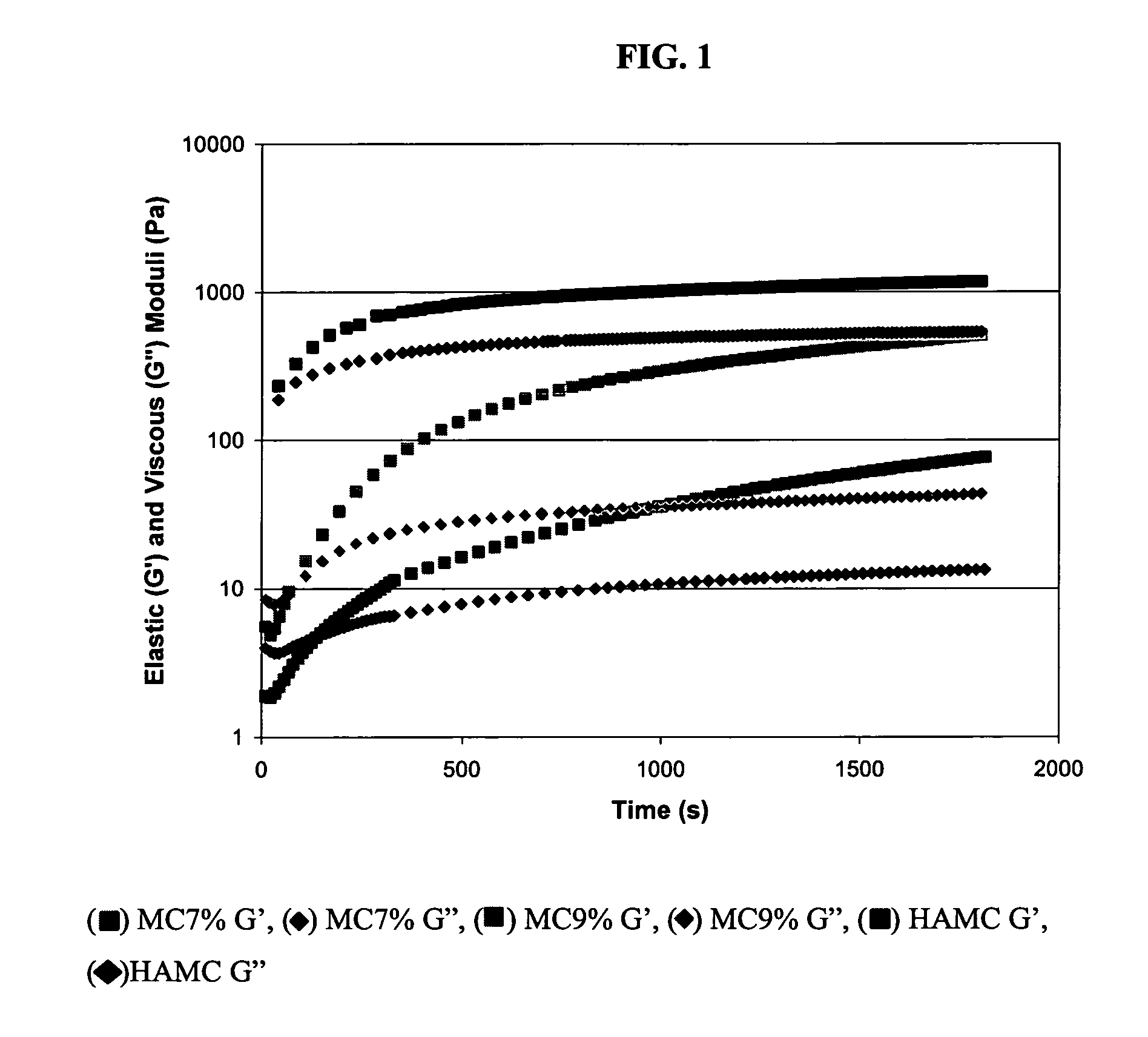
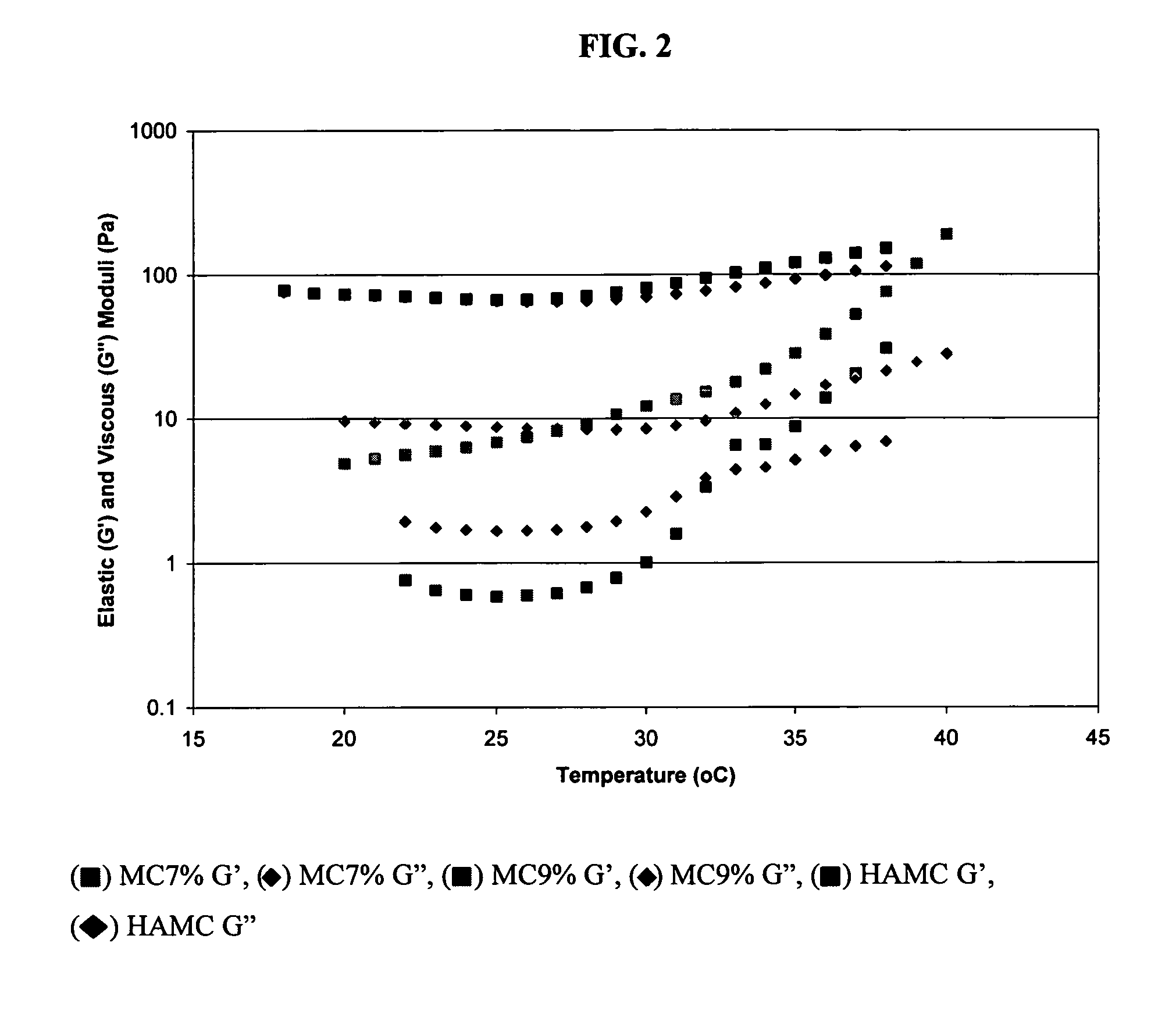
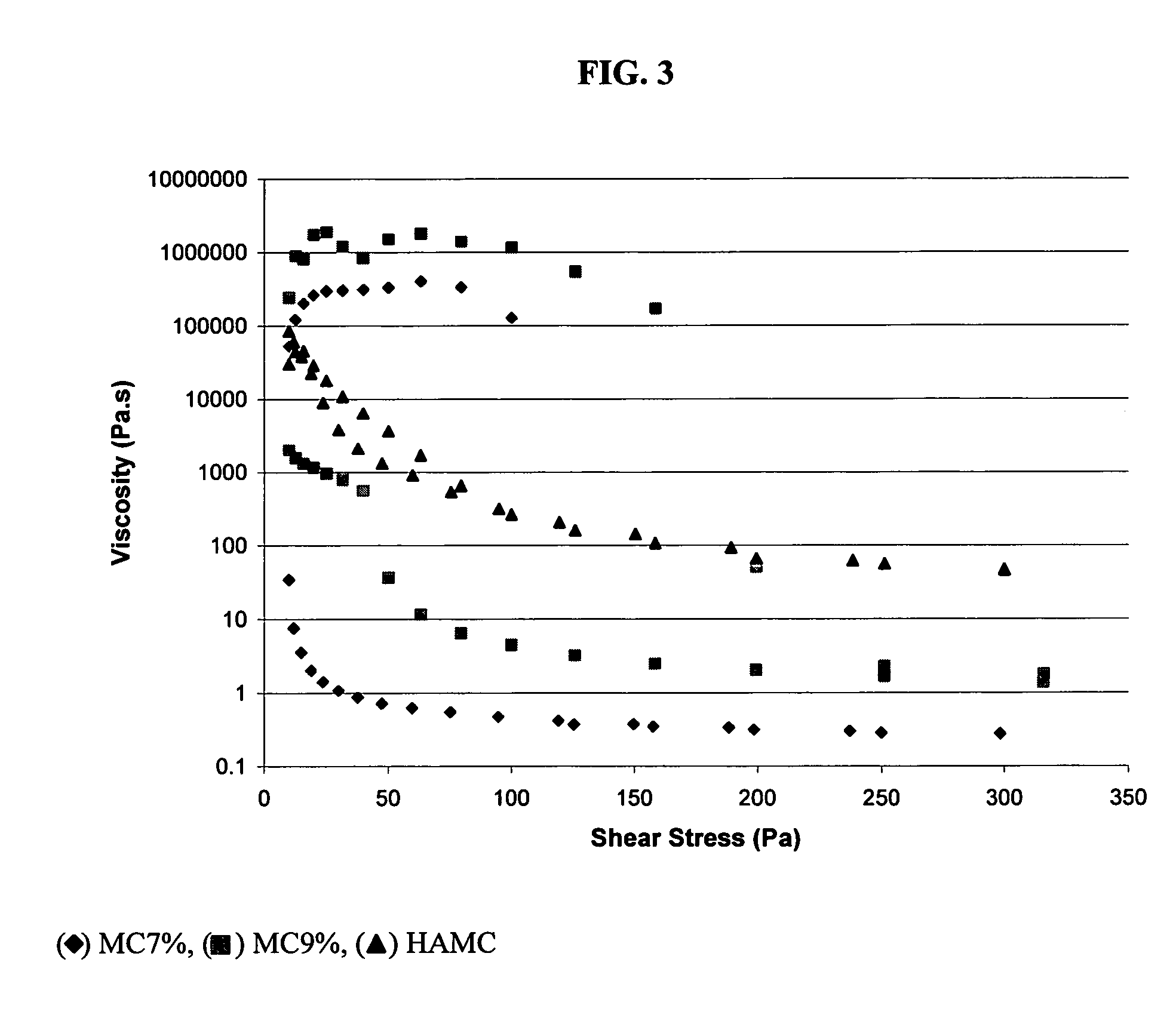
Comparative Study of Injectable Hydrogel vs Natural Polymers in Drug Delivery
OCT 15, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Injectable Hydrogel Evolution and Research Objectives
Injectable hydrogels have emerged as a revolutionary platform in drug delivery systems over the past three decades. Initially developed in the 1980s as simple cross-linked polymer networks, these materials have evolved into sophisticated biomaterials with tunable properties specifically designed for controlled release applications. The progression from first-generation hydrogels with basic swelling properties to current smart responsive systems represents a significant technological advancement in pharmaceutical sciences.
The evolution of injectable hydrogels can be traced through distinct developmental phases. Early research focused primarily on synthetic polymers like polyethylene glycol (PEG) and poly(N-isopropylacrylamide) (PNIPAAm), which offered reproducible properties but limited biocompatibility. The 1990s witnessed a paradigm shift toward natural polymer-based hydrogels, including alginate, chitosan, and hyaluronic acid derivatives, which addressed biocompatibility concerns while maintaining injectability.
Recent advancements have centered on hybrid systems that combine the advantages of both synthetic and natural polymers. These composite hydrogels aim to optimize mechanical strength, biodegradability, biocompatibility, and drug release kinetics simultaneously. The integration of nanotechnology has further expanded capabilities, enabling stimuli-responsive drug release triggered by pH, temperature, enzymes, or external stimuli like light or magnetic fields.
The primary research objectives in this field focus on addressing several persistent challenges. First, achieving precise control over drug release kinetics remains paramount, particularly for therapies requiring pulsatile or sustained delivery profiles. Second, improving mechanical stability while maintaining injectability presents an ongoing engineering challenge, especially for long-term implantation applications. Third, enhancing biocompatibility and reducing foreign body responses continues to drive material innovation.
Comparative analysis between injectable hydrogels and natural polymers in drug delivery constitutes a critical research direction. While natural polymers offer inherent biocompatibility and biodegradability advantages, injectable hydrogels provide superior tunability and often better mechanical properties. Understanding the performance differences between these systems across various therapeutic applications represents a key knowledge gap in the field.
This technical investigation aims to systematically evaluate injectable hydrogels against natural polymer systems across multiple parameters: drug loading capacity, release kinetics, stability, biocompatibility, manufacturing scalability, and clinical translation potential. By establishing quantitative benchmarks for these competing technologies, we seek to develop a comprehensive framework for material selection based on specific therapeutic requirements and delivery contexts.
The evolution of injectable hydrogels can be traced through distinct developmental phases. Early research focused primarily on synthetic polymers like polyethylene glycol (PEG) and poly(N-isopropylacrylamide) (PNIPAAm), which offered reproducible properties but limited biocompatibility. The 1990s witnessed a paradigm shift toward natural polymer-based hydrogels, including alginate, chitosan, and hyaluronic acid derivatives, which addressed biocompatibility concerns while maintaining injectability.
Recent advancements have centered on hybrid systems that combine the advantages of both synthetic and natural polymers. These composite hydrogels aim to optimize mechanical strength, biodegradability, biocompatibility, and drug release kinetics simultaneously. The integration of nanotechnology has further expanded capabilities, enabling stimuli-responsive drug release triggered by pH, temperature, enzymes, or external stimuli like light or magnetic fields.
The primary research objectives in this field focus on addressing several persistent challenges. First, achieving precise control over drug release kinetics remains paramount, particularly for therapies requiring pulsatile or sustained delivery profiles. Second, improving mechanical stability while maintaining injectability presents an ongoing engineering challenge, especially for long-term implantation applications. Third, enhancing biocompatibility and reducing foreign body responses continues to drive material innovation.
Comparative analysis between injectable hydrogels and natural polymers in drug delivery constitutes a critical research direction. While natural polymers offer inherent biocompatibility and biodegradability advantages, injectable hydrogels provide superior tunability and often better mechanical properties. Understanding the performance differences between these systems across various therapeutic applications represents a key knowledge gap in the field.
This technical investigation aims to systematically evaluate injectable hydrogels against natural polymer systems across multiple parameters: drug loading capacity, release kinetics, stability, biocompatibility, manufacturing scalability, and clinical translation potential. By establishing quantitative benchmarks for these competing technologies, we seek to develop a comprehensive framework for material selection based on specific therapeutic requirements and delivery contexts.
Market Analysis of Advanced Drug Delivery Systems
The global advanced drug delivery systems market has witnessed substantial growth, reaching approximately $231.2 billion in 2022 and projected to expand at a CAGR of 8.7% through 2030. This growth is primarily driven by increasing prevalence of chronic diseases, rising demand for targeted drug delivery, and technological advancements in delivery mechanisms.
Injectable hydrogels represent a rapidly growing segment within this market, valued at $14.3 billion in 2022 with expectations to reach $26.8 billion by 2028. These systems offer significant advantages including controlled release profiles, minimized side effects, and improved patient compliance. North America currently dominates this segment with 42% market share, followed by Europe (28%) and Asia-Pacific (21%).
Natural polymers in drug delivery systems constitute another significant market segment valued at $18.7 billion in 2022. This segment is experiencing robust growth due to increasing preference for biocompatible and biodegradable materials. Chitosan, alginate, and collagen-based delivery systems are witnessing particularly strong demand, with applications spanning from oral drug delivery to wound healing products.
The comparative market positioning reveals injectable hydrogels are gaining momentum in oncology and diabetes management, while natural polymers maintain stronger positions in wound care and oral drug delivery. Injectable hydrogels command premium pricing (average 30% higher) compared to natural polymer-based systems, reflecting their technological sophistication and enhanced performance characteristics.
Regional market dynamics show interesting patterns, with North American and European markets favoring injectable hydrogels for their precision and controlled release capabilities. Conversely, Asia-Pacific markets demonstrate stronger adoption of natural polymer-based systems, driven by cost considerations and traditional medicine practices.
Investment trends indicate significant capital flowing into injectable hydrogel technologies, with venture capital funding reaching $1.2 billion in 2022, representing a 35% increase from the previous year. Meanwhile, natural polymer research attracted $780 million in funding, focusing primarily on improving stability and release kinetics.
Market barriers for injectable hydrogels include high development costs, complex regulatory pathways, and manufacturing scalability challenges. Natural polymers face limitations related to batch-to-batch variability, limited mechanical strength, and shorter shelf life. These factors significantly influence market penetration rates across different therapeutic areas.
Consumer and healthcare provider preferences are increasingly favoring minimally invasive delivery systems with reduced administration frequency, positioning injectable hydrogels favorably for future market growth despite their higher costs.
Injectable hydrogels represent a rapidly growing segment within this market, valued at $14.3 billion in 2022 with expectations to reach $26.8 billion by 2028. These systems offer significant advantages including controlled release profiles, minimized side effects, and improved patient compliance. North America currently dominates this segment with 42% market share, followed by Europe (28%) and Asia-Pacific (21%).
Natural polymers in drug delivery systems constitute another significant market segment valued at $18.7 billion in 2022. This segment is experiencing robust growth due to increasing preference for biocompatible and biodegradable materials. Chitosan, alginate, and collagen-based delivery systems are witnessing particularly strong demand, with applications spanning from oral drug delivery to wound healing products.
The comparative market positioning reveals injectable hydrogels are gaining momentum in oncology and diabetes management, while natural polymers maintain stronger positions in wound care and oral drug delivery. Injectable hydrogels command premium pricing (average 30% higher) compared to natural polymer-based systems, reflecting their technological sophistication and enhanced performance characteristics.
Regional market dynamics show interesting patterns, with North American and European markets favoring injectable hydrogels for their precision and controlled release capabilities. Conversely, Asia-Pacific markets demonstrate stronger adoption of natural polymer-based systems, driven by cost considerations and traditional medicine practices.
Investment trends indicate significant capital flowing into injectable hydrogel technologies, with venture capital funding reaching $1.2 billion in 2022, representing a 35% increase from the previous year. Meanwhile, natural polymer research attracted $780 million in funding, focusing primarily on improving stability and release kinetics.
Market barriers for injectable hydrogels include high development costs, complex regulatory pathways, and manufacturing scalability challenges. Natural polymers face limitations related to batch-to-batch variability, limited mechanical strength, and shorter shelf life. These factors significantly influence market penetration rates across different therapeutic areas.
Consumer and healthcare provider preferences are increasingly favoring minimally invasive delivery systems with reduced administration frequency, positioning injectable hydrogels favorably for future market growth despite their higher costs.
Current Challenges in Hydrogel vs Natural Polymer Technologies
Despite significant advancements in drug delivery systems, both injectable hydrogels and natural polymers face substantial technical challenges that limit their widespread clinical application. Injectable hydrogels struggle with inconsistent mechanical properties, often exhibiting either excessive rigidity that impedes injectability or insufficient structural integrity post-injection. This mechanical paradox remains unresolved, particularly for applications requiring precise degradation profiles aligned with therapeutic release schedules.
Biocompatibility issues persist for synthetic hydrogels, with some formulations triggering foreign body responses or inflammation at injection sites. The incorporation of crosslinking agents necessary for in situ gelation introduces toxicity concerns, especially when residual unreacted components remain in the final matrix. Additionally, achieving homogeneous drug distribution within hydrogel networks continues to challenge researchers, as drug molecules often cluster during gelation processes.
Natural polymers, while inherently biocompatible, face significant batch-to-batch variability that complicates regulatory approval pathways. Extraction and purification processes directly impact their performance characteristics, creating reproducibility challenges in manufacturing settings. Their typically rapid degradation rates in physiological environments frequently result in premature drug release, limiting their utility for sustained delivery applications.
The sterilization of natural polymers presents another substantial hurdle, as conventional methods like heat or radiation often compromise their structural integrity and functional properties. This necessitates the development of specialized sterilization protocols that add complexity and cost to production processes.
Scale-up manufacturing represents a critical challenge for both material categories. Hydrogel production often involves complex chemistry that proves difficult to maintain consistently at industrial scales. Natural polymers face supply chain uncertainties, with raw material availability subject to seasonal variations and geographical limitations.
Regulatory pathways remain particularly complex for these advanced delivery systems. Hydrogels incorporating novel crosslinking mechanisms face extensive safety evaluations, while natural polymers extracted from animal or plant sources require comprehensive characterization to address potential immunogenicity concerns.
Storage stability presents ongoing challenges, with both material types exhibiting sensitivity to environmental conditions. Hydrogel precursors may undergo premature crosslinking during storage, while natural polymers frequently demonstrate susceptibility to microbial contamination and degradation over time, necessitating specialized preservation techniques and cold chain logistics.
Biocompatibility issues persist for synthetic hydrogels, with some formulations triggering foreign body responses or inflammation at injection sites. The incorporation of crosslinking agents necessary for in situ gelation introduces toxicity concerns, especially when residual unreacted components remain in the final matrix. Additionally, achieving homogeneous drug distribution within hydrogel networks continues to challenge researchers, as drug molecules often cluster during gelation processes.
Natural polymers, while inherently biocompatible, face significant batch-to-batch variability that complicates regulatory approval pathways. Extraction and purification processes directly impact their performance characteristics, creating reproducibility challenges in manufacturing settings. Their typically rapid degradation rates in physiological environments frequently result in premature drug release, limiting their utility for sustained delivery applications.
The sterilization of natural polymers presents another substantial hurdle, as conventional methods like heat or radiation often compromise their structural integrity and functional properties. This necessitates the development of specialized sterilization protocols that add complexity and cost to production processes.
Scale-up manufacturing represents a critical challenge for both material categories. Hydrogel production often involves complex chemistry that proves difficult to maintain consistently at industrial scales. Natural polymers face supply chain uncertainties, with raw material availability subject to seasonal variations and geographical limitations.
Regulatory pathways remain particularly complex for these advanced delivery systems. Hydrogels incorporating novel crosslinking mechanisms face extensive safety evaluations, while natural polymers extracted from animal or plant sources require comprehensive characterization to address potential immunogenicity concerns.
Storage stability presents ongoing challenges, with both material types exhibiting sensitivity to environmental conditions. Hydrogel precursors may undergo premature crosslinking during storage, while natural polymers frequently demonstrate susceptibility to microbial contamination and degradation over time, necessitating specialized preservation techniques and cold chain logistics.
Comparative Analysis of Current Drug Delivery Solutions
01 Injectable hydrogels based on natural polymers
Injectable hydrogels derived from natural polymers such as collagen, hyaluronic acid, and alginate offer biocompatible matrices for drug delivery. These hydrogels can be administered minimally invasively and form a gel in situ, providing sustained release of therapeutic agents. The natural origin of these polymers enhances biocompatibility and biodegradability, making them suitable for various medical applications including wound healing and tissue regeneration.- Natural polymer-based injectable hydrogels: Injectable hydrogels derived from natural polymers such as chitosan, alginate, and hyaluronic acid offer biocompatible matrices for drug delivery. These polymers can form in-situ gelling systems when injected into the body, providing sustained release of therapeutic agents. Their biodegradability, biocompatibility, and similarity to the extracellular matrix make them ideal candidates for minimally invasive drug delivery applications.
- Stimuli-responsive hydrogel systems: These advanced hydrogel formulations respond to specific environmental triggers such as temperature, pH, or enzymatic activity to release drugs in a controlled manner. The stimuli-responsive behavior allows for targeted drug delivery at specific sites or under particular physiological conditions. These systems can be engineered using natural polymers modified with responsive elements to enhance their functionality for precise therapeutic delivery.
- Composite hydrogels with enhanced mechanical properties: Composite hydrogels combine natural polymers with other materials such as nanoparticles, synthetic polymers, or crosslinking agents to improve mechanical strength, stability, and drug release kinetics. These reinforced systems maintain the biocompatibility of natural polymers while addressing limitations like rapid degradation or poor mechanical integrity. The composite approach enables tailored drug delivery profiles and extended release durations.
- Cell-laden hydrogels for tissue engineering and drug delivery: These specialized hydrogels incorporate living cells alongside therapeutic agents, creating a multifunctional platform for both tissue regeneration and drug delivery. Natural polymers provide an ideal microenvironment for cell encapsulation while simultaneously delivering bioactive molecules. This approach is particularly valuable for regenerative medicine applications where both cellular therapy and pharmaceutical intervention are required.
- Targeted delivery systems using functionalized natural polymers: Natural polymers can be chemically modified or functionalized to enhance their targeting capabilities for specific tissues or cells. These modifications may include conjugation with antibodies, peptides, or other targeting moieties that recognize specific receptors or disease markers. Functionalized natural polymer hydrogels enable more precise drug delivery to diseased tissues while minimizing systemic exposure and side effects.
02 Stimuli-responsive hydrogel drug delivery systems
Stimuli-responsive hydrogels can change their properties in response to environmental triggers such as temperature, pH, or enzymatic activity. These smart delivery systems allow for controlled release of drugs at specific sites or under specific conditions. By incorporating natural polymers into these responsive systems, the resulting hydrogels combine the benefits of biocompatibility with targeted drug delivery capabilities, enhancing therapeutic efficacy while reducing side effects.Expand Specific Solutions03 Composite hydrogels with enhanced mechanical properties
Composite hydrogels combining natural polymers with synthetic materials or nanoparticles exhibit improved mechanical strength and stability. These reinforced systems maintain the biocompatibility of natural polymers while addressing limitations such as rapid degradation or poor mechanical properties. The enhanced structural integrity allows for better control over drug release kinetics and extends the functional lifetime of the delivery system in vivo.Expand Specific Solutions04 Targeted delivery using functionalized natural polymer hydrogels
Natural polymer hydrogels can be functionalized with targeting moieties to direct drug delivery to specific tissues or cells. By modifying polymers such as chitosan, alginate, or hyaluronic acid with ligands that bind to specific receptors, these systems achieve site-specific drug delivery. This approach minimizes systemic exposure to drugs and enhances therapeutic efficacy by increasing local drug concentration at the target site, particularly beneficial for cancer treatment and other localized diseases.Expand Specific Solutions05 Sustained release formulations using crosslinked natural polymers
Crosslinked natural polymer hydrogels provide sustained release of therapeutic agents over extended periods. Various crosslinking methods, including chemical, enzymatic, and physical crosslinking, can be employed to control the network structure and consequently the drug release profile. These systems are particularly valuable for delivering drugs that require consistent therapeutic levels over time, reducing dosing frequency and improving patient compliance while maintaining the biocompatibility advantages of natural polymers.Expand Specific Solutions
Leading Companies and Research Institutions in Biomaterials
The injectable hydrogel versus natural polymer drug delivery market is currently in a growth phase, characterized by increasing research activities and commercial development. The global market size for advanced drug delivery systems is expanding rapidly, projected to reach significant valuation as pharmaceutical companies seek more effective delivery mechanisms. From a technological maturity perspective, the field shows varying degrees of development across applications. Companies like Ocular Therapeutix and MedinCell have established commercial platforms utilizing proprietary hydrogel technologies for sustained drug release, while Baxter International and Grifols leverage their extensive healthcare infrastructure to advance polymer-based delivery systems. Academic institutions including Sichuan University and IIT Bombay are driving fundamental research, while specialized firms such as Contraline and Viking Scientific focus on niche applications with novel hydrogel formulations. The competitive landscape reflects a blend of established pharmaceutical players and innovative startups pursuing differentiated technological approaches.
MedinCell SA
Technical Solution: MedinCell has developed BEPO® technology, a proprietary injectable hydrogel platform for controlled drug delivery. This system utilizes biodegradable copolymers that form a depot when injected subcutaneously, allowing for sustained release of active pharmaceutical ingredients over periods ranging from days to several months. The technology creates a polymeric matrix that undergoes controlled degradation, gradually releasing the encapsulated drug. MedinCell's approach involves dissolving the drug and polymers in a biocompatible solvent that, upon contact with physiological fluids, precipitates to form a solid or semi-solid implant. This in-situ forming depot system enables precise control over drug release kinetics through careful selection of polymer composition, molecular weight, and drug loading parameters. The company has successfully applied this technology to various therapeutic areas including psychiatry, pain management, and contraception.
Strengths: Provides long-acting drug delivery (up to several months), eliminates daily dosing requirements, improves patient compliance, and allows for biodegradable formulations. Weaknesses: Potential initial burst release of drug, limited to certain types of active ingredients, possible inflammatory responses at injection site, and manufacturing complexity requiring specialized equipment and expertise.
Baxter International, Inc.
Technical Solution: Baxter International has developed advanced hydrogel-based drug delivery systems utilizing their expertise in biomaterials and medical solutions. Their technology focuses on injectable hydrogels composed of modified hyaluronic acid and other biocompatible polymers that form three-dimensional networks upon administration. These systems are designed to encapsulate therapeutic agents ranging from small molecules to biologics, providing controlled release over extended periods. Baxter's approach incorporates stimuli-responsive elements that can modulate drug release based on environmental factors such as pH, temperature, or enzymatic activity. Their hydrogel platforms feature tunable mechanical properties that can be optimized for specific anatomical locations and therapeutic applications. The company has applied this technology to develop treatments for chronic conditions requiring sustained drug delivery, including pain management, inflammatory diseases, and certain oncology applications. Baxter's hydrogel systems are engineered to undergo predictable biodegradation, with degradation rates matched to the desired duration of drug release.
Strengths: Versatile platform compatible with various drug types, customizable release profiles, biocompatible materials with established safety profiles, and potential for localized delivery reducing systemic exposure. Weaknesses: Complex manufacturing processes affecting scalability, potential variability in in vivo performance due to patient-specific factors, limited stability of certain biologics in hydrogel environments, and higher production costs compared to conventional formulations.
Key Patents and Breakthroughs in Injectable Biomaterials
Injectable hydrogel for drug delivery
PatentWO2024061673A1
Innovation
- Development of an injectable hydrogel based on AB and/or ABA block co-polymers crosslinked via disulphide bonds, allowing for biodegradable and minimally invasive delivery of dexamethasone through a 30G needle, with self-healing properties and controlled release over several months, facilitated by redox-sensitive degradation in the vitreous environment.
Blends of temperature sensitive and anionic polymers for drug delivery
PatentActiveUS7767656B2
Innovation
- A polymer matrix comprising an inverse thermal gelling polymer and an anionic gelling polymer, such as methylcellulose and hyaluronic acid, which combines to form a fast-gelling, shear-thinning formulation that can be injected for localized delivery, allowing for sustained release of therapeutic agents, including those that typically do not cross the blood-spinal cord barrier.
Biocompatibility and Immunological Considerations
Biocompatibility represents a critical factor in the evaluation of drug delivery systems, particularly when comparing injectable hydrogels with natural polymers. The host immune response to biomaterials can significantly impact therapeutic efficacy and patient safety. Injectable hydrogels, whether synthetic or semi-synthetic, generally demonstrate good biocompatibility profiles due to their high water content and structural similarity to extracellular matrix components. However, certain synthetic polymers like polyacrylamide or polyethylene glycol (PEG) derivatives may trigger mild foreign body reactions upon degradation.
Natural polymers, including collagen, hyaluronic acid, alginate, and chitosan, typically exhibit superior biocompatibility compared to their synthetic counterparts. These materials are recognized by the body as familiar substances, often resulting in minimal immunological responses. For instance, hyaluronic acid, a glycosaminoglycan naturally present in human tissues, rarely elicits significant immune reactions when used in drug delivery applications. Similarly, collagen-based delivery systems benefit from the ubiquitous presence of collagen in the human body.
The immunological considerations for both delivery systems extend beyond mere biocompatibility. Injectable hydrogels may incorporate immunomodulatory components that can actively suppress inflammatory responses or promote tissue regeneration. Recent research has demonstrated that hydrogels functionalized with anti-inflammatory peptides or cytokine-neutralizing antibodies can create immunoprivileged microenvironments for sustained drug release.
Natural polymers often possess inherent immunomodulatory properties. Chitosan, derived from crustacean shells, exhibits antimicrobial and anti-inflammatory activities that can be advantageous in certain therapeutic contexts. However, its non-human origin may occasionally trigger allergic reactions in sensitive individuals. Similarly, alginate, extracted from seaweed, generally demonstrates excellent biocompatibility but may contain impurities that elicit immune responses if not properly purified.
The degradation kinetics of both delivery systems significantly influence their immunological profiles. Rapidly degrading materials may release degradation products that overwhelm local clearance mechanisms, potentially triggering inflammation. Conversely, extremely slow-degrading materials might lead to chronic foreign body reactions. Injectable hydrogels often offer more precisely controllable degradation rates through chemical modifications, whereas natural polymers typically follow more biologically determined breakdown pathways.
Recent advances in biomaterial engineering have focused on developing "stealth" properties for injectable hydrogels, incorporating elements that actively evade immune surveillance. These innovations include PEGylation strategies and biomimetic surface modifications that minimize protein adsorption and subsequent immune cell recognition. Natural polymers, while inherently more biocompatible, are increasingly being modified to enhance their immunomodulatory capabilities while preserving their fundamental biocompatibility advantages.
Natural polymers, including collagen, hyaluronic acid, alginate, and chitosan, typically exhibit superior biocompatibility compared to their synthetic counterparts. These materials are recognized by the body as familiar substances, often resulting in minimal immunological responses. For instance, hyaluronic acid, a glycosaminoglycan naturally present in human tissues, rarely elicits significant immune reactions when used in drug delivery applications. Similarly, collagen-based delivery systems benefit from the ubiquitous presence of collagen in the human body.
The immunological considerations for both delivery systems extend beyond mere biocompatibility. Injectable hydrogels may incorporate immunomodulatory components that can actively suppress inflammatory responses or promote tissue regeneration. Recent research has demonstrated that hydrogels functionalized with anti-inflammatory peptides or cytokine-neutralizing antibodies can create immunoprivileged microenvironments for sustained drug release.
Natural polymers often possess inherent immunomodulatory properties. Chitosan, derived from crustacean shells, exhibits antimicrobial and anti-inflammatory activities that can be advantageous in certain therapeutic contexts. However, its non-human origin may occasionally trigger allergic reactions in sensitive individuals. Similarly, alginate, extracted from seaweed, generally demonstrates excellent biocompatibility but may contain impurities that elicit immune responses if not properly purified.
The degradation kinetics of both delivery systems significantly influence their immunological profiles. Rapidly degrading materials may release degradation products that overwhelm local clearance mechanisms, potentially triggering inflammation. Conversely, extremely slow-degrading materials might lead to chronic foreign body reactions. Injectable hydrogels often offer more precisely controllable degradation rates through chemical modifications, whereas natural polymers typically follow more biologically determined breakdown pathways.
Recent advances in biomaterial engineering have focused on developing "stealth" properties for injectable hydrogels, incorporating elements that actively evade immune surveillance. These innovations include PEGylation strategies and biomimetic surface modifications that minimize protein adsorption and subsequent immune cell recognition. Natural polymers, while inherently more biocompatible, are increasingly being modified to enhance their immunomodulatory capabilities while preserving their fundamental biocompatibility advantages.
Regulatory Pathway for Injectable Biomaterial Approval
The regulatory landscape for injectable biomaterials represents a complex and rigorous pathway that manufacturers must navigate before bringing products to market. For injectable hydrogels and natural polymers used in drug delivery systems, regulatory approval typically follows different tracks depending on their classification as drugs, devices, or combination products.
In the United States, the FDA oversees the approval process through three primary centers: the Center for Drug Evaluation and Research (CDER), the Center for Biologics Evaluation and Research (CBER), and the Center for Devices and Radiological Health (CDRH). Injectable biomaterials often fall under combination product regulations, requiring coordination between these centers through the Office of Combination Products.
The regulatory pathway generally begins with preclinical testing, which includes in vitro and in vivo studies to establish safety profiles and preliminary efficacy. For injectable hydrogels, particular attention is paid to degradation kinetics, potential inflammatory responses, and interaction with surrounding tissues. Natural polymers face additional scrutiny regarding source material consistency and potential immunogenicity.
Clinical trials for injectable biomaterials follow a phased approach. Phase I focuses on safety and dosage in a small group of subjects, Phase II expands to evaluate effectiveness and side effects, while Phase III involves larger populations to confirm effectiveness and monitor adverse reactions. Throughout these phases, injectable hydrogels and natural polymers must demonstrate consistent drug release profiles and stability under physiological conditions.
European regulatory frameworks, governed by the European Medicines Agency (EMA), classify most injectable drug delivery systems under the Medical Device Regulation (MDR) or as medicinal products under Directive 2001/83/EC. The classification depends on the primary mode of action, with additional requirements for materials of biological origin under specific directives.
Post-market surveillance represents a critical component of the regulatory pathway, requiring manufacturers to monitor long-term safety and performance. This includes adverse event reporting, periodic safety update reports, and potential post-approval studies to address specific safety concerns or expand indications.
Regulatory requirements for injectable biomaterials continue to evolve, with increasing emphasis on real-world evidence and patient-reported outcomes. Recent regulatory trends show greater acceptance of in silico modeling and alternative testing methods to reduce animal testing, particularly relevant for biomaterial characterization.
For manufacturers developing injectable hydrogels or natural polymer-based drug delivery systems, early engagement with regulatory authorities through pre-submission meetings can provide valuable guidance on specific testing requirements and potential regulatory challenges, ultimately streamlining the approval process.
In the United States, the FDA oversees the approval process through three primary centers: the Center for Drug Evaluation and Research (CDER), the Center for Biologics Evaluation and Research (CBER), and the Center for Devices and Radiological Health (CDRH). Injectable biomaterials often fall under combination product regulations, requiring coordination between these centers through the Office of Combination Products.
The regulatory pathway generally begins with preclinical testing, which includes in vitro and in vivo studies to establish safety profiles and preliminary efficacy. For injectable hydrogels, particular attention is paid to degradation kinetics, potential inflammatory responses, and interaction with surrounding tissues. Natural polymers face additional scrutiny regarding source material consistency and potential immunogenicity.
Clinical trials for injectable biomaterials follow a phased approach. Phase I focuses on safety and dosage in a small group of subjects, Phase II expands to evaluate effectiveness and side effects, while Phase III involves larger populations to confirm effectiveness and monitor adverse reactions. Throughout these phases, injectable hydrogels and natural polymers must demonstrate consistent drug release profiles and stability under physiological conditions.
European regulatory frameworks, governed by the European Medicines Agency (EMA), classify most injectable drug delivery systems under the Medical Device Regulation (MDR) or as medicinal products under Directive 2001/83/EC. The classification depends on the primary mode of action, with additional requirements for materials of biological origin under specific directives.
Post-market surveillance represents a critical component of the regulatory pathway, requiring manufacturers to monitor long-term safety and performance. This includes adverse event reporting, periodic safety update reports, and potential post-approval studies to address specific safety concerns or expand indications.
Regulatory requirements for injectable biomaterials continue to evolve, with increasing emphasis on real-world evidence and patient-reported outcomes. Recent regulatory trends show greater acceptance of in silico modeling and alternative testing methods to reduce animal testing, particularly relevant for biomaterial characterization.
For manufacturers developing injectable hydrogels or natural polymer-based drug delivery systems, early engagement with regulatory authorities through pre-submission meetings can provide valuable guidance on specific testing requirements and potential regulatory challenges, ultimately streamlining the approval process.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!