How to Track Phosphorylation Shifts During Cell Aging
SEP 23, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Phosphorylation Tracking Background and Objectives
Phosphorylation, a post-translational modification involving the addition of phosphate groups to proteins, plays a critical role in cellular signaling pathways and regulatory mechanisms. The historical trajectory of phosphorylation research dates back to the 1950s when Fischer and Krebs discovered the enzymatic phosphorylation of proteins. Since then, our understanding of phosphorylation dynamics has evolved significantly, revealing its fundamental importance in cellular processes including metabolism, gene expression, cell cycle progression, and apoptosis.
The field has witnessed remarkable technological advancements, transitioning from basic biochemical assays to sophisticated mass spectrometry-based approaches and fluorescent biosensors. These innovations have enabled researchers to monitor phosphorylation events with unprecedented temporal and spatial resolution. However, tracking phosphorylation shifts specifically during cellular aging represents a frontier that remains incompletely explored despite its profound implications for understanding age-related diseases and potential interventions.
Cellular aging, characterized by progressive functional decline and increased vulnerability to stress, involves complex alterations in signaling networks. Phosphorylation patterns undergo substantial remodeling during aging, affecting critical pathways such as insulin/IGF-1 signaling, mTOR, AMPK, and sirtuins. These changes contribute to hallmarks of aging including genomic instability, epigenetic alterations, and dysregulated nutrient sensing.
The technical objectives of phosphorylation tracking in aging cells encompass several dimensions. First, developing methodologies capable of longitudinal monitoring of phosphorylation events in individual cells throughout their lifespan. Second, establishing comprehensive phosphoproteomic profiles that capture the dynamic nature of age-related phosphorylation shifts across different cellular compartments. Third, correlating these molecular signatures with functional outcomes and phenotypic manifestations of cellular senescence.
Recent trends indicate growing interest in integrating multi-omics approaches to contextualize phosphorylation data within broader cellular processes. The emergence of artificial intelligence and machine learning algorithms has further enhanced our ability to interpret complex phosphorylation patterns and predict their functional consequences. Additionally, there is increasing recognition of the heterogeneity in aging processes across different cell types and tissues, necessitating more nuanced analytical frameworks.
The ultimate goal of this technological pursuit extends beyond basic scientific understanding to practical applications in healthcare. By deciphering the phosphorylation code of aging, researchers aim to identify potential biomarkers for aging-related conditions and develop targeted interventions that modulate specific phosphorylation events to promote cellular resilience and longevity.
The field has witnessed remarkable technological advancements, transitioning from basic biochemical assays to sophisticated mass spectrometry-based approaches and fluorescent biosensors. These innovations have enabled researchers to monitor phosphorylation events with unprecedented temporal and spatial resolution. However, tracking phosphorylation shifts specifically during cellular aging represents a frontier that remains incompletely explored despite its profound implications for understanding age-related diseases and potential interventions.
Cellular aging, characterized by progressive functional decline and increased vulnerability to stress, involves complex alterations in signaling networks. Phosphorylation patterns undergo substantial remodeling during aging, affecting critical pathways such as insulin/IGF-1 signaling, mTOR, AMPK, and sirtuins. These changes contribute to hallmarks of aging including genomic instability, epigenetic alterations, and dysregulated nutrient sensing.
The technical objectives of phosphorylation tracking in aging cells encompass several dimensions. First, developing methodologies capable of longitudinal monitoring of phosphorylation events in individual cells throughout their lifespan. Second, establishing comprehensive phosphoproteomic profiles that capture the dynamic nature of age-related phosphorylation shifts across different cellular compartments. Third, correlating these molecular signatures with functional outcomes and phenotypic manifestations of cellular senescence.
Recent trends indicate growing interest in integrating multi-omics approaches to contextualize phosphorylation data within broader cellular processes. The emergence of artificial intelligence and machine learning algorithms has further enhanced our ability to interpret complex phosphorylation patterns and predict their functional consequences. Additionally, there is increasing recognition of the heterogeneity in aging processes across different cell types and tissues, necessitating more nuanced analytical frameworks.
The ultimate goal of this technological pursuit extends beyond basic scientific understanding to practical applications in healthcare. By deciphering the phosphorylation code of aging, researchers aim to identify potential biomarkers for aging-related conditions and develop targeted interventions that modulate specific phosphorylation events to promote cellular resilience and longevity.
Market Analysis for Cell Aging Research Technologies
The cell aging research technology market is experiencing robust growth, driven by increasing focus on age-related diseases and longevity research. Currently valued at approximately $3.5 billion, this market segment is projected to grow at a CAGR of 8.7% through 2028, with phosphorylation tracking technologies representing a significant portion of this expansion.
Demand for advanced phosphorylation tracking tools stems primarily from three sectors: academic research institutions (42% of market share), pharmaceutical companies (35%), and biotechnology firms (18%). Government research facilities and contract research organizations constitute the remaining 5%. This distribution reflects the critical role of phosphorylation analysis in understanding cellular senescence mechanisms.
Regionally, North America dominates with 45% market share, followed by Europe (30%) and Asia-Pacific (20%). The Asia-Pacific region, particularly China and Japan, demonstrates the fastest growth rate at 12.3% annually, driven by increasing research investments and aging population concerns.
Key market drivers include the rising global aging population, increased prevalence of age-related disorders, and growing research funding for cellular aging mechanisms. The WHO reports that by 2050, one in six people worldwide will be over 65, creating urgent demand for aging-related research technologies. Additionally, phosphorylation tracking tools benefit from the broader precision medicine trend, which reached $66 billion in 2022.
Customer needs analysis reveals distinct requirements across segments. Academic researchers prioritize cost-effectiveness and versatility, while pharmaceutical companies demand high throughput capabilities and reproducibility. Biotechnology firms seek integration capabilities with existing platforms and customization options.
Price sensitivity varies significantly by segment, with academic institutions showing high price sensitivity compared to pharmaceutical companies. The average investment for comprehensive phosphorylation tracking systems ranges from $75,000 to $350,000, depending on capabilities and throughput requirements.
Market challenges include high equipment costs, technical complexity requiring specialized training, and regulatory hurdles for clinical applications. Additionally, the market faces standardization issues across different phosphorylation detection methodologies, creating interoperability challenges.
Emerging opportunities include integration with AI and machine learning for data analysis, development of high-throughput screening capabilities, and creation of more accessible point-of-care testing options. The convergence of phosphorylation tracking with other omics technologies represents a particularly promising growth avenue, potentially expanding the addressable market by 30% over the next five years.
Demand for advanced phosphorylation tracking tools stems primarily from three sectors: academic research institutions (42% of market share), pharmaceutical companies (35%), and biotechnology firms (18%). Government research facilities and contract research organizations constitute the remaining 5%. This distribution reflects the critical role of phosphorylation analysis in understanding cellular senescence mechanisms.
Regionally, North America dominates with 45% market share, followed by Europe (30%) and Asia-Pacific (20%). The Asia-Pacific region, particularly China and Japan, demonstrates the fastest growth rate at 12.3% annually, driven by increasing research investments and aging population concerns.
Key market drivers include the rising global aging population, increased prevalence of age-related disorders, and growing research funding for cellular aging mechanisms. The WHO reports that by 2050, one in six people worldwide will be over 65, creating urgent demand for aging-related research technologies. Additionally, phosphorylation tracking tools benefit from the broader precision medicine trend, which reached $66 billion in 2022.
Customer needs analysis reveals distinct requirements across segments. Academic researchers prioritize cost-effectiveness and versatility, while pharmaceutical companies demand high throughput capabilities and reproducibility. Biotechnology firms seek integration capabilities with existing platforms and customization options.
Price sensitivity varies significantly by segment, with academic institutions showing high price sensitivity compared to pharmaceutical companies. The average investment for comprehensive phosphorylation tracking systems ranges from $75,000 to $350,000, depending on capabilities and throughput requirements.
Market challenges include high equipment costs, technical complexity requiring specialized training, and regulatory hurdles for clinical applications. Additionally, the market faces standardization issues across different phosphorylation detection methodologies, creating interoperability challenges.
Emerging opportunities include integration with AI and machine learning for data analysis, development of high-throughput screening capabilities, and creation of more accessible point-of-care testing options. The convergence of phosphorylation tracking with other omics technologies represents a particularly promising growth avenue, potentially expanding the addressable market by 30% over the next five years.
Current Challenges in Phosphorylation Detection Methods
Despite significant advancements in phosphorylation detection technologies, researchers face substantial challenges when tracking phosphorylation shifts during cellular aging processes. Traditional methods such as Western blotting, while reliable for detecting phosphorylated proteins, lack the temporal resolution necessary to capture dynamic changes occurring throughout the aging process. This limitation becomes particularly problematic when studying slow-progressing cellular aging, where phosphorylation events may occur subtly over extended periods.
Mass spectrometry-based approaches offer improved sensitivity but struggle with quantification accuracy when comparing samples across different time points in aging studies. The inherent variability in sample preparation and instrument performance can introduce artifacts that mask genuine biological changes, especially when phosphorylation shifts are modest but biologically significant during aging progression.
Antibody-based detection methods face specificity issues, as many commercial antibodies exhibit cross-reactivity with multiple phosphorylation sites or fail to distinguish between closely related phosphorylated residues. This problem is exacerbated in aging research where researchers often need to monitor multiple phosphorylation events simultaneously across various signaling pathways affected by the aging process.
Live-cell imaging techniques, while promising for real-time monitoring, suffer from limited spatial resolution and signal-to-noise ratios when tracking phosphorylation events in aging cells. The accumulation of autofluorescent materials in aging cells further complicates fluorescence-based detection methods, creating background interference that reduces detection sensitivity for phosphorylation-specific probes.
Sample heterogeneity presents another significant challenge, as aging cell populations typically display considerable variability in their phosphorylation profiles. Current bulk analysis methods often mask this heterogeneity, providing averaged measurements that fail to capture the complex phosphorylation dynamics occurring at the single-cell level during aging.
Temporal resolution remains inadequate with existing technologies, as many phosphorylation events occur rapidly and transiently in response to aging-related stressors. Current methods typically provide snapshots rather than continuous monitoring, missing critical transition states in phosphorylation patterns during cellular aging progression.
Integration of multi-omics data represents a growing challenge, as researchers increasingly recognize that phosphorylation shifts during aging must be interpreted within the broader context of other post-translational modifications, gene expression changes, and metabolic alterations. Current analytical frameworks struggle to integrate these diverse data types into cohesive models that accurately represent the complex molecular landscape of aging cells.
Mass spectrometry-based approaches offer improved sensitivity but struggle with quantification accuracy when comparing samples across different time points in aging studies. The inherent variability in sample preparation and instrument performance can introduce artifacts that mask genuine biological changes, especially when phosphorylation shifts are modest but biologically significant during aging progression.
Antibody-based detection methods face specificity issues, as many commercial antibodies exhibit cross-reactivity with multiple phosphorylation sites or fail to distinguish between closely related phosphorylated residues. This problem is exacerbated in aging research where researchers often need to monitor multiple phosphorylation events simultaneously across various signaling pathways affected by the aging process.
Live-cell imaging techniques, while promising for real-time monitoring, suffer from limited spatial resolution and signal-to-noise ratios when tracking phosphorylation events in aging cells. The accumulation of autofluorescent materials in aging cells further complicates fluorescence-based detection methods, creating background interference that reduces detection sensitivity for phosphorylation-specific probes.
Sample heterogeneity presents another significant challenge, as aging cell populations typically display considerable variability in their phosphorylation profiles. Current bulk analysis methods often mask this heterogeneity, providing averaged measurements that fail to capture the complex phosphorylation dynamics occurring at the single-cell level during aging.
Temporal resolution remains inadequate with existing technologies, as many phosphorylation events occur rapidly and transiently in response to aging-related stressors. Current methods typically provide snapshots rather than continuous monitoring, missing critical transition states in phosphorylation patterns during cellular aging progression.
Integration of multi-omics data represents a growing challenge, as researchers increasingly recognize that phosphorylation shifts during aging must be interpreted within the broader context of other post-translational modifications, gene expression changes, and metabolic alterations. Current analytical frameworks struggle to integrate these diverse data types into cohesive models that accurately represent the complex molecular landscape of aging cells.
Established Phosphorylation Monitoring Techniques
01 Methods for detecting phosphorylation changes
Various techniques have been developed to detect and track changes in protein phosphorylation states. These methods include mass spectrometry-based approaches, immunoassays, and fluorescence-based detection systems that can identify phosphorylation shifts in target proteins. These techniques allow researchers to monitor dynamic phosphorylation events in real-time and understand how these modifications affect protein function and cellular signaling pathways.- Methods for detecting phosphorylation changes: Various techniques have been developed to detect and track changes in protein phosphorylation states. These methods include mass spectrometry-based approaches, immunoassays, and fluorescence-based detection systems that can identify phosphorylation shifts in target proteins. These techniques allow researchers to monitor dynamic phosphorylation events in real-time or in fixed samples, providing insights into cellular signaling pathways and protein regulation mechanisms.
- Phosphorylation-specific antibodies and probes: Specialized antibodies and molecular probes have been developed that specifically recognize phosphorylated residues (typically serine, threonine, or tyrosine) on target proteins. These reagents can be used in various assay formats including Western blotting, ELISA, immunohistochemistry, and flow cytometry to track phosphorylation shifts. Some advanced probes incorporate fluorescent or luminescent reporters that change their signal output upon binding to phosphorylated targets, enabling real-time monitoring of phosphorylation dynamics.
- Kinase activity assays for phosphorylation tracking: Assays designed to measure the activity of protein kinases provide indirect methods for tracking phosphorylation shifts. These assays monitor the transfer of phosphate groups from ATP to substrate proteins or peptides, allowing researchers to quantify phosphorylation events. High-throughput kinase activity assays can be used to screen compounds that modulate phosphorylation pathways, with applications in drug discovery and development for diseases involving dysregulated phosphorylation.
- Phosphoproteomics for large-scale phosphorylation analysis: Phosphoproteomics approaches combine advanced mass spectrometry techniques with phosphopeptide enrichment methods to enable large-scale identification and quantification of phosphorylation sites across the proteome. These methods can track global phosphorylation shifts in response to stimuli, disease states, or drug treatments. Bioinformatics tools are often integrated with phosphoproteomics data to identify regulated phosphorylation networks and predict functional consequences of phosphorylation changes.
- Cellular imaging techniques for phosphorylation dynamics: Advanced imaging techniques have been developed to visualize phosphorylation events within living cells. These include FRET-based biosensors, phosphorylation-sensitive fluorescent proteins, and other optical probes that change their properties upon phosphorylation. Such techniques allow researchers to track phosphorylation shifts with high spatial and temporal resolution, revealing compartmentalized signaling events and phosphorylation dynamics at the single-cell level.
02 Phosphorylation biomarkers for disease diagnosis
Phosphorylation patterns of specific proteins can serve as biomarkers for various diseases, including cancer, neurodegenerative disorders, and metabolic conditions. Changes in phosphorylation states of key proteins can indicate disease progression or treatment response. Detection and tracking of these phosphorylation shifts enable early diagnosis, patient stratification, and personalized treatment approaches based on specific phosphorylation profiles.Expand Specific Solutions03 Kinase activity assays for phosphorylation monitoring
Specialized assays have been developed to monitor kinase activity, which is directly responsible for protein phosphorylation. These assays can track phosphorylation shifts by measuring the transfer of phosphate groups to substrate proteins. High-throughput screening platforms allow for simultaneous analysis of multiple kinases and their substrates, providing comprehensive phosphorylation profiles and enabling the identification of novel phosphorylation sites and regulatory mechanisms.Expand Specific Solutions04 Phosphoproteomic analysis technologies
Advanced phosphoproteomic technologies enable comprehensive analysis of the phosphoproteome, allowing researchers to track global phosphorylation shifts across thousands of proteins simultaneously. These technologies combine enrichment strategies for phosphopeptides with high-resolution mass spectrometry and sophisticated data analysis algorithms. This approach provides insights into complex phosphorylation networks and signaling cascades that regulate cellular processes and disease mechanisms.Expand Specific Solutions05 Phosphorylation-specific antibodies and probes
Specialized antibodies and molecular probes have been developed that specifically recognize phosphorylated residues on target proteins. These tools enable visualization and quantification of phosphorylation shifts in various experimental contexts, including cell cultures, tissue samples, and in vivo models. The development of phospho-specific antibodies with high specificity and sensitivity has revolutionized the ability to track dynamic phosphorylation events and understand their functional significance in normal physiology and disease states.Expand Specific Solutions
Leading Research Institutions and Biotech Companies
The phosphorylation tracking during cell aging market is in its growth phase, with increasing research focus on understanding cellular senescence mechanisms. The global market for cell aging research tools is estimated at $3-4 billion, expanding at 8-10% annually as longevity research gains momentum. Technologically, the field shows moderate maturity with established techniques but significant room for innovation. Leading players include Illumina and Revvity Health Sciences providing advanced sequencing and detection platforms, while academic institutions like The University of California and Fudan University drive fundamental research. Pharmaceutical companies such as By-health and Shiseido are increasingly investing in aging biomarkers, while specialized firms like Cell2in are developing novel cell quality assessment technologies targeting age-related cellular changes.
Illumina, Inc.
Technical Solution: Illumina has developed advanced next-generation sequencing (NGS) platforms integrated with phosphoproteomics analysis for tracking phosphorylation changes during cellular aging. Their technology combines high-throughput sequencing with mass spectrometry-based phosphopeptide enrichment to create comprehensive phosphorylation profiles across the aging cell proteome. The TruSeq Phospho-Profiling workflow enables researchers to identify thousands of phosphorylation sites simultaneously while quantifying subtle changes in phosphorylation levels that occur during senescence. This approach incorporates stable isotope labeling with amino acids in cell culture (SILAC) for precise quantification of phosphorylation dynamics across different timepoints in the aging process. Illumina's bioinformatics pipeline further enhances this technology by providing sophisticated algorithms that can detect statistically significant phosphorylation shifts even in complex biological samples with high background noise.
Strengths: Unparalleled throughput capacity allowing whole-phosphoproteome analysis; exceptional sensitivity for detecting low-abundance phosphorylation events; comprehensive bioinformatics support. Weaknesses: High equipment costs; requires significant technical expertise; sample preparation complexity can introduce variability in results.
Revvity Health Sciences, Inc.
Technical Solution: Revvity Health Sciences has pioneered multiplexed phosphorylation tracking systems specifically optimized for aging research. Their AlphaLISA SureFire Ultra phosphorylation assay platform enables researchers to monitor multiple phosphorylation events simultaneously in aging cell populations with minimal sample requirements. The technology employs proximity-based detection methods using donor and acceptor beads conjugated with specific antibodies against phosphorylated proteins of interest. When phosphorylation occurs, the beads come into proximity, generating a quantifiable signal. Revvity's innovation includes developing aging-specific phosphorylation signature panels that target key pathways implicated in cellular senescence, including mTOR, AMPK, and p53 signaling networks. Their automated high-content imaging systems further complement these assays by providing spatial resolution of phosphorylation events within individual cells, allowing researchers to distinguish between chronologically young cells and those exhibiting senescence markers within heterogeneous populations.
Strengths: High-throughput capability with minimal sample requirements; excellent for longitudinal studies tracking phosphorylation changes over time; robust reproducibility across experiments. Weaknesses: Limited to known phosphorylation targets; requires specialized detection equipment; higher cost per data point compared to some alternative methods.
Key Innovations in Temporal Phosphoproteomics
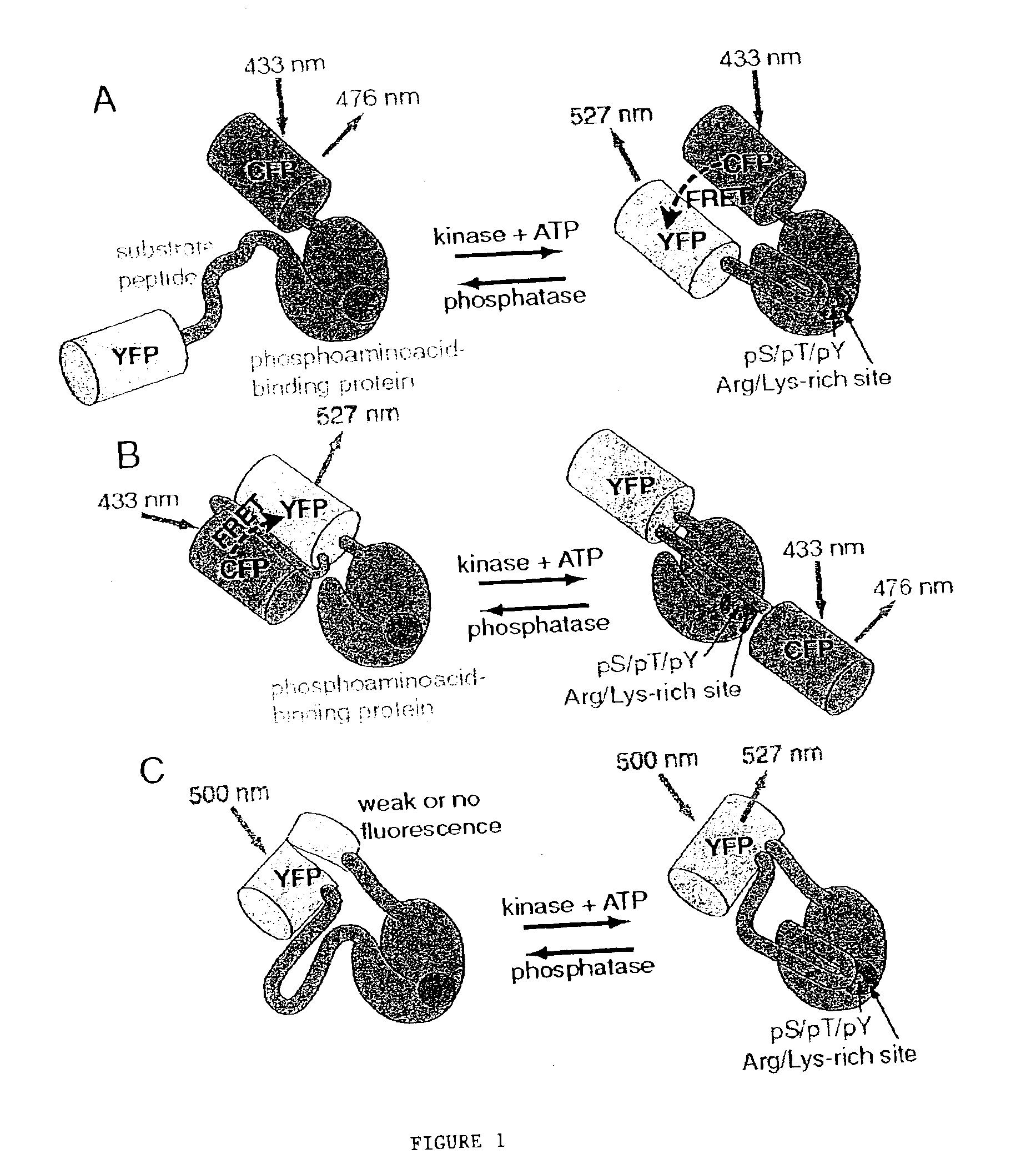
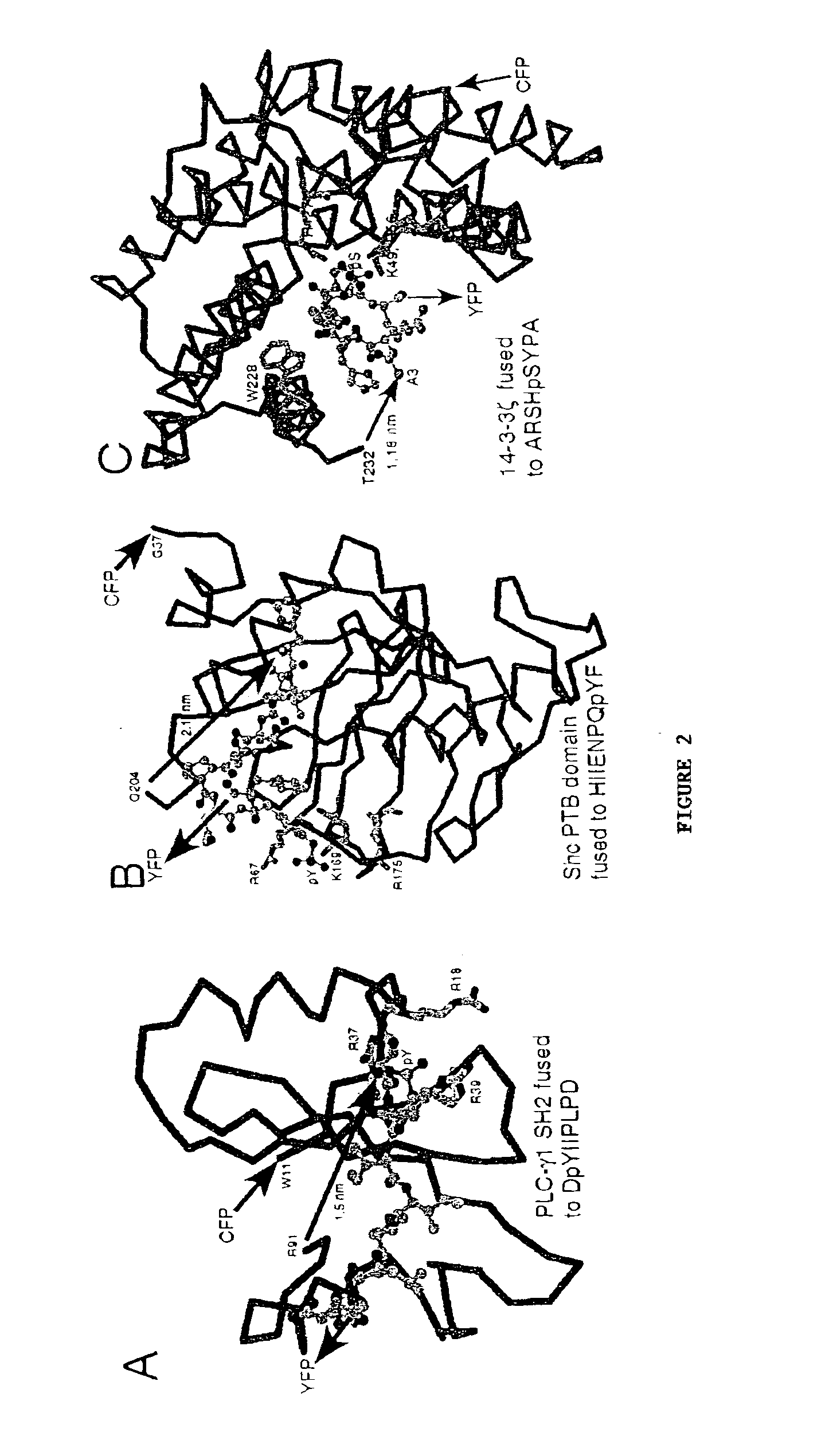
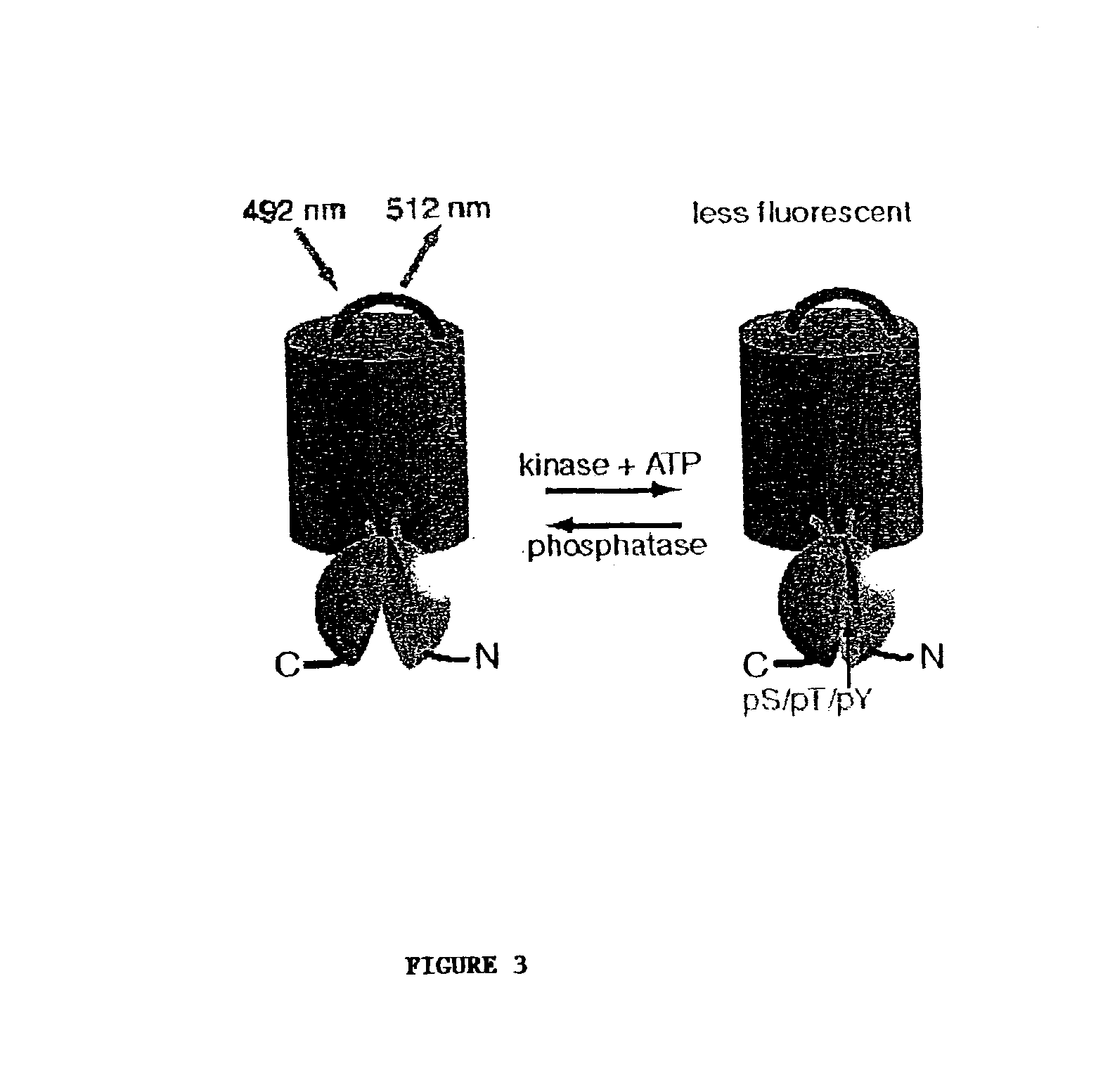
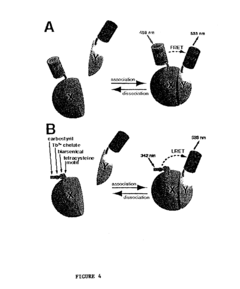
Emission ratiometric indicators of phosphorylation
PatentInactiveUS6900304B2
Innovation
- Development of chimeric phosphorylation indicators containing a donor molecule, a phosphorylatable domain, and a phosphoaminoacid binding domain, which exhibit resonance energy transfer changes upon phosphorylation or dephosphorylation, allowing for the detection of kinase or phosphatase activity through fluorescence or luminescence resonance energy transfer.
C. a. m. detector
PatentWO2006089341A1
Innovation
- The device employs fluorophore labeling and electronic assessment of intracellular polarization changes, combined with morphological observations via microscope or 96-well plate analysis, utilizing a computed program to titrate and quantify these changes, and is designed for high-throughput screening of blood, skin, and tissue samples.
Data Integration and Computational Analysis Approaches
The integration of multi-omics data represents a critical approach for comprehensive tracking of phosphorylation shifts during cellular aging. Modern phosphoproteomics generates vast datasets requiring sophisticated computational frameworks to extract meaningful biological insights. High-throughput mass spectrometry techniques produce complex data matrices that necessitate normalization procedures to account for technical variations and batch effects before meaningful integration can occur.
Machine learning algorithms have emerged as powerful tools for analyzing phosphorylation dynamics across the aging continuum. Supervised learning methods can identify phosphorylation signatures associated with specific aging timepoints, while unsupervised clustering approaches reveal co-regulated phosphorylation networks that change during senescence. Deep learning frameworks, particularly those utilizing convolutional neural networks, have demonstrated superior performance in detecting subtle phosphorylation pattern shifts that traditional statistical methods might overlook.
Network-based computational approaches provide essential context for interpreting phosphorylation data. Kinase-substrate interaction networks help identify master regulatory kinases whose activity changes during aging. Pathway enrichment analysis contextualizes phosphorylation events within biological processes, revealing how signaling cascades become dysregulated over time. Temporal network analysis further captures the dynamic nature of phosphorylation events, identifying early phosphorylation changes that may trigger downstream aging phenotypes.
Data visualization tools have evolved to address the multidimensional nature of phosphoproteomics data. Interactive dashboards now enable researchers to explore phosphorylation dynamics across multiple experimental conditions simultaneously. Trajectory inference algorithms map phosphorylation changes along a pseudotemporal aging axis, providing insights into the progressive nature of cellular aging at the post-translational level.
Integration of phosphoproteomics with other omics layers presents both opportunities and challenges. Correlative analysis between transcriptomics and phosphoproteomics can distinguish between changes in phosphorylation resulting from altered protein expression versus kinase activity shifts. Multi-modal data integration frameworks such as MOFA (Multi-Omics Factor Analysis) and DIABLO (Data Integration Analysis for Biomarker discovery using Latent cOmponents) enable holistic views of cellular aging processes, revealing how phosphorylation networks interact with other molecular systems during senescence.
Cloud-based computational platforms have democratized access to advanced phosphoproteomics analysis. Services like Galaxy and Skyline provide user-friendly interfaces for researchers without extensive computational expertise. Meanwhile, standardized data formats and repositories such as PRIDE and PhosphoSitePlus facilitate data sharing and meta-analyses across multiple aging studies, accelerating discovery in this rapidly evolving field.
Machine learning algorithms have emerged as powerful tools for analyzing phosphorylation dynamics across the aging continuum. Supervised learning methods can identify phosphorylation signatures associated with specific aging timepoints, while unsupervised clustering approaches reveal co-regulated phosphorylation networks that change during senescence. Deep learning frameworks, particularly those utilizing convolutional neural networks, have demonstrated superior performance in detecting subtle phosphorylation pattern shifts that traditional statistical methods might overlook.
Network-based computational approaches provide essential context for interpreting phosphorylation data. Kinase-substrate interaction networks help identify master regulatory kinases whose activity changes during aging. Pathway enrichment analysis contextualizes phosphorylation events within biological processes, revealing how signaling cascades become dysregulated over time. Temporal network analysis further captures the dynamic nature of phosphorylation events, identifying early phosphorylation changes that may trigger downstream aging phenotypes.
Data visualization tools have evolved to address the multidimensional nature of phosphoproteomics data. Interactive dashboards now enable researchers to explore phosphorylation dynamics across multiple experimental conditions simultaneously. Trajectory inference algorithms map phosphorylation changes along a pseudotemporal aging axis, providing insights into the progressive nature of cellular aging at the post-translational level.
Integration of phosphoproteomics with other omics layers presents both opportunities and challenges. Correlative analysis between transcriptomics and phosphoproteomics can distinguish between changes in phosphorylation resulting from altered protein expression versus kinase activity shifts. Multi-modal data integration frameworks such as MOFA (Multi-Omics Factor Analysis) and DIABLO (Data Integration Analysis for Biomarker discovery using Latent cOmponents) enable holistic views of cellular aging processes, revealing how phosphorylation networks interact with other molecular systems during senescence.
Cloud-based computational platforms have democratized access to advanced phosphoproteomics analysis. Services like Galaxy and Skyline provide user-friendly interfaces for researchers without extensive computational expertise. Meanwhile, standardized data formats and repositories such as PRIDE and PhosphoSitePlus facilitate data sharing and meta-analyses across multiple aging studies, accelerating discovery in this rapidly evolving field.
Ethical and Regulatory Considerations in Aging Research
The ethical landscape surrounding aging research, particularly in tracking phosphorylation shifts, presents complex challenges that require careful consideration. Research involving human subjects necessitates strict adherence to informed consent protocols, ensuring participants fully understand the nature, risks, and potential benefits of their involvement. This becomes especially critical when studying vulnerable populations such as the elderly, where cognitive impairment may affect consent capacity.
Privacy concerns represent another significant ethical dimension, as phosphorylation tracking often involves collecting sensitive genetic and proteomic data. Researchers must implement robust data protection measures while balancing the scientific need for data sharing with confidentiality requirements. The long-term storage of biological samples raises additional questions regarding future use and consent parameters.
Regulatory frameworks governing aging research vary considerably across jurisdictions, creating compliance challenges for international collaborations. In the United States, research falls under FDA and NIH oversight, while the European Union applies the General Data Protection Regulation (GDPR) alongside specific biomedical research directives. Asian countries, particularly Japan and Singapore, have developed specialized regulatory approaches to aging research given their demographic challenges.
The commercialization potential of phosphorylation tracking technologies introduces further ethical considerations regarding equitable access. As these technologies may lead to interventions that extend healthspan or lifespan, questions arise about distributive justice and whether such benefits would be accessible only to privileged populations, potentially exacerbating existing health inequalities.
Animal testing remains controversial yet essential in phosphorylation research. Regulatory bodies increasingly emphasize the 3Rs principle (Replacement, Reduction, Refinement) to minimize animal suffering while maintaining scientific validity. Researchers must justify animal use through ethical review processes and demonstrate that alternatives have been considered.
Emerging technologies like AI-assisted phosphorylation analysis and CRISPR-based interventions targeting age-related phosphorylation patterns introduce novel ethical questions regarding human enhancement and the boundaries of medical intervention. These technologies blur the line between treatment and enhancement, prompting discussions about the fundamental aims of medicine and society's relationship with aging.
Professional scientific bodies have responded by developing specialized ethical guidelines for aging research, emphasizing transparency, responsible innovation, and stakeholder engagement. These frameworks acknowledge that aging research carries unique implications for human identity, intergenerational equity, and societal structures.
Privacy concerns represent another significant ethical dimension, as phosphorylation tracking often involves collecting sensitive genetic and proteomic data. Researchers must implement robust data protection measures while balancing the scientific need for data sharing with confidentiality requirements. The long-term storage of biological samples raises additional questions regarding future use and consent parameters.
Regulatory frameworks governing aging research vary considerably across jurisdictions, creating compliance challenges for international collaborations. In the United States, research falls under FDA and NIH oversight, while the European Union applies the General Data Protection Regulation (GDPR) alongside specific biomedical research directives. Asian countries, particularly Japan and Singapore, have developed specialized regulatory approaches to aging research given their demographic challenges.
The commercialization potential of phosphorylation tracking technologies introduces further ethical considerations regarding equitable access. As these technologies may lead to interventions that extend healthspan or lifespan, questions arise about distributive justice and whether such benefits would be accessible only to privileged populations, potentially exacerbating existing health inequalities.
Animal testing remains controversial yet essential in phosphorylation research. Regulatory bodies increasingly emphasize the 3Rs principle (Replacement, Reduction, Refinement) to minimize animal suffering while maintaining scientific validity. Researchers must justify animal use through ethical review processes and demonstrate that alternatives have been considered.
Emerging technologies like AI-assisted phosphorylation analysis and CRISPR-based interventions targeting age-related phosphorylation patterns introduce novel ethical questions regarding human enhancement and the boundaries of medical intervention. These technologies blur the line between treatment and enhancement, prompting discussions about the fundamental aims of medicine and society's relationship with aging.
Professional scientific bodies have responded by developing specialized ethical guidelines for aging research, emphasizing transparency, responsible innovation, and stakeholder engagement. These frameworks acknowledge that aging research carries unique implications for human identity, intergenerational equity, and societal structures.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!