Compare Phosphorylation and Isomerization in Cellular Contexts
SEP 23, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Phosphorylation and Isomerization Background and Objectives
Phosphorylation and isomerization represent two fundamental post-translational modifications (PTMs) that regulate protein function within cellular environments. The historical trajectory of phosphorylation research dates back to the 1950s with the pioneering work of Edmond Fischer and Edwin Krebs, who discovered reversible protein phosphorylation as a regulatory mechanism. This discovery eventually earned them the Nobel Prize in Physiology or Medicine in 1992, highlighting the significance of this modification in cellular signaling pathways.
Isomerization research, particularly protein isomerization, gained prominence in the 1980s with the identification of peptidyl-prolyl isomerases (PPIases) and their role in protein folding. The discovery of cyclophilins and FK506-binding proteins (FKBPs) as immunosuppressant targets further elevated the importance of isomerization in cellular processes and pharmaceutical development.
The technological evolution in studying these modifications has progressed from basic biochemical assays to sophisticated mass spectrometry techniques, enabling comprehensive phosphoproteome and isomerome mapping. Recent advances in cryo-electron microscopy and computational modeling have further enhanced our understanding of the structural dynamics associated with these modifications.
Current research indicates that while phosphorylation primarily functions as an on/off switch in signaling cascades, isomerization often serves as a conformational regulator that can influence protein-protein interactions and subcellular localization. The interplay between these modifications represents an emerging area of interest, with evidence suggesting coordinated regulation in various cellular contexts.
The global research landscape shows a significant imbalance, with phosphorylation studies dominating the literature (approximately 250,000 publications) compared to isomerization (roughly 15,000 publications). This disparity highlights the need for more focused research on isomerization mechanisms and their functional implications.
The primary objective of this technical investigation is to systematically compare phosphorylation and isomerization in terms of their biochemical mechanisms, regulatory dynamics, and functional consequences within cellular environments. Specifically, we aim to: (1) evaluate the kinetics and energetics of both modifications under physiological conditions; (2) assess their respective roles in signal transduction, protein stability, and cellular homeostasis; (3) identify potential crosstalk mechanisms between these modifications; and (4) explore emerging technologies for simultaneous detection and quantification of both modifications in complex biological samples.
By establishing a comprehensive comparative framework, this research seeks to bridge knowledge gaps and provide insights that could inform future therapeutic strategies targeting post-translational modification networks in various disease contexts.
Isomerization research, particularly protein isomerization, gained prominence in the 1980s with the identification of peptidyl-prolyl isomerases (PPIases) and their role in protein folding. The discovery of cyclophilins and FK506-binding proteins (FKBPs) as immunosuppressant targets further elevated the importance of isomerization in cellular processes and pharmaceutical development.
The technological evolution in studying these modifications has progressed from basic biochemical assays to sophisticated mass spectrometry techniques, enabling comprehensive phosphoproteome and isomerome mapping. Recent advances in cryo-electron microscopy and computational modeling have further enhanced our understanding of the structural dynamics associated with these modifications.
Current research indicates that while phosphorylation primarily functions as an on/off switch in signaling cascades, isomerization often serves as a conformational regulator that can influence protein-protein interactions and subcellular localization. The interplay between these modifications represents an emerging area of interest, with evidence suggesting coordinated regulation in various cellular contexts.
The global research landscape shows a significant imbalance, with phosphorylation studies dominating the literature (approximately 250,000 publications) compared to isomerization (roughly 15,000 publications). This disparity highlights the need for more focused research on isomerization mechanisms and their functional implications.
The primary objective of this technical investigation is to systematically compare phosphorylation and isomerization in terms of their biochemical mechanisms, regulatory dynamics, and functional consequences within cellular environments. Specifically, we aim to: (1) evaluate the kinetics and energetics of both modifications under physiological conditions; (2) assess their respective roles in signal transduction, protein stability, and cellular homeostasis; (3) identify potential crosstalk mechanisms between these modifications; and (4) explore emerging technologies for simultaneous detection and quantification of both modifications in complex biological samples.
By establishing a comprehensive comparative framework, this research seeks to bridge knowledge gaps and provide insights that could inform future therapeutic strategies targeting post-translational modification networks in various disease contexts.
Market Applications and Demand Analysis
The market for technologies related to phosphorylation and isomerization detection, manipulation, and analysis has experienced significant growth in recent years, driven primarily by advancements in pharmaceutical research, personalized medicine, and biotechnology applications. Current market estimates value the global protein post-translational modification analysis market at approximately $2.8 billion, with phosphorylation-specific technologies representing nearly 40% of this segment.
Pharmaceutical companies demonstrate the highest demand for phosphorylation analysis technologies, as protein kinases remain one of the most important drug target classes, with over 30 FDA-approved kinase inhibitors currently on the market. The clinical success of these drugs has intensified research efforts to understand phosphorylation dynamics in disease contexts, creating sustained demand for advanced analytical tools.
In contrast, isomerization technologies represent a smaller but rapidly growing market segment. With increasing recognition of isomerization's role in protein misfolding diseases such as Alzheimer's and Parkinson's, demand for isomerization detection methods has grown at approximately 15% annually over the past five years. This growth trajectory is expected to continue as researchers uncover additional biological significance of protein isomerization events.
Diagnostic applications represent another expanding market sector, particularly for phosphorylation biomarkers. Cancer diagnostics utilizing phosphorylation signatures have gained significant traction, with several commercial assays now available for clinical use. The global cancer biomarker market, valued at $11.5 billion, increasingly incorporates phosphorylation-based detection methods, creating substantial commercial opportunities.
Geographically, North America dominates the market with approximately 45% share, followed by Europe (30%) and Asia-Pacific (20%). However, the Asia-Pacific region shows the fastest growth rate due to increasing research investments in China, Japan, and South Korea, particularly in pharmaceutical and biotechnology sectors.
Industry surveys indicate that research institutions and pharmaceutical companies prioritize technologies offering higher sensitivity, throughput, and compatibility with limited sample quantities. Mass spectrometry-based approaches currently dominate the market, but antibody-based detection methods maintain significant market share due to their accessibility and established protocols.
Emerging market opportunities include technologies that can simultaneously analyze both phosphorylation and isomerization events within the same cellular context, addressing the growing recognition of their interconnected roles in signaling networks. Additionally, real-time monitoring technologies for these modifications in living cells represent a high-demand segment with limited current market offerings.
Pharmaceutical companies demonstrate the highest demand for phosphorylation analysis technologies, as protein kinases remain one of the most important drug target classes, with over 30 FDA-approved kinase inhibitors currently on the market. The clinical success of these drugs has intensified research efforts to understand phosphorylation dynamics in disease contexts, creating sustained demand for advanced analytical tools.
In contrast, isomerization technologies represent a smaller but rapidly growing market segment. With increasing recognition of isomerization's role in protein misfolding diseases such as Alzheimer's and Parkinson's, demand for isomerization detection methods has grown at approximately 15% annually over the past five years. This growth trajectory is expected to continue as researchers uncover additional biological significance of protein isomerization events.
Diagnostic applications represent another expanding market sector, particularly for phosphorylation biomarkers. Cancer diagnostics utilizing phosphorylation signatures have gained significant traction, with several commercial assays now available for clinical use. The global cancer biomarker market, valued at $11.5 billion, increasingly incorporates phosphorylation-based detection methods, creating substantial commercial opportunities.
Geographically, North America dominates the market with approximately 45% share, followed by Europe (30%) and Asia-Pacific (20%). However, the Asia-Pacific region shows the fastest growth rate due to increasing research investments in China, Japan, and South Korea, particularly in pharmaceutical and biotechnology sectors.
Industry surveys indicate that research institutions and pharmaceutical companies prioritize technologies offering higher sensitivity, throughput, and compatibility with limited sample quantities. Mass spectrometry-based approaches currently dominate the market, but antibody-based detection methods maintain significant market share due to their accessibility and established protocols.
Emerging market opportunities include technologies that can simultaneously analyze both phosphorylation and isomerization events within the same cellular context, addressing the growing recognition of their interconnected roles in signaling networks. Additionally, real-time monitoring technologies for these modifications in living cells represent a high-demand segment with limited current market offerings.
Current Technical Challenges in PTM Research
Post-translational modifications (PTMs) research faces significant technical challenges that limit our comprehensive understanding of cellular processes. The detection and quantification of PTMs, particularly phosphorylation and isomerization, remain problematic due to their dynamic nature and low abundance in cellular environments. Current mass spectrometry techniques, while powerful, struggle with sensitivity limitations when analyzing low-abundance PTMs, especially in complex biological samples without extensive enrichment procedures.
The temporal dynamics of PTMs present another major challenge. Phosphorylation events can occur within seconds to minutes, while isomerization may take hours to manifest. Existing technologies lack sufficient temporal resolution to capture these rapid modifications in real-time, creating significant blind spots in our understanding of signaling cascades and regulatory networks.
Cross-talk between different PTMs adds another layer of complexity. Phosphorylation at one site can influence isomerization at another, creating intricate regulatory networks that are difficult to decipher with current analytical approaches. The field lacks integrated methodologies that can simultaneously monitor multiple PTM types on the same protein to elucidate these interdependencies.
Site-specific analysis presents technical hurdles as well. While phosphorylation sites can be relatively well characterized using phospho-specific antibodies, isomerization detection lacks comparable tools. The subtle structural changes in isomerization events often escape detection by conventional antibody-based methods, necessitating more sophisticated analytical approaches.
Computational challenges compound these experimental limitations. Current algorithms struggle to accurately predict PTM sites, particularly for isomerization events which involve conformational changes rather than chemical additions. Machine learning approaches show promise but require extensive training datasets that are currently insufficient, especially for less-studied modifications like isomerization.
The biological context dependency of PTMs further complicates research efforts. The same protein may exhibit different modification patterns depending on cell type, developmental stage, or disease state. Current technologies lack the throughput and flexibility to systematically analyze these context-dependent variations across diverse biological systems.
Standardization issues also plague the field, with different laboratories employing varied protocols for PTM enrichment, detection, and quantification. This heterogeneity complicates data comparison across studies and impedes the development of comprehensive PTM databases that could accelerate research progress through meta-analyses and pattern recognition.
The temporal dynamics of PTMs present another major challenge. Phosphorylation events can occur within seconds to minutes, while isomerization may take hours to manifest. Existing technologies lack sufficient temporal resolution to capture these rapid modifications in real-time, creating significant blind spots in our understanding of signaling cascades and regulatory networks.
Cross-talk between different PTMs adds another layer of complexity. Phosphorylation at one site can influence isomerization at another, creating intricate regulatory networks that are difficult to decipher with current analytical approaches. The field lacks integrated methodologies that can simultaneously monitor multiple PTM types on the same protein to elucidate these interdependencies.
Site-specific analysis presents technical hurdles as well. While phosphorylation sites can be relatively well characterized using phospho-specific antibodies, isomerization detection lacks comparable tools. The subtle structural changes in isomerization events often escape detection by conventional antibody-based methods, necessitating more sophisticated analytical approaches.
Computational challenges compound these experimental limitations. Current algorithms struggle to accurately predict PTM sites, particularly for isomerization events which involve conformational changes rather than chemical additions. Machine learning approaches show promise but require extensive training datasets that are currently insufficient, especially for less-studied modifications like isomerization.
The biological context dependency of PTMs further complicates research efforts. The same protein may exhibit different modification patterns depending on cell type, developmental stage, or disease state. Current technologies lack the throughput and flexibility to systematically analyze these context-dependent variations across diverse biological systems.
Standardization issues also plague the field, with different laboratories employing varied protocols for PTM enrichment, detection, and quantification. This heterogeneity complicates data comparison across studies and impedes the development of comprehensive PTM databases that could accelerate research progress through meta-analyses and pattern recognition.
Established Methodologies for PTM Analysis
01 Protein phosphorylation and isomerization in cellular signaling
Phosphorylation and isomerization of proteins play crucial roles in cellular signaling pathways. These post-translational modifications regulate protein function, activity, and interactions. Phosphorylation involves the addition of phosphate groups to specific amino acid residues, while isomerization involves structural changes in protein conformation. These processes are essential for signal transduction, cell cycle regulation, and various cellular responses to environmental stimuli.- Protein phosphorylation detection methods: Various methods for detecting protein phosphorylation have been developed, including antibody-based assays, mass spectrometry techniques, and fluorescence-based detection systems. These methods allow for the identification and quantification of phosphorylated proteins, which is crucial for understanding cellular signaling pathways and protein regulation mechanisms. The detection of phosphorylation states can provide insights into disease mechanisms and potential therapeutic targets.
- Enzymatic isomerization processes: Enzymatic isomerization processes involve the use of specific enzymes to catalyze the conversion of one isomer to another. These processes are particularly important in industrial applications, such as the production of high-fructose corn syrup through glucose isomerization. Enzymatic isomerization offers advantages over chemical methods, including higher specificity, milder reaction conditions, and reduced formation of unwanted by-products.
- Phosphorylation in cell signaling pathways: Phosphorylation plays a critical role in cell signaling pathways by regulating protein activity, localization, and interactions. The addition of phosphate groups to proteins by kinases and their removal by phosphatases constitute a key mechanism for signal transduction in cells. Dysregulation of phosphorylation processes has been implicated in various diseases, including cancer, diabetes, and neurodegenerative disorders, making these pathways important targets for therapeutic intervention.
- Chemical isomerization catalysts and methods: Chemical catalysts and methods for isomerization reactions have been developed for various applications, particularly in the petrochemical industry. These include metal-based catalysts, acid catalysts, and base catalysts that facilitate the conversion between structural isomers, stereoisomers, or positional isomers. The development of selective and efficient isomerization catalysts is important for industrial processes such as petroleum refining, pharmaceutical synthesis, and fine chemical production.
- Combined phosphorylation and isomerization in biological systems: The interplay between phosphorylation and isomerization in biological systems represents an important regulatory mechanism. Certain proteins undergo conformational changes through isomerization following phosphorylation events, which can alter their function, stability, or interactions with other molecules. This sequential modification process is particularly relevant in protein folding, cell cycle regulation, and signal transduction pathways. Understanding these combined processes provides insights into complex cellular regulatory networks and potential therapeutic approaches.
02 Detection methods for phosphorylation and isomerization events
Various analytical techniques have been developed to detect and quantify phosphorylation and isomerization events in biological samples. These methods include mass spectrometry-based approaches, immunoassays, chromatographic techniques, and fluorescence-based detection systems. These technologies enable researchers to identify specific phosphorylation sites, measure isomerization rates, and monitor dynamic changes in these modifications under different physiological conditions or in response to drug treatments.Expand Specific Solutions03 Enzymatic mechanisms of phosphorylation and isomerization
Enzymes that catalyze phosphorylation (kinases) and isomerization (isomerases) are critical for regulating cellular processes. Kinases transfer phosphate groups from ATP to substrate proteins, while isomerases catalyze the conversion between different isomeric forms of molecules. These enzymes exhibit specificity for particular substrates and are themselves regulated through various mechanisms including phosphorylation, protein-protein interactions, and allosteric modulation. Understanding these enzymatic mechanisms is important for developing targeted therapeutics.Expand Specific Solutions04 Chemical isomerization and phosphorylation in synthetic processes
Chemical methods for isomerization and phosphorylation are important in the synthesis of pharmaceuticals, agrochemicals, and other industrial compounds. These processes involve the use of catalysts, specific reaction conditions, and protective group strategies to achieve selective modifications. Techniques such as stereoselective isomerization and regioselective phosphorylation enable the production of compounds with desired properties and biological activities.Expand Specific Solutions05 Therapeutic applications targeting phosphorylation and isomerization pathways
Dysregulation of phosphorylation and isomerization processes is implicated in various diseases including cancer, neurodegenerative disorders, and inflammatory conditions. Therapeutic strategies targeting these pathways include kinase inhibitors, phosphatase modulators, and compounds that affect isomerization events. Drug development efforts focus on achieving specificity for particular enzymes or pathways to minimize off-target effects while maximizing therapeutic benefits.Expand Specific Solutions
Key Research Institutions and Biotech Companies
The phosphorylation and isomerization landscape in cellular contexts is evolving rapidly, with the market currently in a growth phase characterized by increasing research investments and expanding applications in drug development. The global market size for technologies addressing these post-translational modifications is estimated to be substantial, driven by their critical roles in cellular signaling and protein function regulation. Leading pharmaceutical companies like Novartis AG, Ionis Pharmaceuticals, and Millennium Pharmaceuticals (Takeda) are advancing the technical maturity of phosphorylation research, while academic institutions including University of California, University of Michigan, and Caltech are pioneering isomerization studies. Research collaborations between industry players and academic institutions are accelerating technological innovations, particularly in developing targeted therapies that modulate these cellular processes for treating cancer and neurological disorders.
The Regents of the University of California
Technical Solution: The University of California has developed advanced mass spectrometry-based techniques for comprehensive phosphorylation analysis in cellular contexts. Their approach combines phosphoproteomics with computational modeling to map kinase-substrate relationships and phosphorylation dynamics. They've pioneered methods using stable isotope labeling with amino acids in cell culture (SILAC) combined with titanium dioxide (TiO2) enrichment to quantitatively measure thousands of phosphorylation sites simultaneously. Their research has revealed how phosphorylation networks respond to various cellular stimuli and how these networks are dysregulated in disease states. Additionally, they've developed novel chemical biology approaches to study isomerization events, particularly focusing on prolyl isomerization and its impact on protein function and signaling pathways. Their comparative studies have demonstrated how phosphorylation and isomerization can work in concert to regulate protein function, with phosphorylation often serving as a rapid, reversible modification while isomerization provides conformational control that can lock proteins in specific functional states.
Strengths: Their integrated multi-omics approach allows for comprehensive mapping of both modifications in complex cellular environments. Their advanced mass spectrometry techniques provide exceptional sensitivity for detecting low-abundance modifications. Weaknesses: The high-end instrumentation required limits accessibility, and computational challenges remain in accurately predicting the functional consequences of identified modifications across diverse cellular contexts.
Novartis AG
Technical Solution: Novartis has developed a proprietary platform called KinobeadsTM that enables systematic profiling of kinase inhibitors against native kinases in cell lysates, allowing comprehensive analysis of phosphorylation networks. Their technology combines chemical proteomics with quantitative mass spectrometry to map drug-target interactions and downstream phosphorylation events. For studying isomerization, Novartis employs nuclear magnetic resonance (NMR) spectroscopy and X-ray crystallography to visualize conformational changes in proteins. They've pioneered the development of selective inhibitors targeting peptidyl-prolyl isomerases (PPIases) like Pin1, which has revealed critical roles for isomerization in cancer and neurodegeneration. Their comparative studies between phosphorylation and isomerization have demonstrated how these modifications can work synergistically, with phosphorylation often preceding and enabling specific isomerization events. Novartis researchers have shown that in certain signaling pathways, phosphorylation provides the initial rapid response while isomerization acts as a molecular timer that extends and modulates the signal duration, offering potential for developing dual-targeting therapeutic strategies that address both modifications simultaneously.
Strengths: Their integrated drug discovery platform allows direct translation of basic phosphorylation/isomerization research into therapeutic applications. Their extensive compound libraries enable rapid screening for modulators of both processes. Weaknesses: Their research primarily focuses on modifications relevant to their therapeutic areas of interest rather than providing a comprehensive understanding across all cellular contexts and protein types.
Critical Mechanisms and Regulatory Pathways
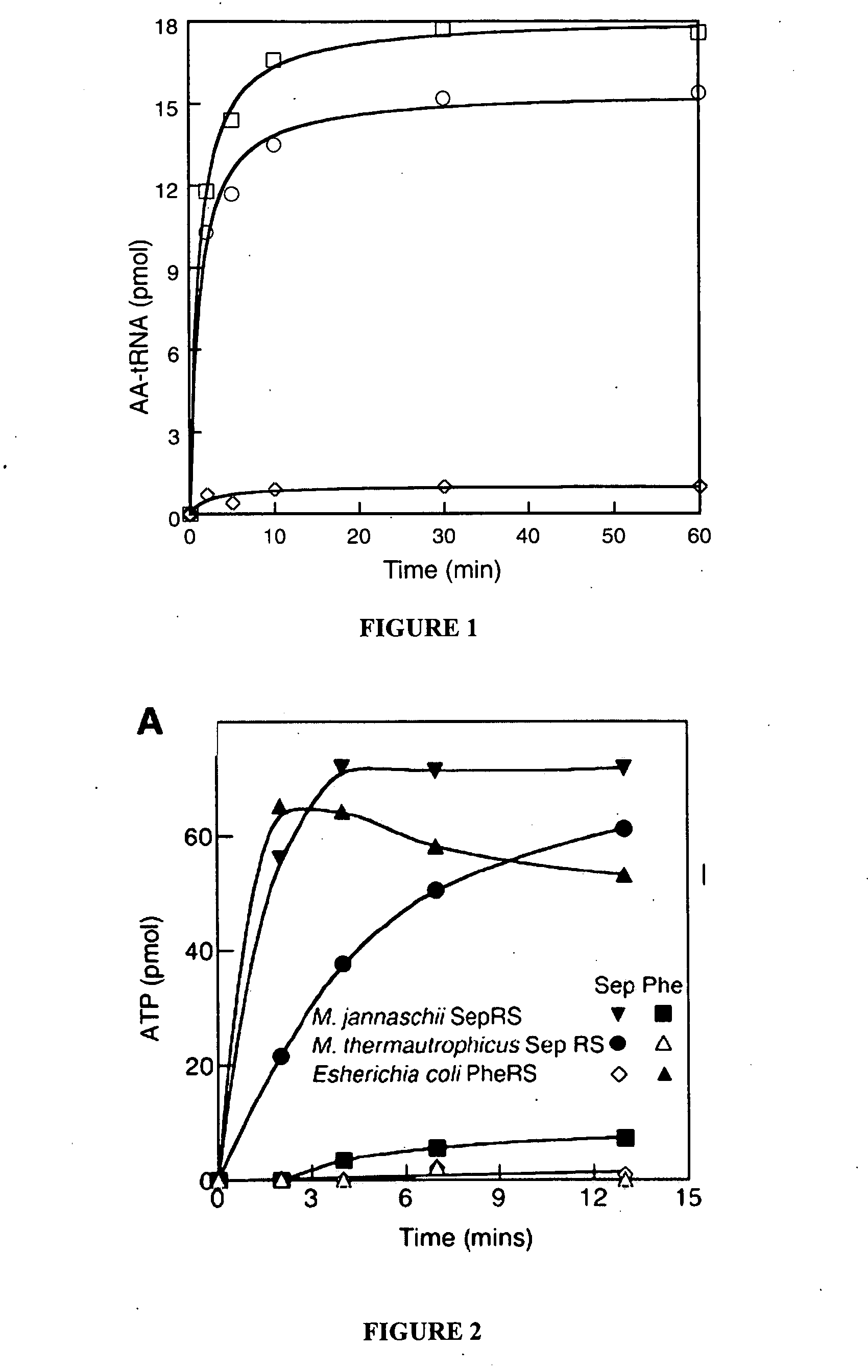
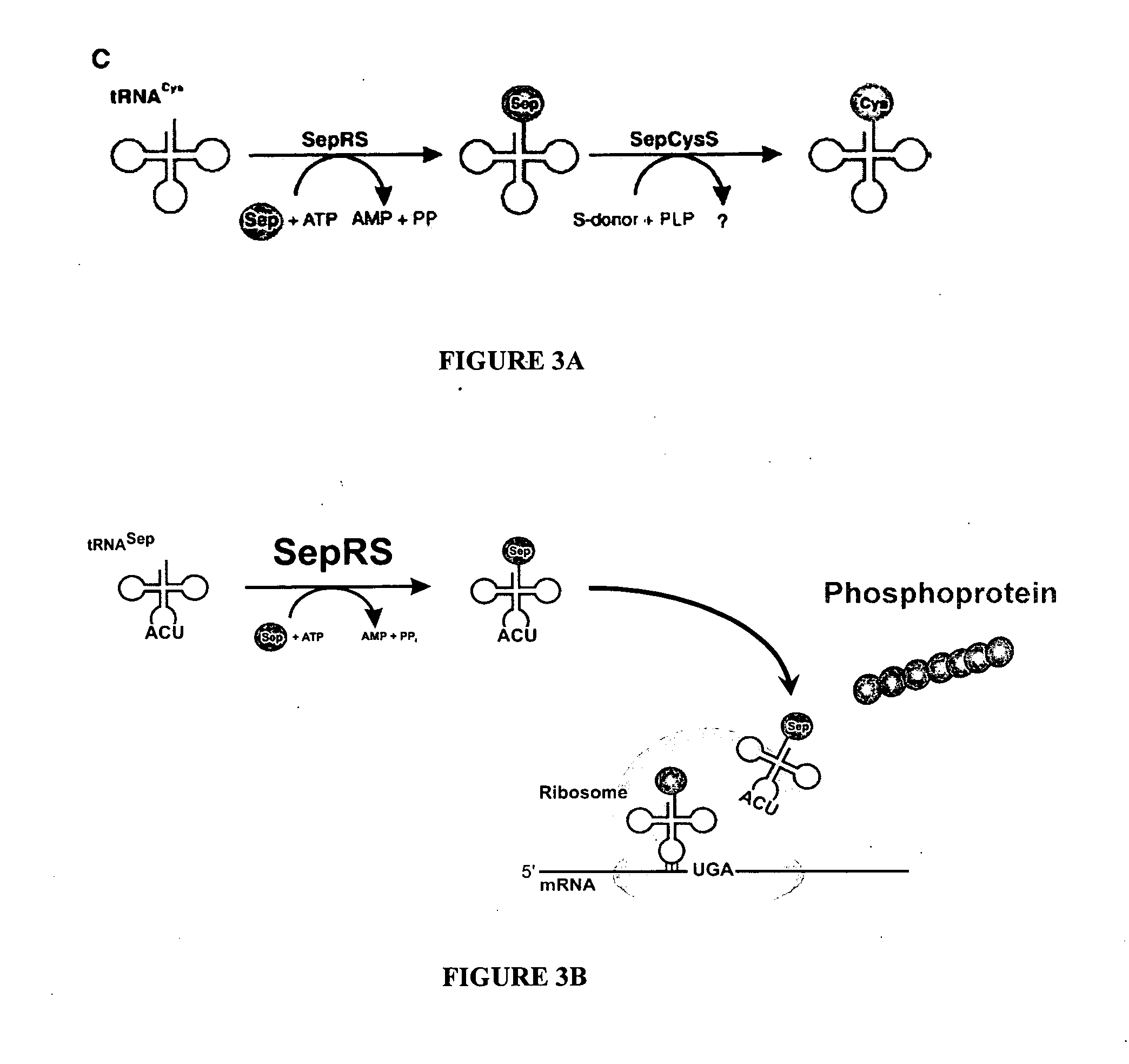
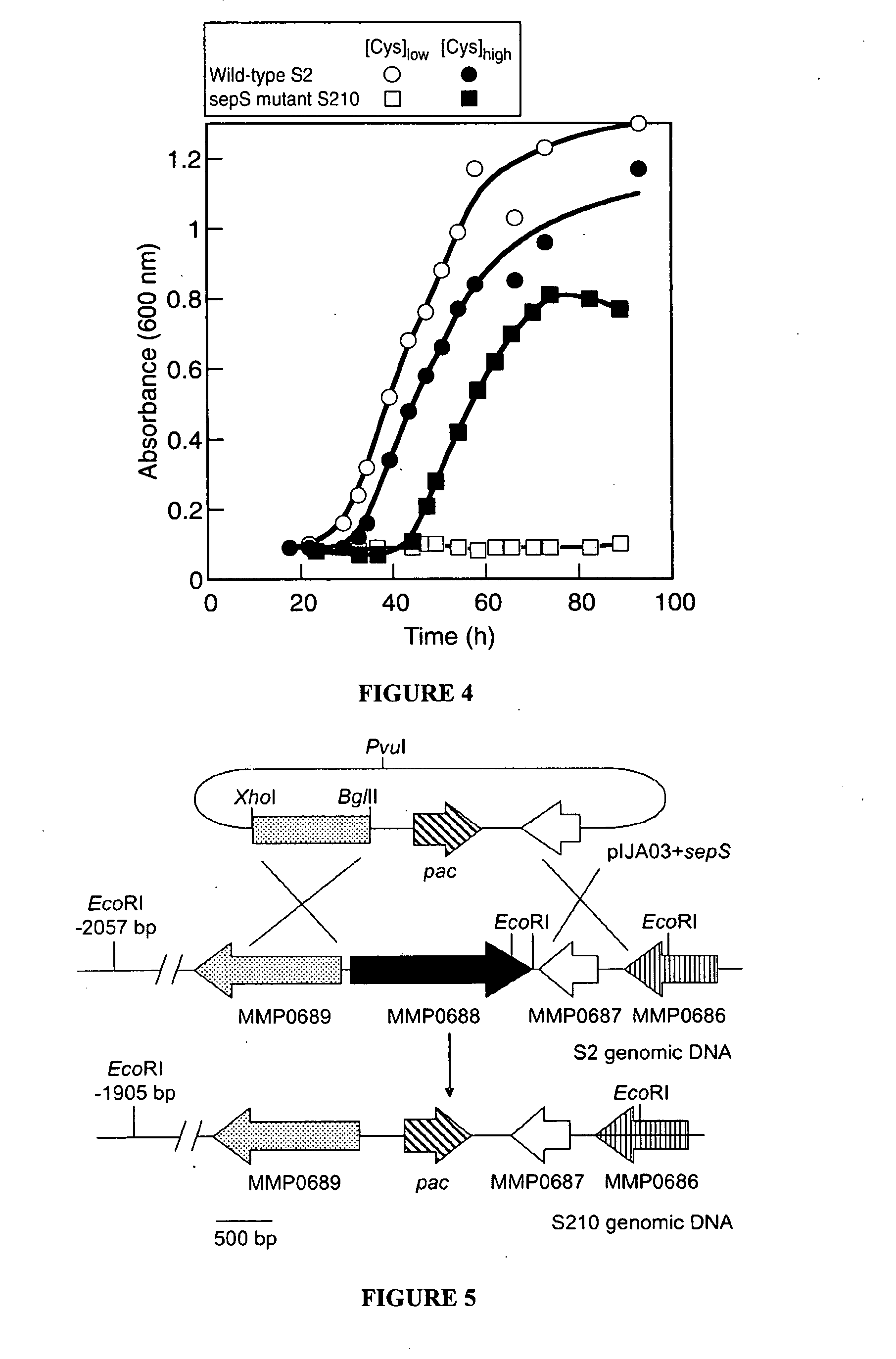
Site specific incorporation of phosphoserine into polypeptides using phosphoseryl-tRNA synthetase
PatentActiveUS20070077620A1
Innovation
- The use of nucleic acids encoding genes with SepRS and tRNASep activity, specifically the cysteinyl-tRNA from Methanocaldococcus jannaschii and the class II-type O-phosphoseryl-tRNA synthetase, to preferentially aminoacylate tRNASep with O-phosphoserine, allowing for the targeted incorporation of phosphoserine into proteins during translation.
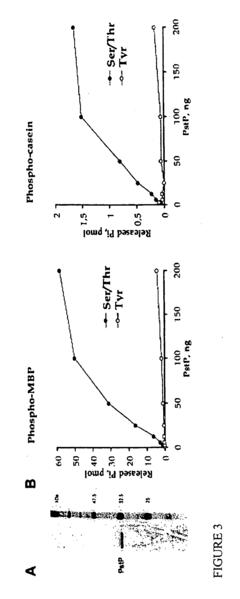
PknB kinase and pstP phosphatase and methods of identifying inhibitory substances
PatentInactiveUS20060019324A1
Innovation
- The pknB kinase and pstP phosphatase, which are part of a conserved operon with genes involved in peptidoglycan synthesis, are identified and characterized for their role in signal transduction pathways, with PstP dephosphorylating PknB, thereby regulating its kinase activity, suggesting a potential target for antibacterial agents.
Computational Tools for PTM Prediction
The field of post-translational modification (PTM) prediction has witnessed significant advancements in computational tools, particularly for phosphorylation and isomerization prediction. These sophisticated algorithms leverage machine learning, statistical models, and structural analysis to identify potential modification sites with increasing accuracy.
For phosphorylation prediction, tools like NetPhos, GPS, and PhosphoSitePlus have become industry standards. NetPhos employs neural networks trained on experimentally verified phosphorylation sites to predict serine, threonine, and tyrosine phosphorylation with sensitivity exceeding 80%. GPS (Group-based Prediction System) utilizes a group-based approach that considers the biochemical properties of amino acids surrounding potential phosphorylation sites, achieving higher specificity for kinase-specific predictions.
More recent developments include DeepPhos and MusiteDeep, which implement deep learning architectures to capture complex sequence patterns. These tools have demonstrated superior performance, with area under ROC curve values approaching 0.90 for certain kinase families, representing a significant improvement over traditional methods.
Isomerization prediction tools remain less developed compared to phosphorylation predictors, reflecting the more challenging nature of detecting these conformational changes. Tools like PISRED and IsoPredict focus primarily on proline isomerization, employing support vector machines and random forest algorithms to identify potential cis-trans isomerization sites based on sequence context and structural features.
Integration approaches that combine multiple prediction methods have shown particular promise. The PTMPred suite, for instance, incorporates both sequence-based and structure-based features to improve prediction accuracy across different PTM types, including both phosphorylation and isomerization events.
Recent innovations include context-aware prediction tools that consider cellular compartmentalization, tissue specificity, and temporal dynamics. PTM-X incorporates subcellular localization data to refine predictions, while CellPTM integrates cell-type specific expression data to provide context-dependent modification predictions.
The accuracy gap between phosphorylation and isomerization prediction tools highlights the need for more comprehensive experimental validation datasets for isomerization events. Current isomerization prediction tools typically achieve precision rates of 65-75%, significantly lower than the 80-90% commonly observed for phosphorylation prediction tools.
Future development trends point toward multi-modal integration approaches that combine genomic, proteomic, and structural data to improve prediction accuracy, particularly for challenging modifications like isomerization. The application of transformer-based architectures, which have revolutionized other areas of computational biology, represents a promising direction for next-generation PTM prediction tools.
For phosphorylation prediction, tools like NetPhos, GPS, and PhosphoSitePlus have become industry standards. NetPhos employs neural networks trained on experimentally verified phosphorylation sites to predict serine, threonine, and tyrosine phosphorylation with sensitivity exceeding 80%. GPS (Group-based Prediction System) utilizes a group-based approach that considers the biochemical properties of amino acids surrounding potential phosphorylation sites, achieving higher specificity for kinase-specific predictions.
More recent developments include DeepPhos and MusiteDeep, which implement deep learning architectures to capture complex sequence patterns. These tools have demonstrated superior performance, with area under ROC curve values approaching 0.90 for certain kinase families, representing a significant improvement over traditional methods.
Isomerization prediction tools remain less developed compared to phosphorylation predictors, reflecting the more challenging nature of detecting these conformational changes. Tools like PISRED and IsoPredict focus primarily on proline isomerization, employing support vector machines and random forest algorithms to identify potential cis-trans isomerization sites based on sequence context and structural features.
Integration approaches that combine multiple prediction methods have shown particular promise. The PTMPred suite, for instance, incorporates both sequence-based and structure-based features to improve prediction accuracy across different PTM types, including both phosphorylation and isomerization events.
Recent innovations include context-aware prediction tools that consider cellular compartmentalization, tissue specificity, and temporal dynamics. PTM-X incorporates subcellular localization data to refine predictions, while CellPTM integrates cell-type specific expression data to provide context-dependent modification predictions.
The accuracy gap between phosphorylation and isomerization prediction tools highlights the need for more comprehensive experimental validation datasets for isomerization events. Current isomerization prediction tools typically achieve precision rates of 65-75%, significantly lower than the 80-90% commonly observed for phosphorylation prediction tools.
Future development trends point toward multi-modal integration approaches that combine genomic, proteomic, and structural data to improve prediction accuracy, particularly for challenging modifications like isomerization. The application of transformer-based architectures, which have revolutionized other areas of computational biology, represents a promising direction for next-generation PTM prediction tools.
Therapeutic Implications and Drug Development
The therapeutic landscape is being revolutionized by our deepening understanding of phosphorylation and isomerization processes. Phosphorylation-targeting therapies have established a significant foothold in modern medicine, with kinase inhibitors representing one of the most successful drug classes developed in recent decades. Currently, over 70 FDA-approved kinase inhibitors are available for treating various cancers and inflammatory diseases, with hundreds more in clinical trials.
Emerging research suggests that combination therapies targeting both phosphorylation and isomerization pathways may offer synergistic benefits. For instance, drugs inhibiting both CDK phosphorylation and PIN1-mediated isomerization have shown enhanced efficacy in preclinical cancer models compared to single-pathway interventions. This dual-targeting approach addresses the compensatory mechanisms that often lead to treatment resistance.
The development of isomerization-targeting therapeutics presents unique opportunities and challenges. Unlike kinase inhibitors, which often struggle with specificity due to conserved ATP-binding pockets, isomerase inhibitors can potentially achieve greater selectivity. Several PIN1 inhibitors, including KPT-6566 and API-1, have demonstrated promising results in preclinical studies for cancer treatment, particularly in cases where phosphorylation-based therapies have failed.
Phosphorylation-isomerization crosstalk also offers novel therapeutic windows. Time-dependent drug administration strategies that account for the sequential nature of these modifications have shown improved efficacy in experimental settings. For example, administering a kinase inhibitor followed by an isomerase inhibitor at specific intervals has demonstrated enhanced tumor suppression in xenograft models compared to simultaneous administration.
Biomarker development represents another critical aspect of this therapeutic frontier. Phosphorylation-isomerization signatures are emerging as powerful predictive tools for treatment response. Multi-omic approaches that simultaneously assess phosphorylation states and isomerization profiles can identify patient subgroups most likely to benefit from specific therapeutic interventions, advancing the precision medicine paradigm.
Drug delivery innovations are addressing the unique challenges of targeting these distinct post-translational modifications. Nanoparticle-based delivery systems capable of sequential release of kinase and isomerase inhibitors have shown promise in maintaining therapeutic concentrations while minimizing off-target effects. Additionally, peptide-based therapeutics designed to disrupt specific phosphorylation-dependent isomerization events offer a highly targeted approach with potentially fewer side effects than traditional small molecule inhibitors.
Emerging research suggests that combination therapies targeting both phosphorylation and isomerization pathways may offer synergistic benefits. For instance, drugs inhibiting both CDK phosphorylation and PIN1-mediated isomerization have shown enhanced efficacy in preclinical cancer models compared to single-pathway interventions. This dual-targeting approach addresses the compensatory mechanisms that often lead to treatment resistance.
The development of isomerization-targeting therapeutics presents unique opportunities and challenges. Unlike kinase inhibitors, which often struggle with specificity due to conserved ATP-binding pockets, isomerase inhibitors can potentially achieve greater selectivity. Several PIN1 inhibitors, including KPT-6566 and API-1, have demonstrated promising results in preclinical studies for cancer treatment, particularly in cases where phosphorylation-based therapies have failed.
Phosphorylation-isomerization crosstalk also offers novel therapeutic windows. Time-dependent drug administration strategies that account for the sequential nature of these modifications have shown improved efficacy in experimental settings. For example, administering a kinase inhibitor followed by an isomerase inhibitor at specific intervals has demonstrated enhanced tumor suppression in xenograft models compared to simultaneous administration.
Biomarker development represents another critical aspect of this therapeutic frontier. Phosphorylation-isomerization signatures are emerging as powerful predictive tools for treatment response. Multi-omic approaches that simultaneously assess phosphorylation states and isomerization profiles can identify patient subgroups most likely to benefit from specific therapeutic interventions, advancing the precision medicine paradigm.
Drug delivery innovations are addressing the unique challenges of targeting these distinct post-translational modifications. Nanoparticle-based delivery systems capable of sequential release of kinase and isomerase inhibitors have shown promise in maintaining therapeutic concentrations while minimizing off-target effects. Additionally, peptide-based therapeutics designed to disrupt specific phosphorylation-dependent isomerization events offer a highly targeted approach with potentially fewer side effects than traditional small molecule inhibitors.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!