How to Optimize Phosphorylation for Enzyme Activation
SEP 23, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Phosphorylation Mechanisms and Enzyme Activation Goals
Phosphorylation represents one of the most critical post-translational modifications in biological systems, playing a fundamental role in enzyme regulation across all domains of life. The historical trajectory of phosphorylation research began with the pioneering work of Edmond Fischer and Edwin Krebs in the 1950s, who discovered that glycogen phosphorylase activation required a phosphorylation event. This discovery eventually earned them the Nobel Prize and established phosphorylation as a central mechanism in cellular signaling.
The evolution of phosphorylation research has progressed through several distinct phases. Initially focused on basic biochemical characterization, the field expanded to include structural biology approaches in the 1980s and 1990s, revealing the three-dimensional configurations of kinases and their substrates. The genomic era subsequently identified the complete "kinome" - the full complement of protein kinases encoded in various genomes - revealing approximately 518 kinases in humans alone.
Recent technological advances in mass spectrometry have revolutionized phosphoproteomics, enabling the identification of thousands of phosphorylation sites simultaneously. This has shifted the field toward systems-level understanding of phosphorylation networks and their dynamic regulation. Computational approaches have further enhanced our ability to predict phosphorylation sites and model kinase-substrate interactions.
The primary goal in optimizing phosphorylation for enzyme activation is to achieve precise temporal and spatial control over enzymatic activity. This includes developing strategies to enhance phosphorylation efficiency, specificity, and reversibility. Specific objectives include identifying optimal amino acid sequences surrounding phosphorylation sites, understanding the structural determinants that influence kinase-substrate recognition, and developing methods to modulate phosphorylation rates in response to specific cellular conditions.
Another critical aim is to elucidate the mechanistic details of how phosphorylation induces conformational changes that activate enzymes. This involves characterizing the energetic landscapes of enzymes before and after phosphorylation, identifying allosteric networks that transmit conformational changes, and developing predictive models of phosphorylation-induced activation.
The technological trajectory points toward increasingly precise tools for controlling phosphorylation events, including optogenetic approaches that enable light-controlled kinase activation, chemical genetics strategies for specific kinase targeting, and synthetic biology approaches to engineer novel phosphorylation circuits with desired properties. These advances aim to transform phosphorylation from a descriptive field to a predictive and manipulable science with broad applications in biotechnology and medicine.
The evolution of phosphorylation research has progressed through several distinct phases. Initially focused on basic biochemical characterization, the field expanded to include structural biology approaches in the 1980s and 1990s, revealing the three-dimensional configurations of kinases and their substrates. The genomic era subsequently identified the complete "kinome" - the full complement of protein kinases encoded in various genomes - revealing approximately 518 kinases in humans alone.
Recent technological advances in mass spectrometry have revolutionized phosphoproteomics, enabling the identification of thousands of phosphorylation sites simultaneously. This has shifted the field toward systems-level understanding of phosphorylation networks and their dynamic regulation. Computational approaches have further enhanced our ability to predict phosphorylation sites and model kinase-substrate interactions.
The primary goal in optimizing phosphorylation for enzyme activation is to achieve precise temporal and spatial control over enzymatic activity. This includes developing strategies to enhance phosphorylation efficiency, specificity, and reversibility. Specific objectives include identifying optimal amino acid sequences surrounding phosphorylation sites, understanding the structural determinants that influence kinase-substrate recognition, and developing methods to modulate phosphorylation rates in response to specific cellular conditions.
Another critical aim is to elucidate the mechanistic details of how phosphorylation induces conformational changes that activate enzymes. This involves characterizing the energetic landscapes of enzymes before and after phosphorylation, identifying allosteric networks that transmit conformational changes, and developing predictive models of phosphorylation-induced activation.
The technological trajectory points toward increasingly precise tools for controlling phosphorylation events, including optogenetic approaches that enable light-controlled kinase activation, chemical genetics strategies for specific kinase targeting, and synthetic biology approaches to engineer novel phosphorylation circuits with desired properties. These advances aim to transform phosphorylation from a descriptive field to a predictive and manipulable science with broad applications in biotechnology and medicine.
Market Applications of Optimized Enzyme Phosphorylation
The optimization of enzyme phosphorylation has created significant market opportunities across multiple industries. In the pharmaceutical sector, enhanced phosphorylation techniques have revolutionized drug development processes by enabling more precise control over enzyme activity. This has led to the development of novel therapeutics targeting kinase-dependent pathways, particularly in oncology where aberrant phosphorylation is a hallmark of many cancers. Companies like Roche, Novartis, and Pfizer have incorporated optimized phosphorylation techniques into their drug discovery platforms, resulting in several successful targeted therapies.
The biotechnology industry has leveraged optimized enzyme phosphorylation to develop more efficient biocatalysts for industrial processes. These enhanced enzymes demonstrate improved stability, activity, and specificity, reducing production costs and environmental impact. For instance, optimized phosphorylation of amylases has increased their efficiency in starch processing by up to 40%, significantly reducing energy consumption in food production.
In agriculture, phosphorylation optimization has enabled the development of more resilient crops through targeted modification of key metabolic enzymes. Companies like Bayer and Syngenta have utilized these techniques to create crops with enhanced stress tolerance and improved nutrient utilization efficiency. The market for such enhanced agricultural products is projected to grow substantially as climate change challenges traditional farming practices.
The diagnostic sector has benefited from advances in phosphorylation optimization through the development of more sensitive and specific enzyme-linked immunosorbent assays (ELISAs). These improved diagnostics offer earlier detection of diseases, particularly those involving dysregulated phosphorylation pathways such as neurodegenerative disorders and diabetes. The global market for such advanced diagnostic tools continues to expand as healthcare systems increasingly focus on preventive medicine.
Cosmetic and personal care industries have also adopted optimized enzyme phosphorylation techniques to develop anti-aging products that target specific cellular pathways. Enzymes with enhanced activity through controlled phosphorylation are being incorporated into formulations that more effectively address skin aging processes at the molecular level.
The biofuel industry represents another significant market application, where optimized phosphorylation of key enzymes involved in cellulose breakdown has improved the efficiency of converting biomass to ethanol. This advancement has helped address one of the major bottlenecks in sustainable biofuel production, potentially reducing production costs and increasing yield.
As these market applications continue to evolve, cross-industry collaborations are emerging to leverage optimized phosphorylation techniques across different sectors, creating new market opportunities and innovative solutions to complex challenges in healthcare, agriculture, and industrial biotechnology.
The biotechnology industry has leveraged optimized enzyme phosphorylation to develop more efficient biocatalysts for industrial processes. These enhanced enzymes demonstrate improved stability, activity, and specificity, reducing production costs and environmental impact. For instance, optimized phosphorylation of amylases has increased their efficiency in starch processing by up to 40%, significantly reducing energy consumption in food production.
In agriculture, phosphorylation optimization has enabled the development of more resilient crops through targeted modification of key metabolic enzymes. Companies like Bayer and Syngenta have utilized these techniques to create crops with enhanced stress tolerance and improved nutrient utilization efficiency. The market for such enhanced agricultural products is projected to grow substantially as climate change challenges traditional farming practices.
The diagnostic sector has benefited from advances in phosphorylation optimization through the development of more sensitive and specific enzyme-linked immunosorbent assays (ELISAs). These improved diagnostics offer earlier detection of diseases, particularly those involving dysregulated phosphorylation pathways such as neurodegenerative disorders and diabetes. The global market for such advanced diagnostic tools continues to expand as healthcare systems increasingly focus on preventive medicine.
Cosmetic and personal care industries have also adopted optimized enzyme phosphorylation techniques to develop anti-aging products that target specific cellular pathways. Enzymes with enhanced activity through controlled phosphorylation are being incorporated into formulations that more effectively address skin aging processes at the molecular level.
The biofuel industry represents another significant market application, where optimized phosphorylation of key enzymes involved in cellulose breakdown has improved the efficiency of converting biomass to ethanol. This advancement has helped address one of the major bottlenecks in sustainable biofuel production, potentially reducing production costs and increasing yield.
As these market applications continue to evolve, cross-industry collaborations are emerging to leverage optimized phosphorylation techniques across different sectors, creating new market opportunities and innovative solutions to complex challenges in healthcare, agriculture, and industrial biotechnology.
Current Challenges in Phosphorylation-Mediated Enzyme Activation
Despite significant advances in understanding phosphorylation mechanisms, several critical challenges persist in optimizing phosphorylation for enzyme activation. One fundamental obstacle is the complexity of phosphorylation site specificity. Enzymes often contain multiple potential phosphorylation sites, and determining which sites are functionally relevant for activation versus those that may inhibit or have no effect remains difficult. This complexity is compounded by the context-dependent nature of phosphorylation, where the same modification may produce different outcomes depending on the cellular environment.
Temporal regulation presents another significant challenge. The precise timing of phosphorylation events is crucial for proper enzyme function, particularly in signaling cascades where sequential activation is required. Current technologies lack sufficient temporal resolution to fully capture and manipulate these dynamics, limiting our ability to optimize phosphorylation-mediated activation in complex biological systems.
The reversible nature of phosphorylation creates additional complications. The interplay between kinases (which add phosphate groups) and phosphatases (which remove them) establishes a dynamic equilibrium that is difficult to predict and control. This balance varies across different cellular compartments and physiological states, making standardized optimization approaches challenging to implement.
Structural constraints further complicate optimization efforts. Phosphorylation often induces conformational changes that are essential for enzyme activation, but predicting these structural rearrangements remains imprecise. Current computational models struggle to accurately simulate the complex allosteric effects that propagate through protein structures following phosphorylation events.
Technical limitations in detection and quantification methods represent another significant hurdle. While mass spectrometry has revolutionized phosphoproteomic analysis, challenges persist in detecting low-abundance phosphorylation events and distinguishing between closely related phosphorylation sites. This impedes comprehensive mapping of phosphorylation networks necessary for targeted optimization.
The heterogeneity of enzyme populations within cells creates additional complexity. At any given time, enzymes exist in various phosphorylation states, making it difficult to achieve uniform activation across an entire population. This heterogeneity complicates both experimental analysis and therapeutic applications targeting phosphorylation-dependent enzyme activation.
Finally, translating in vitro optimization strategies to in vivo applications remains problematic. Phosphorylation dynamics in living systems are influenced by numerous factors including subcellular localization, interaction with scaffolding proteins, and cross-talk with other post-translational modifications. These contextual factors are difficult to recapitulate in simplified experimental systems, creating a significant gap between laboratory findings and practical applications.
Temporal regulation presents another significant challenge. The precise timing of phosphorylation events is crucial for proper enzyme function, particularly in signaling cascades where sequential activation is required. Current technologies lack sufficient temporal resolution to fully capture and manipulate these dynamics, limiting our ability to optimize phosphorylation-mediated activation in complex biological systems.
The reversible nature of phosphorylation creates additional complications. The interplay between kinases (which add phosphate groups) and phosphatases (which remove them) establishes a dynamic equilibrium that is difficult to predict and control. This balance varies across different cellular compartments and physiological states, making standardized optimization approaches challenging to implement.
Structural constraints further complicate optimization efforts. Phosphorylation often induces conformational changes that are essential for enzyme activation, but predicting these structural rearrangements remains imprecise. Current computational models struggle to accurately simulate the complex allosteric effects that propagate through protein structures following phosphorylation events.
Technical limitations in detection and quantification methods represent another significant hurdle. While mass spectrometry has revolutionized phosphoproteomic analysis, challenges persist in detecting low-abundance phosphorylation events and distinguishing between closely related phosphorylation sites. This impedes comprehensive mapping of phosphorylation networks necessary for targeted optimization.
The heterogeneity of enzyme populations within cells creates additional complexity. At any given time, enzymes exist in various phosphorylation states, making it difficult to achieve uniform activation across an entire population. This heterogeneity complicates both experimental analysis and therapeutic applications targeting phosphorylation-dependent enzyme activation.
Finally, translating in vitro optimization strategies to in vivo applications remains problematic. Phosphorylation dynamics in living systems are influenced by numerous factors including subcellular localization, interaction with scaffolding proteins, and cross-talk with other post-translational modifications. These contextual factors are difficult to recapitulate in simplified experimental systems, creating a significant gap between laboratory findings and practical applications.
Established Protocols for Optimizing Phosphorylation Efficiency
01 Phosphorylation-based enzyme activation mechanisms
Phosphorylation serves as a key regulatory mechanism for enzyme activation in cellular signaling pathways. This process involves the transfer of phosphate groups to specific amino acid residues, typically serine, threonine, or tyrosine, resulting in conformational changes that alter enzyme activity. These phosphorylation events can either activate or inhibit enzymes depending on the specific protein and cellular context, providing a versatile mechanism for signal transduction and metabolic regulation.- Phosphorylation-based enzyme activation mechanisms: Phosphorylation serves as a key regulatory mechanism for enzyme activation in cellular signaling pathways. This process involves the transfer of phosphate groups to specific amino acid residues, typically serine, threonine, or tyrosine, resulting in conformational changes that alter enzyme activity. These modifications can either activate or inhibit enzymatic function, providing a versatile control mechanism for cellular processes including metabolism, gene expression, and cell cycle progression.
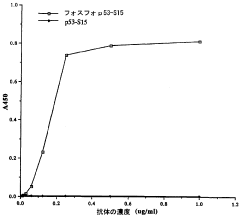
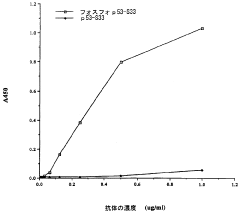
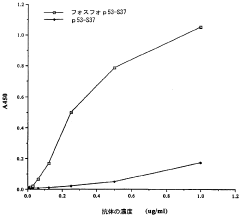
- Detection methods for phosphorylation-mediated enzyme activation: Various analytical techniques have been developed to detect and quantify phosphorylation events associated with enzyme activation. These methods include immunoassays using phospho-specific antibodies, mass spectrometry-based approaches, fluorescence resonance energy transfer (FRET) techniques, and phospho-proteomic analyses. Such detection systems enable researchers to monitor enzyme activation states in real-time, facilitating the study of signaling cascades and the development of targeted therapeutics for diseases involving dysregulated phosphorylation.
- Kinase inhibitors and modulators of enzyme phosphorylation: Compounds that modulate the phosphorylation status of enzymes represent important therapeutic agents for various diseases. Kinase inhibitors can block the phosphorylation-dependent activation of enzymes involved in pathological processes, while phosphatase inhibitors can prevent the removal of activating phosphate groups. These modulators can be designed to target specific enzymes within signaling pathways, offering potential treatments for cancer, inflammatory disorders, and neurodegenerative diseases where abnormal enzyme activation contributes to disease progression.
- Phosphorylation in cellular signaling cascades: Phosphorylation events often occur in sequential cascades, where one activated enzyme phosphorylates and activates downstream enzymes, amplifying the initial signal. These signaling cascades regulate critical cellular functions including growth, differentiation, and apoptosis. Mitogen-activated protein kinase (MAPK) pathways and phosphoinositide 3-kinase (PI3K)/Akt pathways are examples of such cascades where phosphorylation-mediated enzyme activation plays a central role in transmitting signals from cell surface receptors to nuclear transcription factors.
- Engineered phosphorylation systems for biotechnological applications: Engineered systems that control enzyme activation through phosphorylation have applications in biotechnology and synthetic biology. These include biosensors that detect analytes through phosphorylation-induced conformational changes, engineered kinase circuits for programmed cellular responses, and phosphorylation-dependent protein switches for controlled release of therapeutic agents. Such systems can be designed with specific phosphorylation sites to create enzymes with tunable activity, enabling precise control over biological processes in research and industrial applications.
02 Detection methods for phosphorylation-mediated enzyme activation
Various analytical techniques have been developed to detect and quantify phosphorylation-mediated enzyme activation. These methods include phospho-specific antibodies, mass spectrometry, fluorescence-based assays, and kinase activity assays. Such detection systems enable researchers to monitor phosphorylation events in real-time, identify phosphorylation sites, and measure the resulting changes in enzyme activity, providing valuable tools for studying signal transduction pathways and developing targeted therapeutics.Expand Specific Solutions03 Therapeutic applications targeting phosphorylation-dependent enzyme activation
Dysregulation of phosphorylation-dependent enzyme activation is implicated in various diseases, including cancer, diabetes, and neurodegenerative disorders. Therapeutic approaches targeting these pathways include kinase inhibitors, phosphatase modulators, and compounds that disrupt protein-protein interactions involved in phosphorylation-mediated signaling. By selectively modulating phosphorylation events, these therapeutics aim to restore normal enzyme function and cellular homeostasis in disease states.Expand Specific Solutions04 Role of phosphorylation in enzyme complex formation and substrate recognition
Phosphorylation events often facilitate the assembly of multi-protein enzyme complexes and influence substrate recognition. The addition of phosphate groups can create binding sites for proteins containing phospho-binding domains, such as SH2, PTB, or 14-3-3 domains, leading to the recruitment of downstream effectors. Additionally, phosphorylation can alter enzyme-substrate interactions by changing the electrostatic properties or conformation of the active site, thereby regulating catalytic efficiency and substrate specificity.Expand Specific Solutions05 Systems biology approaches to understand phosphorylation networks in enzyme activation
Systems biology approaches, including computational modeling, high-throughput screening, and network analysis, have been employed to understand the complex interplay of phosphorylation events in enzyme activation. These methods help elucidate the dynamics, feedback mechanisms, and cross-talk between different phosphorylation pathways, providing a comprehensive view of cellular signaling networks. By integrating multiple data types, researchers can predict the effects of perturbations to phosphorylation networks and identify potential therapeutic targets.Expand Specific Solutions
Key Research Groups and Companies in Enzyme Engineering
The enzyme phosphorylation optimization market is currently in a growth phase, characterized by increasing research activities and technological advancements. The competitive landscape features a mix of academic institutions (Yale University, Brown University, Duke University) and commercial entities (Bio-Rad, New England Biolabs, Eppendorf SE) driving innovation. The market size is expanding due to growing applications in pharmaceutical development, biotechnology, and medical research. From a technological maturity perspective, companies like Merck Patent GmbH and Ionis Pharmaceuticals are advancing proprietary phosphorylation techniques, while research institutions like The Regents of the University of California are establishing fundamental scientific frameworks. Specialized players such as Cube Biotech and AM-Pharma are developing niche applications, creating a diverse ecosystem of solutions for enzyme activation through optimized phosphorylation processes.
Merck Patent GmbH
Technical Solution: Merck has developed the PhosphoOptimize™ platform, a comprehensive system for enzyme phosphorylation optimization across pharmaceutical and biotechnology applications. Their approach integrates computational modeling with experimental validation to identify optimal phosphorylation conditions for diverse enzyme classes. Merck's technology incorporates a library of over 200 engineered kinases with modified specificity and activity profiles, enabling precise targeting of specific phosphorylation sites. Their system features microfluidic reaction chambers that allow parallel optimization of multiple phosphorylation parameters simultaneously, dramatically accelerating the development process. Merck has also pioneered mass spectrometry-based phosphorylation mapping techniques that provide detailed insights into phosphorylation patterns and their effects on enzyme structure and function. Their platform includes proprietary stabilizing agents that protect enzymes during phosphorylation, maintaining structural integrity and preventing aggregation even under challenging reaction conditions.
Strengths: Exceptional breadth of applications across different enzyme classes; strong integration with downstream purification workflows; excellent scalability from research to production. Weaknesses: Complex system requiring significant expertise; substantial initial investment; some components have limited compatibility with non-Merck systems.
Ajinomoto Co., Inc.
Technical Solution: Ajinomoto has pioneered industrial-scale enzyme phosphorylation optimization through their AJIPHASE® technology platform. Their approach focuses on controlling phosphorylation for large-scale enzyme production in food and pharmaceutical applications. The company has developed proprietary bioreactor systems that maintain precise control over phosphorylation conditions during enzyme production, including innovative feedback control mechanisms that adjust reaction parameters in real-time based on phosphorylation progress. Ajinomoto's technology incorporates specialized ATP regeneration systems that maintain optimal ATP levels throughout the phosphorylation process, significantly improving efficiency and reducing costs in industrial applications. Their process also features proprietary stabilizing agents that protect enzymes during the phosphorylation process, preventing denaturation and maintaining activity. This integrated approach has enabled Ajinomoto to achieve phosphorylation yields exceeding 90% in industrial settings, substantially higher than conventional methods.
Strengths: Unmatched expertise in industrial-scale applications; cost-effective for large production volumes; excellent process consistency and reproducibility. Weaknesses: Less suitable for research-scale applications; limited flexibility for novel enzyme types; requires significant infrastructure investment.
Critical Innovations in Site-Specific Phosphorylation
Method for assaying phosphorylation enzyme activity
PatentWO2000072011A1
Innovation
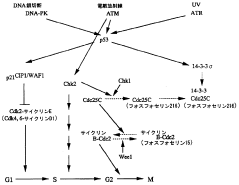
- A method using substrate peptides phosphorylated by DNA-PK, ATM, and ATR, with immunological measurement of phosphorylation levels to assess kinase activity, allowing for the screening of compounds that inhibit or promote phosphorylation or dephosphorylation, and the development of kits for these measurements.
Assay for detecting the enzymatic activity of a phosphorylation enzyme using enhanced signal generation
PatentInactiveUS6548266B1
Innovation
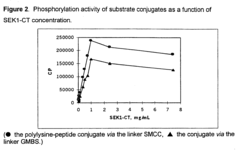
- The method involves immobilizing substrates on a solid support by covalent linkage to a polymer, enhancing enzyme-substrate contact and sensitivity, allowing for a one-plate assay procedure that increases throughput and reduces background noise, and using signal-enhancing polymers like polylysine to improve substrate availability and detection.
Computational Approaches to Phosphorylation Site Prediction
Computational approaches to phosphorylation site prediction have emerged as essential tools in the optimization of enzyme activation processes. These methods leverage machine learning algorithms, statistical models, and structural analysis to identify potential phosphorylation sites with increasing accuracy. Traditional sequence-based prediction tools like NetPhos, GPS, and DISPHOS utilize neural networks and support vector machines to analyze amino acid sequences surrounding potential phosphorylation sites, identifying patterns associated with kinase recognition.
More advanced computational approaches incorporate three-dimensional structural information of proteins, significantly enhancing prediction accuracy. Tools such as Predikin and KinasePhos 2.0 consider the spatial arrangement of amino acids and accessibility of potential phosphorylation sites, providing more biologically relevant predictions. These structure-based methods help researchers understand not only where phosphorylation might occur but also the mechanistic implications for enzyme activation.
Recent developments in deep learning have revolutionized phosphorylation site prediction. Convolutional neural networks and recurrent neural networks can now process vast amounts of phosphoproteomic data to identify subtle patterns that traditional algorithms might miss. DeepPhos and MusiteDeep represent this new generation of prediction tools, offering improved sensitivity and specificity across diverse protein families.
Integration of multiple data types has further enhanced predictive capabilities. Modern approaches combine sequence analysis, structural information, evolutionary conservation, and experimental phosphoproteomic data. Meta-predictors like PhosphoSVM and iPhos-PseEn aggregate results from multiple prediction algorithms, reducing false positives and increasing confidence in predicted sites.
Kinase-specific prediction tools represent another significant advancement in the field. These specialized algorithms are trained on phosphorylation events mediated by specific kinases, allowing researchers to predict not only potential phosphorylation sites but also the likely kinases responsible. This information is crucial when designing targeted approaches to optimize enzyme activation through phosphorylation.
Computational validation through molecular dynamics simulations provides insights into how predicted phosphorylation events might affect protein conformation and function. These simulations can model the structural changes induced by phosphorylation, helping researchers understand the mechanistic basis of enzyme activation and informing experimental design for validation studies.
More advanced computational approaches incorporate three-dimensional structural information of proteins, significantly enhancing prediction accuracy. Tools such as Predikin and KinasePhos 2.0 consider the spatial arrangement of amino acids and accessibility of potential phosphorylation sites, providing more biologically relevant predictions. These structure-based methods help researchers understand not only where phosphorylation might occur but also the mechanistic implications for enzyme activation.
Recent developments in deep learning have revolutionized phosphorylation site prediction. Convolutional neural networks and recurrent neural networks can now process vast amounts of phosphoproteomic data to identify subtle patterns that traditional algorithms might miss. DeepPhos and MusiteDeep represent this new generation of prediction tools, offering improved sensitivity and specificity across diverse protein families.
Integration of multiple data types has further enhanced predictive capabilities. Modern approaches combine sequence analysis, structural information, evolutionary conservation, and experimental phosphoproteomic data. Meta-predictors like PhosphoSVM and iPhos-PseEn aggregate results from multiple prediction algorithms, reducing false positives and increasing confidence in predicted sites.
Kinase-specific prediction tools represent another significant advancement in the field. These specialized algorithms are trained on phosphorylation events mediated by specific kinases, allowing researchers to predict not only potential phosphorylation sites but also the likely kinases responsible. This information is crucial when designing targeted approaches to optimize enzyme activation through phosphorylation.
Computational validation through molecular dynamics simulations provides insights into how predicted phosphorylation events might affect protein conformation and function. These simulations can model the structural changes induced by phosphorylation, helping researchers understand the mechanistic basis of enzyme activation and informing experimental design for validation studies.
Regulatory Considerations for Engineered Enzyme Applications
The regulatory landscape for engineered enzymes with optimized phosphorylation mechanisms presents significant considerations for both research and commercial applications. Regulatory bodies such as the FDA in the United States, the EMA in Europe, and similar organizations worldwide have established frameworks that govern the development, testing, and deployment of engineered enzymes across various sectors including pharmaceuticals, food processing, and industrial manufacturing.
For pharmaceutical applications, engineered enzymes with enhanced phosphorylation-based activation must undergo rigorous safety assessments following ICH (International Council for Harmonisation) guidelines. These assessments typically include evaluation of potential immunogenicity, toxicity profiles, and off-target effects that might arise from altered phosphorylation patterns. The FDA's guidance for enzyme products requires comprehensive characterization of phosphorylation sites and their impact on enzyme kinetics and stability.
In food processing applications, enzymes with optimized phosphorylation mechanisms must comply with food additive regulations. The GRAS (Generally Recognized As Safe) status determination involves thorough documentation of the phosphorylation optimization process and its impact on enzyme functionality and safety. The European Food Safety Authority (EFSA) similarly requires detailed molecular characterization of engineered enzymes, with particular attention to modifications affecting post-translational processes like phosphorylation.
Environmental regulations present another critical dimension, particularly for industrial applications. Engineered enzymes released into the environment must be assessed for ecological impact, biodegradability, and potential gene transfer risks. The EPA in the United States and the European Chemicals Agency have established protocols for evaluating the environmental safety of biocatalysts, including those with modified phosphorylation patterns.
Intellectual property considerations also intersect with regulatory frameworks. Patents covering specific phosphorylation optimization techniques may influence regulatory strategy, particularly regarding biosimilars and generic enzyme products. Companies must navigate both patent exclusivity periods and regulatory approval pathways when developing competitive enzyme technologies.
Emerging regulatory trends indicate increasing scrutiny of synthetic biology approaches to enzyme engineering. Regulatory bodies are developing new frameworks to address CRISPR-based enzyme modifications and directed evolution techniques that target phosphorylation sites. These frameworks aim to balance innovation with appropriate risk assessment methodologies tailored to novel engineering approaches.
Cross-border regulatory harmonization remains challenging, with significant variations in how different jurisdictions approach engineered enzyme regulation. Companies pursuing global markets for phosphorylation-optimized enzymes must develop comprehensive regulatory strategies that address these regional differences while maintaining consistent quality and safety standards.
For pharmaceutical applications, engineered enzymes with enhanced phosphorylation-based activation must undergo rigorous safety assessments following ICH (International Council for Harmonisation) guidelines. These assessments typically include evaluation of potential immunogenicity, toxicity profiles, and off-target effects that might arise from altered phosphorylation patterns. The FDA's guidance for enzyme products requires comprehensive characterization of phosphorylation sites and their impact on enzyme kinetics and stability.
In food processing applications, enzymes with optimized phosphorylation mechanisms must comply with food additive regulations. The GRAS (Generally Recognized As Safe) status determination involves thorough documentation of the phosphorylation optimization process and its impact on enzyme functionality and safety. The European Food Safety Authority (EFSA) similarly requires detailed molecular characterization of engineered enzymes, with particular attention to modifications affecting post-translational processes like phosphorylation.
Environmental regulations present another critical dimension, particularly for industrial applications. Engineered enzymes released into the environment must be assessed for ecological impact, biodegradability, and potential gene transfer risks. The EPA in the United States and the European Chemicals Agency have established protocols for evaluating the environmental safety of biocatalysts, including those with modified phosphorylation patterns.
Intellectual property considerations also intersect with regulatory frameworks. Patents covering specific phosphorylation optimization techniques may influence regulatory strategy, particularly regarding biosimilars and generic enzyme products. Companies must navigate both patent exclusivity periods and regulatory approval pathways when developing competitive enzyme technologies.
Emerging regulatory trends indicate increasing scrutiny of synthetic biology approaches to enzyme engineering. Regulatory bodies are developing new frameworks to address CRISPR-based enzyme modifications and directed evolution techniques that target phosphorylation sites. These frameworks aim to balance innovation with appropriate risk assessment methodologies tailored to novel engineering approaches.
Cross-border regulatory harmonization remains challenging, with significant variations in how different jurisdictions approach engineered enzyme regulation. Companies pursuing global markets for phosphorylation-optimized enzymes must develop comprehensive regulatory strategies that address these regional differences while maintaining consistent quality and safety standards.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!