM systems, on the other hand, are susceptible to membrane degradation and catalyst poisoning, especially when operating at high current densities or with impure water feeds.
Energy efficiency remains a critical challenge for both technologies. While significant improvements have been made, further advancements are needed to reduce the overall energy consumption per unit of hydrogen produced. This is particularly important for the integration of electrolyzers with renewable energy sources, where maximizing efficiency is crucial for economic viability.
Scalability presents another significant challenge, especially for PEM electrolyzers. The current manufacturing processes for key components, such as membranes and catalyst-coated membranes, are not easily scalable to the levels required for large-scale hydrogen production. This limitation hampers the technology's ability to meet the growing demand for green hydrogen in industrial applications.
Environmental concerns also pose challenges, particularly for PEM electrolyzers. The use of perfluorinated membranes raises questions about the long-term environmental impact and end-of-life disposal of these materials. For alkaline systems, the handling and disposal of large volumes of corrosive electrolytes present safety and environmental challenges.
Addressing these multifaceted challenges requires concerted efforts in materials science, process engineering, and system integration. Innovations in electrode and catalyst design, membrane materials, and cell architectures are crucial for overcoming the current limitations and unlocking the full potential of both alkaline and PEM electrolytic technologies in the transition to a hydrogen-based economy.
One of the primary technical challenges for alkaline electrolyzers is the limited current density achievable, which impacts overall system efficiency. This limitation is primarily due to the formation of gas bubbles on electrode surfaces, impeding the electrolyte's contact with the electrodes. Additionally, the corrosive nature of the alkaline environment poses material degradation issues, particularly for the diaphragm and electrode materials, leading to reduced longevity and increased maintenance requirements.
PEM electrolyzers, while offering higher current densities and efficiencies, face their own set of challenges. The most significant hurdle is the high cost of materials, particularly the platinum-group metal catalysts and the perfluorosulfonic acid membranes. These expensive components contribute substantially to the overall system cost, making PEM electrolyzers less economically viable for large-scale hydrogen production.
Both technologies struggle with durability issues, albeit for different reasons. Alkaline systems suffer from carbonate formation when exposed to atmospheric CO2, which can degrade the electrolyte and reduce efficiency over time. PEM systems, on the other hand, are susceptible to membrane degradation and catalyst poisoning, especially when operating at high current densities or with impure water feeds.
Energy efficiency remains a critical challenge for both technologies. While significant improvements have been made, further advancements are needed to reduce the overall energy consumption per unit of hydrogen produced. This is particularly important for the integration of electrolyzers with renewable energy sources, where maximizing efficiency is crucial for economic viability.
Scalability presents another significant challenge, especially for PEM electrolyzers. The current manufacturing processes for key components, such as membranes and catalyst-coated membranes, are not easily scalable to the levels required for large-scale hydrogen production. This limitation hampers the technology's ability to meet the growing demand for green hydrogen in industrial applications.
Environmental concerns also pose challenges, particularly for PEM electrolyzers. The use of perfluorinated membranes raises questions about the long-term environmental impact and end-of-life disposal of these materials. For alkaline systems, the handling and disposal of large volumes of corrosive electrolytes present safety and environmental challenges.
Addressing these multifaceted challenges requires concerted efforts in materials science, process engineering, and system integration. Innovations in electrode and catalyst design, membrane materials, and cell architectures are crucial for overcoming the current limitations and unlocking the full potential of both alkaline and PEM electrolytic technologies in the transition to a hydrogen-based economy.
Alkaline vs PEM Solutions
01 Membrane composition and structure
Advanced membrane materials and structures are crucial for both alkaline and proton exchange membrane electrolytic cells. These membranes are designed to enhance ion conductivity, mechanical strength, and chemical stability. Innovations include composite membranes, reinforced structures, and novel polymer blends to improve performance and durability in electrolytic processes.- Membrane composition and structure: Advancements in membrane composition and structure for both alkaline and proton exchange membrane electrolytic cells. This includes novel polymer materials, composite membranes, and nanostructured designs to enhance ion conductivity, mechanical stability, and overall cell performance.
- Electrode materials and catalysts: Development of innovative electrode materials and catalysts for improved efficiency in alkaline and proton exchange membrane electrolytic cells. This encompasses novel metal alloys, nanoparticles, and carbon-based materials to enhance catalytic activity, reduce overpotential, and increase durability.
- Cell design and configuration: Optimization of cell design and configuration for both alkaline and proton exchange membrane electrolytic cells. This includes innovations in flow field designs, electrode assemblies, and overall cell architecture to improve mass transport, reduce ohmic losses, and enhance overall system efficiency.
- Electrolyte composition and management: Advancements in electrolyte composition and management systems for alkaline and proton exchange membrane electrolytic cells. This involves developing novel electrolyte formulations, additives, and circulation strategies to optimize ion transport, minimize degradation, and maintain stable operating conditions.
- System integration and control: Innovations in system integration and control strategies for alkaline and proton exchange membrane electrolytic cells. This includes advanced monitoring systems, predictive maintenance algorithms, and intelligent control mechanisms to optimize performance, extend cell lifetime, and improve overall system reliability.
02 Electrode materials and catalysts
Development of high-performance electrode materials and catalysts is essential for efficient electrolysis. This includes nanostructured catalysts, precious metal alloys, and non-precious metal alternatives. These materials aim to reduce overpotential, increase reaction kinetics, and improve the overall efficiency of both alkaline and PEM electrolytic cells.Expand Specific Solutions03 System design and integration
Innovative system designs focus on integrating alkaline and PEM electrolytic cells into larger energy systems. This includes optimizing cell stacks, developing advanced control systems, and improving balance-of-plant components. The goal is to enhance overall system efficiency, reduce costs, and improve scalability for various applications.Expand Specific Solutions04 Electrolyte composition and management
Advancements in electrolyte formulations and management strategies are crucial for both alkaline and PEM systems. This includes developing stable electrolytes, improving circulation methods, and implementing effective purification techniques. These innovations aim to enhance conductivity, reduce corrosion, and maintain optimal operating conditions.Expand Specific Solutions05 Performance optimization and durability
Research focuses on optimizing the performance and durability of electrolytic cells. This involves developing strategies to mitigate degradation mechanisms, improve operational stability, and extend the lifespan of components. Advanced diagnostic tools and in-situ monitoring techniques are also being developed to enhance long-term performance and reliability.Expand Specific Solutions
Key Industry Players
The comparative study of alkaline vs. proton exchange membrane electrolytic cells is currently in a growth phase, with increasing market size and technological advancements. The global market for these technologies is expanding rapidly, driven by the growing demand for clean hydrogen production. While both technologies are maturing, proton exchange membrane (PEM) electrolyzers are gaining traction due to their higher efficiency and compact design. Key players like Plug Power, Inc. and Asahi Kasei Corp. are investing heavily in research and development to improve performance and reduce costs. Companies such as Renault SA and AUDI AG are exploring these technologies for automotive applications, indicating a broadening market scope. The involvement of research institutions like the University of Connecticut and California Institute of Technology suggests ongoing efforts to further enhance these technologies' capabilities and efficiency.
Plug Power, Inc.
Technical Solution: Plug Power has developed advanced proton exchange membrane (PEM) electrolysis technology for hydrogen production. Their PEM electrolyzers utilize a solid polymer electrolyte membrane to separate hydrogen and oxygen gases. The company's systems can achieve high current densities of over 2 A/cm2 [1], enabling efficient large-scale hydrogen production. Plug Power's PEM electrolyzers operate at lower temperatures (50-80°C) compared to alkaline systems, allowing for faster start-up and dynamic operation. They have also implemented advanced catalyst technologies to reduce precious metal loading and improve long-term stability [2].
Strengths: High efficiency, rapid response to load changes, compact design. Weaknesses: Higher capital costs, sensitivity to water purity.
Asahi Kasei Corp.
Technical Solution: Asahi Kasei has developed innovative alkaline water electrolysis technology for hydrogen production. Their system uses a zero-gap cell structure with high-performance electrodes and a proprietary diaphragm. The company's alkaline electrolyzers can achieve current densities of up to 0.8 A/cm2 [3], which is high for alkaline systems. Asahi Kasei's technology operates at higher temperatures (60-80°C) than PEM systems, potentially improving overall system efficiency. They have also focused on developing durable, low-cost materials to enhance the economic viability of large-scale hydrogen production [4].
Strengths: Lower cost materials, scalability for large installations. Weaknesses: Lower current density compared to PEM, less flexible operation.
Core Innovations
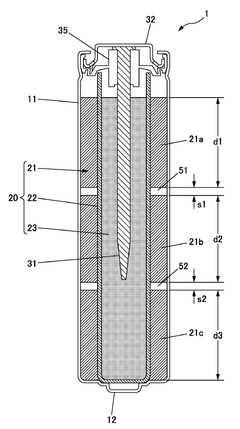
Alkaline cell
PatentWO2016117127A1
Innovation
- The alkaline battery design incorporates a positive electrode mixture with manganese dioxide and graphite, a negative electrode mixture containing zinc alloy powder with particles of 75 μm or less in a 25-40% mass range, and hollow cylindrical pellets with specific gaps and densities to optimize discharge performance, along with a negative electrode current collector and alkaline electrolyte.
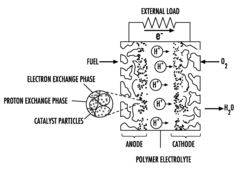
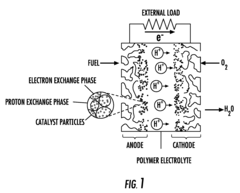
Proton exchange membrane and membrane-electrode assembly (MEA), method for their production and fuel cell using said membrane or assembly
PatentInactiveUS20090136820A1
Innovation
- A proton exchange membrane and membrane-electrode assembly (MEA) using sulfonated acrylamide copolymers, specifically a polyelectrolyte membrane comprising a sulfonated copolymer of acrylate and acrylamide sulfonic acid, which offers improved proton conductivity, chemical stability, and reduced production costs, along with a method for producing these membranes and assemblies that involve harmless reaction reagents and intermediates.
Economic Feasibility
The economic feasibility of alkaline and proton exchange membrane (PEM) electrolytic cells is a critical factor in determining their adoption and scalability in various applications. Alkaline electrolyzers have traditionally been more cost-effective due to their use of less expensive materials and longer operational lifetimes. The capital costs for alkaline systems are generally lower, with estimates ranging from $500 to $1,000 per kilowatt of capacity. These systems also benefit from lower maintenance costs over time, contributing to a more favorable total cost of ownership.
In contrast, PEM electrolyzers have historically been more expensive, with capital costs typically ranging from $1,000 to $1,500 per kilowatt. This higher initial investment is primarily due to the use of precious metal catalysts, such as platinum and iridium, which significantly contribute to the overall system cost. However, recent advancements in PEM technology have led to reduced catalyst loading and improved manufacturing processes, narrowing the cost gap between the two technologies.
Operational costs also play a crucial role in economic feasibility. PEM electrolyzers generally have higher electrical efficiency, operating at around 70-80% efficiency compared to 60-70% for alkaline systems. This increased efficiency can lead to lower electricity costs over the system's lifetime, particularly in regions with high electricity prices. Additionally, PEM systems offer greater flexibility in operation, with the ability to respond quickly to fluctuating power inputs, making them more suitable for integration with renewable energy sources.
The economic landscape is further influenced by the scale of production and deployment. As manufacturing volumes increase, economies of scale are expected to drive down costs for both technologies. PEM electrolyzers, being newer to the market, have more room for cost reduction through technological improvements and increased production scale. Industry projections suggest that PEM system costs could decrease by up to 50% by 2030, potentially making them more competitive with alkaline systems.
Market demand and application requirements also impact economic feasibility. Alkaline electrolyzers are well-suited for large-scale, continuous hydrogen production, such as in industrial applications. PEM systems, with their compact size and rapid response times, are more advantageous in distributed and variable output scenarios, such as hydrogen refueling stations or grid balancing services. The value proposition of each technology can therefore vary significantly depending on the specific use case and local market conditions.
In conclusion, while alkaline electrolyzers currently maintain an edge in terms of overall economic feasibility, particularly for large-scale applications, PEM technology is rapidly closing the gap. The choice between the two technologies will increasingly depend on specific project requirements, local energy prices, and the valuation of system flexibility and performance characteristics. As both technologies continue to evolve and mature, ongoing economic analysis will be crucial for informed decision-making in the hydrogen production sector.
In contrast, PEM electrolyzers have historically been more expensive, with capital costs typically ranging from $1,000 to $1,500 per kilowatt. This higher initial investment is primarily due to the use of precious metal catalysts, such as platinum and iridium, which significantly contribute to the overall system cost. However, recent advancements in PEM technology have led to reduced catalyst loading and improved manufacturing processes, narrowing the cost gap between the two technologies.
Operational costs also play a crucial role in economic feasibility. PEM electrolyzers generally have higher electrical efficiency, operating at around 70-80% efficiency compared to 60-70% for alkaline systems. This increased efficiency can lead to lower electricity costs over the system's lifetime, particularly in regions with high electricity prices. Additionally, PEM systems offer greater flexibility in operation, with the ability to respond quickly to fluctuating power inputs, making them more suitable for integration with renewable energy sources.
The economic landscape is further influenced by the scale of production and deployment. As manufacturing volumes increase, economies of scale are expected to drive down costs for both technologies. PEM electrolyzers, being newer to the market, have more room for cost reduction through technological improvements and increased production scale. Industry projections suggest that PEM system costs could decrease by up to 50% by 2030, potentially making them more competitive with alkaline systems.
Market demand and application requirements also impact economic feasibility. Alkaline electrolyzers are well-suited for large-scale, continuous hydrogen production, such as in industrial applications. PEM systems, with their compact size and rapid response times, are more advantageous in distributed and variable output scenarios, such as hydrogen refueling stations or grid balancing services. The value proposition of each technology can therefore vary significantly depending on the specific use case and local market conditions.
In conclusion, while alkaline electrolyzers currently maintain an edge in terms of overall economic feasibility, particularly for large-scale applications, PEM technology is rapidly closing the gap. The choice between the two technologies will increasingly depend on specific project requirements, local energy prices, and the valuation of system flexibility and performance characteristics. As both technologies continue to evolve and mature, ongoing economic analysis will be crucial for informed decision-making in the hydrogen production sector.
Environmental Impact
The environmental impact of alkaline and proton exchange membrane (PEM) electrolytic cells is a crucial consideration in the comparative study of these two technologies. Both systems play a significant role in the production of hydrogen, a clean energy carrier, but their environmental footprints differ in several aspects.
Alkaline electrolysis, being a more mature technology, has been widely used in industrial applications for decades. Its environmental impact is primarily associated with the materials used in the electrodes and electrolyte. The use of potassium hydroxide as an electrolyte raises concerns about potential leaks and the need for proper disposal. However, the longevity of alkaline systems can offset some of these concerns, as they typically have longer operational lifespans compared to PEM cells.
PEM electrolysis, on the other hand, utilizes more advanced materials, including precious metals like platinum and iridium as catalysts. The extraction and processing of these rare metals have significant environmental implications, including habitat disruption and energy-intensive mining operations. However, PEM cells operate at higher efficiencies, potentially reducing overall energy consumption and associated emissions in the long run.
Water consumption is another environmental factor to consider. Both technologies require ultra-pure water, but PEM cells generally have stricter water quality requirements. This necessitates more intensive water purification processes, which can increase energy consumption and potentially strain local water resources.
In terms of operational emissions, both alkaline and PEM electrolysis produce oxygen as a by-product, which is generally considered environmentally benign. However, the energy source used to power these systems greatly influences their overall environmental impact. When powered by renewable energy sources, both technologies can contribute significantly to reducing greenhouse gas emissions by providing clean hydrogen for various applications.
The end-of-life considerations for these technologies also differ. Alkaline systems, with their simpler construction and less exotic materials, may be easier to recycle. PEM cells, containing precious metals, present both a challenge and an opportunity for recycling and material recovery, which could mitigate some of the environmental impacts associated with their initial production.
Considering the full lifecycle, PEM electrolysis may have a lower environmental impact in scenarios where high efficiency and compact design are prioritized, particularly when coupled with renewable energy sources. Alkaline electrolysis, however, may be preferable in situations where long-term durability and simpler maintenance are key factors, potentially reducing the frequency of system replacements and associated environmental costs.
Alkaline electrolysis, being a more mature technology, has been widely used in industrial applications for decades. Its environmental impact is primarily associated with the materials used in the electrodes and electrolyte. The use of potassium hydroxide as an electrolyte raises concerns about potential leaks and the need for proper disposal. However, the longevity of alkaline systems can offset some of these concerns, as they typically have longer operational lifespans compared to PEM cells.
PEM electrolysis, on the other hand, utilizes more advanced materials, including precious metals like platinum and iridium as catalysts. The extraction and processing of these rare metals have significant environmental implications, including habitat disruption and energy-intensive mining operations. However, PEM cells operate at higher efficiencies, potentially reducing overall energy consumption and associated emissions in the long run.
Water consumption is another environmental factor to consider. Both technologies require ultra-pure water, but PEM cells generally have stricter water quality requirements. This necessitates more intensive water purification processes, which can increase energy consumption and potentially strain local water resources.
In terms of operational emissions, both alkaline and PEM electrolysis produce oxygen as a by-product, which is generally considered environmentally benign. However, the energy source used to power these systems greatly influences their overall environmental impact. When powered by renewable energy sources, both technologies can contribute significantly to reducing greenhouse gas emissions by providing clean hydrogen for various applications.
The end-of-life considerations for these technologies also differ. Alkaline systems, with their simpler construction and less exotic materials, may be easier to recycle. PEM cells, containing precious metals, present both a challenge and an opportunity for recycling and material recovery, which could mitigate some of the environmental impacts associated with their initial production.
Considering the full lifecycle, PEM electrolysis may have a lower environmental impact in scenarios where high efficiency and compact design are prioritized, particularly when coupled with renewable energy sources. Alkaline electrolysis, however, may be preferable in situations where long-term durability and simpler maintenance are key factors, potentially reducing the frequency of system replacements and associated environmental costs.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!