Measure Phosphorylation Turnover in Living Cells
SEP 23, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Phosphorylation Turnover Measurement Background and Objectives
Protein phosphorylation represents one of the most critical post-translational modifications in cellular signaling pathways, regulating diverse biological processes including cell growth, differentiation, metabolism, and apoptosis. The dynamic nature of phosphorylation events, characterized by continuous cycles of phosphorylation and dephosphorylation, forms the foundation of cellular signal transduction mechanisms. Understanding these dynamics, particularly phosphorylation turnover rates, provides crucial insights into cellular regulation under both normal and pathological conditions.
Historically, phosphorylation studies have evolved from static measurements using biochemical assays to more sophisticated approaches enabling temporal resolution. Early techniques relied heavily on radioactive labeling with 32P, followed by protein extraction and analysis, which provided limited temporal information and required cell disruption. The field progressed with the development of phospho-specific antibodies and mass spectrometry techniques, allowing for more comprehensive phosphoproteome analysis but still lacking real-time capabilities in intact cells.
The emergence of fluorescence-based technologies in the late 1990s and early 2000s marked a significant advancement, enabling researchers to visualize phosphorylation events in living cells. Fluorescence resonance energy transfer (FRET)-based biosensors represented a breakthrough, allowing for spatiotemporal monitoring of specific phosphorylation events. However, these approaches primarily focused on detecting phosphorylation status rather than quantifying turnover rates.
Recent technological innovations have shifted focus toward measuring phosphorylation turnover—the rates at which phosphate groups are added and removed from proteins—providing a more dynamic view of cellular signaling. This parameter is particularly important as alterations in phosphorylation turnover, rather than steady-state levels, often underlie disease mechanisms and therapeutic responses.
The primary objective of phosphorylation turnover measurement technologies is to quantitatively assess the kinetics of phosphorylation/dephosphorylation cycles in living cells with minimal perturbation to normal cellular functions. This includes developing methods that offer high temporal resolution, single-cell sensitivity, and the ability to track multiple phosphorylation events simultaneously.
Additional goals include establishing standardized approaches for measuring turnover rates across different cellular contexts, developing computational models to interpret turnover data in the context of signaling networks, and creating tools accessible to researchers without specialized expertise in biophysics or advanced imaging techniques.
Understanding phosphorylation turnover has profound implications for drug discovery and development, particularly for kinase inhibitors and phosphatase modulators. By characterizing how disease states alter phosphorylation dynamics and how therapeutic interventions restore normal turnover rates, researchers aim to develop more effective targeted therapies with reduced off-target effects.
Historically, phosphorylation studies have evolved from static measurements using biochemical assays to more sophisticated approaches enabling temporal resolution. Early techniques relied heavily on radioactive labeling with 32P, followed by protein extraction and analysis, which provided limited temporal information and required cell disruption. The field progressed with the development of phospho-specific antibodies and mass spectrometry techniques, allowing for more comprehensive phosphoproteome analysis but still lacking real-time capabilities in intact cells.
The emergence of fluorescence-based technologies in the late 1990s and early 2000s marked a significant advancement, enabling researchers to visualize phosphorylation events in living cells. Fluorescence resonance energy transfer (FRET)-based biosensors represented a breakthrough, allowing for spatiotemporal monitoring of specific phosphorylation events. However, these approaches primarily focused on detecting phosphorylation status rather than quantifying turnover rates.
Recent technological innovations have shifted focus toward measuring phosphorylation turnover—the rates at which phosphate groups are added and removed from proteins—providing a more dynamic view of cellular signaling. This parameter is particularly important as alterations in phosphorylation turnover, rather than steady-state levels, often underlie disease mechanisms and therapeutic responses.
The primary objective of phosphorylation turnover measurement technologies is to quantitatively assess the kinetics of phosphorylation/dephosphorylation cycles in living cells with minimal perturbation to normal cellular functions. This includes developing methods that offer high temporal resolution, single-cell sensitivity, and the ability to track multiple phosphorylation events simultaneously.
Additional goals include establishing standardized approaches for measuring turnover rates across different cellular contexts, developing computational models to interpret turnover data in the context of signaling networks, and creating tools accessible to researchers without specialized expertise in biophysics or advanced imaging techniques.
Understanding phosphorylation turnover has profound implications for drug discovery and development, particularly for kinase inhibitors and phosphatase modulators. By characterizing how disease states alter phosphorylation dynamics and how therapeutic interventions restore normal turnover rates, researchers aim to develop more effective targeted therapies with reduced off-target effects.
Market Demand for Live-Cell Phosphorylation Analysis
The global market for live-cell phosphorylation analysis technologies has experienced significant growth in recent years, driven primarily by increasing research activities in cell signaling pathways and their role in disease mechanisms. Current market estimates value this segment at approximately 2.5 billion USD, with a projected annual growth rate of 8-10% over the next five years.
Pharmaceutical and biotechnology companies represent the largest market segment, accounting for nearly 45% of the total demand. These organizations require advanced phosphorylation monitoring tools to accelerate drug discovery processes, particularly for kinase inhibitors and signal transduction modulators. The ability to observe phosphorylation dynamics in real-time provides crucial insights into drug efficacy and mechanism of action, significantly reducing development timelines and costs.
Academic and research institutions constitute the second-largest market segment, representing about 35% of the demand. The growing focus on understanding fundamental cellular processes and disease mechanisms has intensified the need for sophisticated phosphorylation analysis tools in basic research settings. Grant funding specifically targeting cellular signaling research has increased by approximately 15% in the past three years across major research economies.
Clinical diagnostics represents an emerging application area with substantial growth potential. The ability to monitor phosphorylation events in patient samples could enable more precise disease classification and treatment selection, particularly in oncology. Several major diagnostic companies have initiated development programs for phosphorylation-based companion diagnostics, signaling future market expansion in this direction.
Geographically, North America dominates the market with approximately 40% share, followed by Europe (30%) and Asia-Pacific (25%). However, the Asia-Pacific region is experiencing the fastest growth rate, driven by increasing R&D investments in China, Japan, and South Korea.
Key market drivers include the rising prevalence of chronic diseases with dysregulated signaling pathways, growing investments in proteomics research, and technological advancements enabling higher sensitivity and throughput. The demand for multiplexed analysis capabilities—allowing simultaneous monitoring of multiple phosphorylation events—has become particularly pronounced as researchers seek to understand complex signaling networks.
Market challenges include the high cost of advanced instrumentation, technical complexity requiring specialized expertise, and competition from alternative technologies such as fixed-cell assays. Additionally, there remains significant unmet need for standardized protocols and reagents that can reliably quantify phosphorylation turnover rates across diverse experimental conditions.
Pharmaceutical and biotechnology companies represent the largest market segment, accounting for nearly 45% of the total demand. These organizations require advanced phosphorylation monitoring tools to accelerate drug discovery processes, particularly for kinase inhibitors and signal transduction modulators. The ability to observe phosphorylation dynamics in real-time provides crucial insights into drug efficacy and mechanism of action, significantly reducing development timelines and costs.
Academic and research institutions constitute the second-largest market segment, representing about 35% of the demand. The growing focus on understanding fundamental cellular processes and disease mechanisms has intensified the need for sophisticated phosphorylation analysis tools in basic research settings. Grant funding specifically targeting cellular signaling research has increased by approximately 15% in the past three years across major research economies.
Clinical diagnostics represents an emerging application area with substantial growth potential. The ability to monitor phosphorylation events in patient samples could enable more precise disease classification and treatment selection, particularly in oncology. Several major diagnostic companies have initiated development programs for phosphorylation-based companion diagnostics, signaling future market expansion in this direction.
Geographically, North America dominates the market with approximately 40% share, followed by Europe (30%) and Asia-Pacific (25%). However, the Asia-Pacific region is experiencing the fastest growth rate, driven by increasing R&D investments in China, Japan, and South Korea.
Key market drivers include the rising prevalence of chronic diseases with dysregulated signaling pathways, growing investments in proteomics research, and technological advancements enabling higher sensitivity and throughput. The demand for multiplexed analysis capabilities—allowing simultaneous monitoring of multiple phosphorylation events—has become particularly pronounced as researchers seek to understand complex signaling networks.
Market challenges include the high cost of advanced instrumentation, technical complexity requiring specialized expertise, and competition from alternative technologies such as fixed-cell assays. Additionally, there remains significant unmet need for standardized protocols and reagents that can reliably quantify phosphorylation turnover rates across diverse experimental conditions.
Current Challenges in Cellular Phosphorylation Detection
Despite significant advancements in phosphorylation detection technologies, researchers continue to face substantial challenges when attempting to measure phosphorylation turnover in living cells. One of the primary obstacles is achieving sufficient temporal resolution to capture rapid phosphorylation events that often occur within seconds to minutes. Current methods frequently require cell fixation or lysis, which only provide static snapshots rather than dynamic information about phosphorylation turnover rates.
Spatial resolution presents another significant challenge, as phosphorylation events are often localized to specific subcellular compartments. Conventional techniques struggle to distinguish between phosphorylation occurring in different cellular regions, limiting our understanding of compartment-specific signaling dynamics. This spatial limitation becomes particularly problematic when studying proteins that shuttle between different cellular locations during signaling events.
Signal-to-noise ratio remains a persistent issue, especially when detecting low-abundance phosphoproteins. Background fluorescence and non-specific binding of detection reagents can mask genuine phosphorylation signals, leading to false positives or negatives. This challenge is magnified in living cells where autofluorescence from cellular components further complicates accurate detection.
The multiplexing capability of current technologies is also limited, making it difficult to simultaneously monitor multiple phosphorylation events within the same cell. Since cellular signaling pathways involve complex networks with numerous phosphorylation events occurring in parallel, this limitation significantly hampers comprehensive pathway analysis in real-time.
Probe development faces considerable hurdles as well. Creating phospho-specific probes that can enter living cells without disrupting normal cellular functions remains technically challenging. Many existing probes suffer from poor cell permeability, cytotoxicity, or insufficient specificity for particular phosphorylation sites, restricting their utility in live-cell applications.
Quantification accuracy represents another major challenge. Converting fluorescence or other detection signals into absolute phosphorylation levels requires complex calibration procedures that are difficult to standardize across different experimental setups. This leads to variability in reported phosphorylation rates between laboratories and complicates data interpretation.
Finally, there are significant technical barriers to long-term imaging of phosphorylation dynamics. Phototoxicity and photobleaching limit the duration of live-cell imaging experiments, while maintaining physiological conditions during extended imaging sessions presents additional complications. These factors collectively restrict our ability to track phosphorylation turnover over biologically relevant timescales.
Spatial resolution presents another significant challenge, as phosphorylation events are often localized to specific subcellular compartments. Conventional techniques struggle to distinguish between phosphorylation occurring in different cellular regions, limiting our understanding of compartment-specific signaling dynamics. This spatial limitation becomes particularly problematic when studying proteins that shuttle between different cellular locations during signaling events.
Signal-to-noise ratio remains a persistent issue, especially when detecting low-abundance phosphoproteins. Background fluorescence and non-specific binding of detection reagents can mask genuine phosphorylation signals, leading to false positives or negatives. This challenge is magnified in living cells where autofluorescence from cellular components further complicates accurate detection.
The multiplexing capability of current technologies is also limited, making it difficult to simultaneously monitor multiple phosphorylation events within the same cell. Since cellular signaling pathways involve complex networks with numerous phosphorylation events occurring in parallel, this limitation significantly hampers comprehensive pathway analysis in real-time.
Probe development faces considerable hurdles as well. Creating phospho-specific probes that can enter living cells without disrupting normal cellular functions remains technically challenging. Many existing probes suffer from poor cell permeability, cytotoxicity, or insufficient specificity for particular phosphorylation sites, restricting their utility in live-cell applications.
Quantification accuracy represents another major challenge. Converting fluorescence or other detection signals into absolute phosphorylation levels requires complex calibration procedures that are difficult to standardize across different experimental setups. This leads to variability in reported phosphorylation rates between laboratories and complicates data interpretation.
Finally, there are significant technical barriers to long-term imaging of phosphorylation dynamics. Phototoxicity and photobleaching limit the duration of live-cell imaging experiments, while maintaining physiological conditions during extended imaging sessions presents additional complications. These factors collectively restrict our ability to track phosphorylation turnover over biologically relevant timescales.
Current Methodologies for Phosphorylation Dynamics Monitoring
01 Methods for measuring protein phosphorylation turnover rates
Various techniques and assays have been developed to measure the rate at which proteins undergo phosphorylation and dephosphorylation cycles. These methods include mass spectrometry-based approaches, fluorescence-based assays, and isotope labeling techniques that can track the addition and removal of phosphate groups on proteins over time. These measurements provide insights into cellular signaling dynamics and protein regulation mechanisms.- Methods for measuring protein phosphorylation turnover rates: Various techniques and assays have been developed to measure the rate at which proteins undergo phosphorylation and dephosphorylation cycles. These methods include mass spectrometry-based approaches, fluorescence-based assays, and isotope labeling techniques that can track the addition and removal of phosphate groups on target proteins. These techniques allow researchers to quantify the dynamic nature of protein phosphorylation and understand how quickly phosphorylation states change under different cellular conditions.
- Regulation of phosphorylation turnover in disease states: Abnormal phosphorylation turnover rates are implicated in various diseases, including cancer, neurodegenerative disorders, and metabolic diseases. The dysregulation of kinase and phosphatase activities can lead to altered phosphorylation dynamics, affecting cellular signaling pathways. Understanding these alterations provides insights into disease mechanisms and potential therapeutic targets. Compounds that can modulate phosphorylation turnover rates have been developed as potential treatments for these conditions.
- Biomarkers based on phosphorylation turnover rates: Phosphorylation turnover rates can serve as biomarkers for disease diagnosis, prognosis, and treatment response monitoring. Changes in the dynamics of protein phosphorylation can indicate cellular dysfunction before other symptoms appear. Technologies have been developed to detect and quantify these changes in clinical samples, enabling earlier and more accurate disease detection. These biomarkers are particularly valuable in cancer diagnostics and monitoring treatment efficacy.
- Computational models for predicting phosphorylation turnover: Advanced computational models have been developed to predict and analyze phosphorylation turnover rates in complex biological systems. These models integrate data from multiple sources, including proteomics, genomics, and metabolomics, to simulate the dynamics of phosphorylation networks. Machine learning algorithms are employed to identify patterns and predict how phosphorylation turnover rates might change under different conditions or in response to specific treatments, aiding in drug development and personalized medicine approaches.
- Tools and reagents for studying phosphorylation dynamics: Specialized tools and reagents have been developed specifically for studying phosphorylation turnover rates in research settings. These include phospho-specific antibodies, engineered kinase and phosphatase variants, phosphorylation-sensitive fluorescent reporters, and chemical probes that can track phosphorylation events in real-time. These tools enable researchers to visualize and quantify phosphorylation dynamics in living cells, providing insights into fundamental biological processes and facilitating drug discovery efforts targeting phosphorylation-dependent pathways.
02 Phosphorylation turnover in disease mechanisms and drug development
Abnormal phosphorylation turnover rates are implicated in various diseases, particularly cancer and neurodegenerative disorders. Research focuses on understanding how altered phosphorylation dynamics contribute to disease progression and identifying compounds that can modulate these rates for therapeutic purposes. Drug development strategies target specific kinases or phosphatases to normalize phosphorylation turnover rates in diseased cells.Expand Specific Solutions03 Computational models for phosphorylation turnover analysis
Advanced computational approaches have been developed to analyze and predict phosphorylation turnover rates in complex biological systems. These models integrate experimental data with mathematical algorithms to simulate phosphorylation dynamics under various conditions. Machine learning and artificial intelligence techniques are increasingly being applied to interpret large-scale phosphoproteomics data and predict how phosphorylation turnover rates affect cellular functions.Expand Specific Solutions04 Biomarkers based on phosphorylation turnover rates
Phosphorylation turnover rates of specific proteins can serve as biomarkers for disease diagnosis, prognosis, and treatment response monitoring. Research has identified signature patterns of phosphorylation dynamics that correlate with particular disease states or therapeutic outcomes. Diagnostic methods based on measuring these turnover rates in patient samples are being developed for clinical applications.Expand Specific Solutions05 Regulation of cellular processes through phosphorylation turnover
Phosphorylation turnover rates play crucial roles in regulating various cellular processes including cell cycle progression, metabolism, and signal transduction. The balance between kinase and phosphatase activities determines these rates, creating dynamic control systems that allow cells to respond to environmental changes. Research has revealed how different proteins exhibit distinct phosphorylation turnover rates that are finely tuned to their specific functions in cellular pathways.Expand Specific Solutions
Key Players in Live-Cell Imaging and Biosensor Development
The phosphorylation turnover measurement in living cells market is currently in a growth phase, with increasing adoption across pharmaceutical research and academic institutions. The global market size is expanding steadily, driven by rising demand for real-time cellular analysis in drug discovery and development. Technologically, this field shows moderate maturity with established players like Agilent Technologies offering analytical instrumentation, while pharmaceutical giants including Bayer AG, F. Hoffmann-La Roche, and Merck Sharp & Dohme invest in application development. Academic institutions such as The Broad Institute and University of California contribute significant research advancements. The competitive landscape features specialized equipment manufacturers alongside research-focused pharmaceutical companies, with emerging collaboration between industry and academic sectors driving innovation in phosphorylation dynamics monitoring technologies.
Agilent Technologies, Inc.
Technical Solution: Agilent Technologies has pioneered the development of integrated mass spectrometry-based solutions for measuring phosphorylation turnover in living cells. Their Phosphorylation Dynamics Monitoring System combines liquid chromatography with triple quadrupole mass spectrometry (LC-MS/MS) to achieve sensitive detection of phosphopeptides. The technology employs ATP analogs containing stable isotopes that can be metabolically incorporated into phosphorylation sites, allowing precise tracking of phosphorylation turnover rates. Agilent's system features their proprietary Jet Stream ESI technology that enhances ionization efficiency for phosphopeptides, improving detection sensitivity by up to 5-10 fold compared to conventional ESI sources. The platform includes specialized software for kinetic modeling of phosphorylation/dephosphorylation rates, enabling researchers to determine half-lives of specific phosphorylation events and identify regulatory mechanisms controlling protein phosphorylation dynamics in cellular signaling networks.
Strengths: Exceptional sensitivity for detecting low-abundance phosphopeptides; comprehensive workflow from sample preparation to data analysis; robust quantification capabilities. Weaknesses: Requires significant technical expertise; sample preparation can be labor-intensive; higher initial investment compared to antibody-based methods.
The Regents of the University of California
Technical Solution: The University of California has pioneered innovative approaches for measuring phosphorylation turnover in living cells through their development of the PhosphoFlow platform. This technology combines flow cytometry with phospho-specific antibodies to quantify phosphorylation states at the single-cell level with high temporal resolution. Their method incorporates metabolic labeling with heavy isotope-containing amino acids (similar to SILAC) but with rapid fixation timepoints to capture phosphorylation dynamics. The UC system has further enhanced this approach by developing cell-permeable phosphatase-resistant ATP analogs that allow pulse-chase experiments to track phosphorylation turnover rates. Their latest innovation includes a microfluidic-based system that enables continuous monitoring of phosphorylation changes in response to stimuli with temporal resolution in the second range. The platform integrates with computational models that can extract rate constants for both phosphorylation and dephosphorylation reactions, providing insights into the kinetics of signaling networks.
Strengths: Single-cell resolution allowing analysis of heterogeneous cell populations; excellent statistical power through high-throughput flow cytometry; ability to simultaneously measure multiple phosphorylation sites. Weaknesses: Limited to phosphorylation sites with available specific antibodies; requires cell fixation for most applications; challenging to obtain spatial information within cells.
Critical Technologies for Real-Time Phosphorylation Analysis
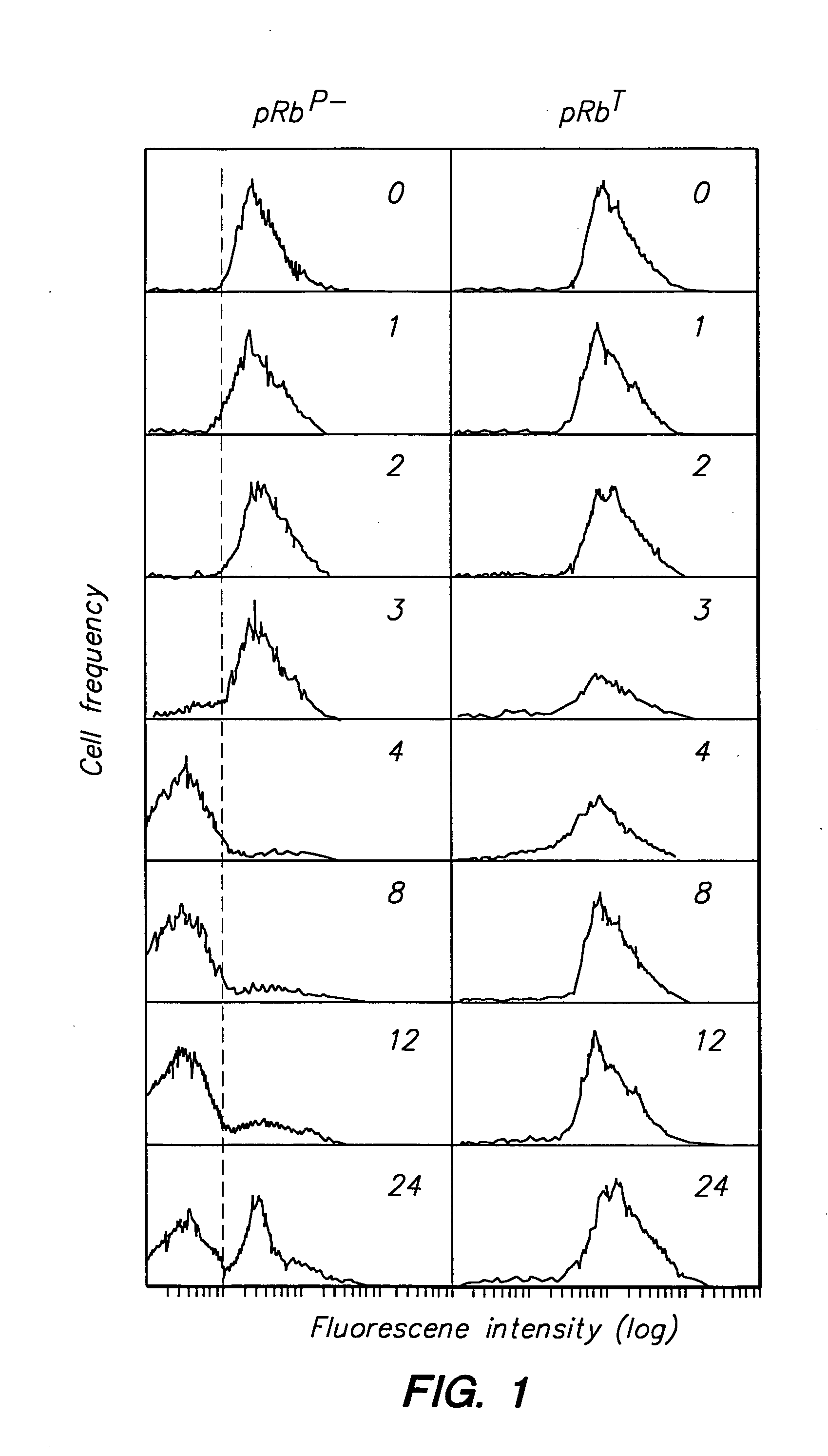
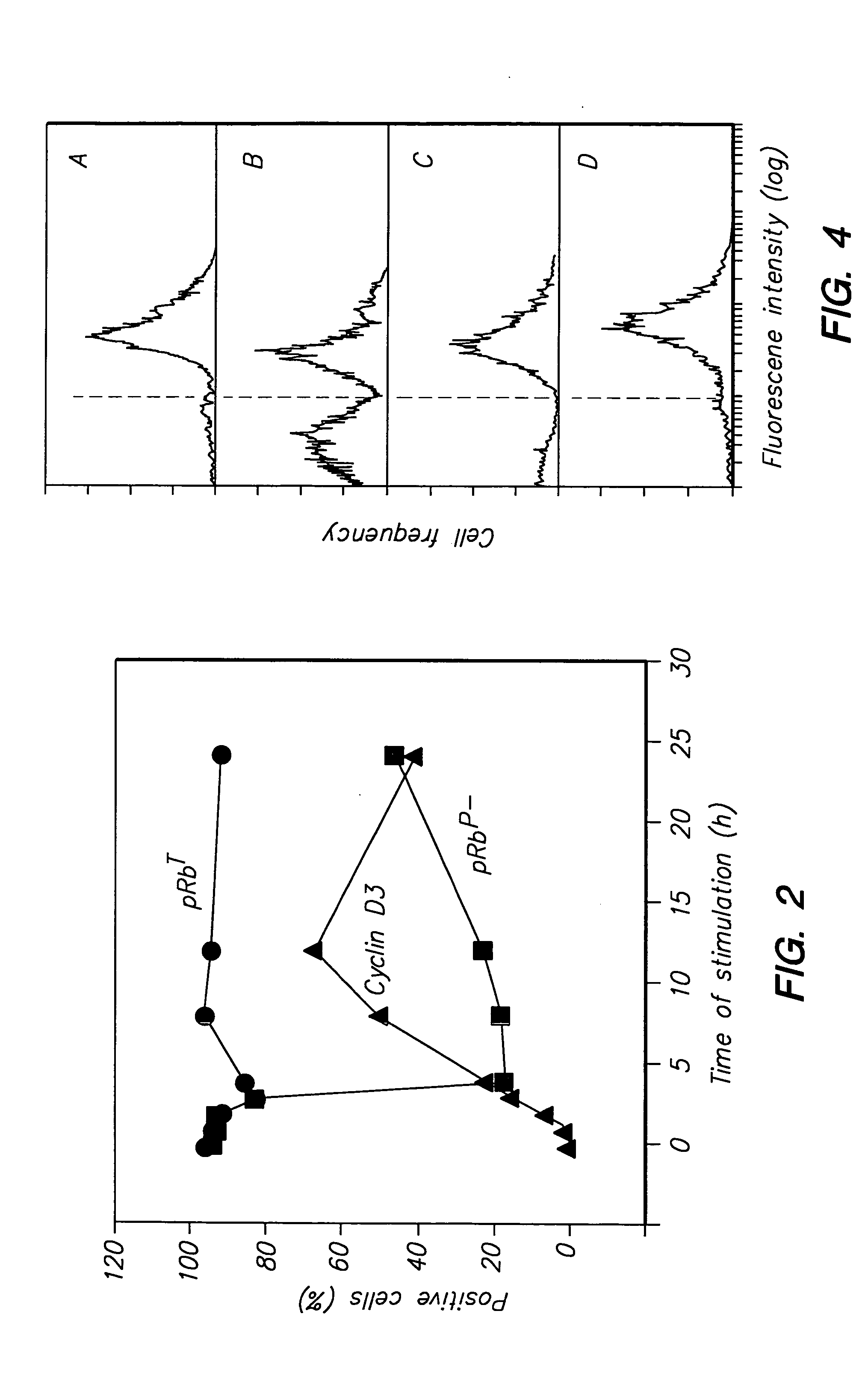
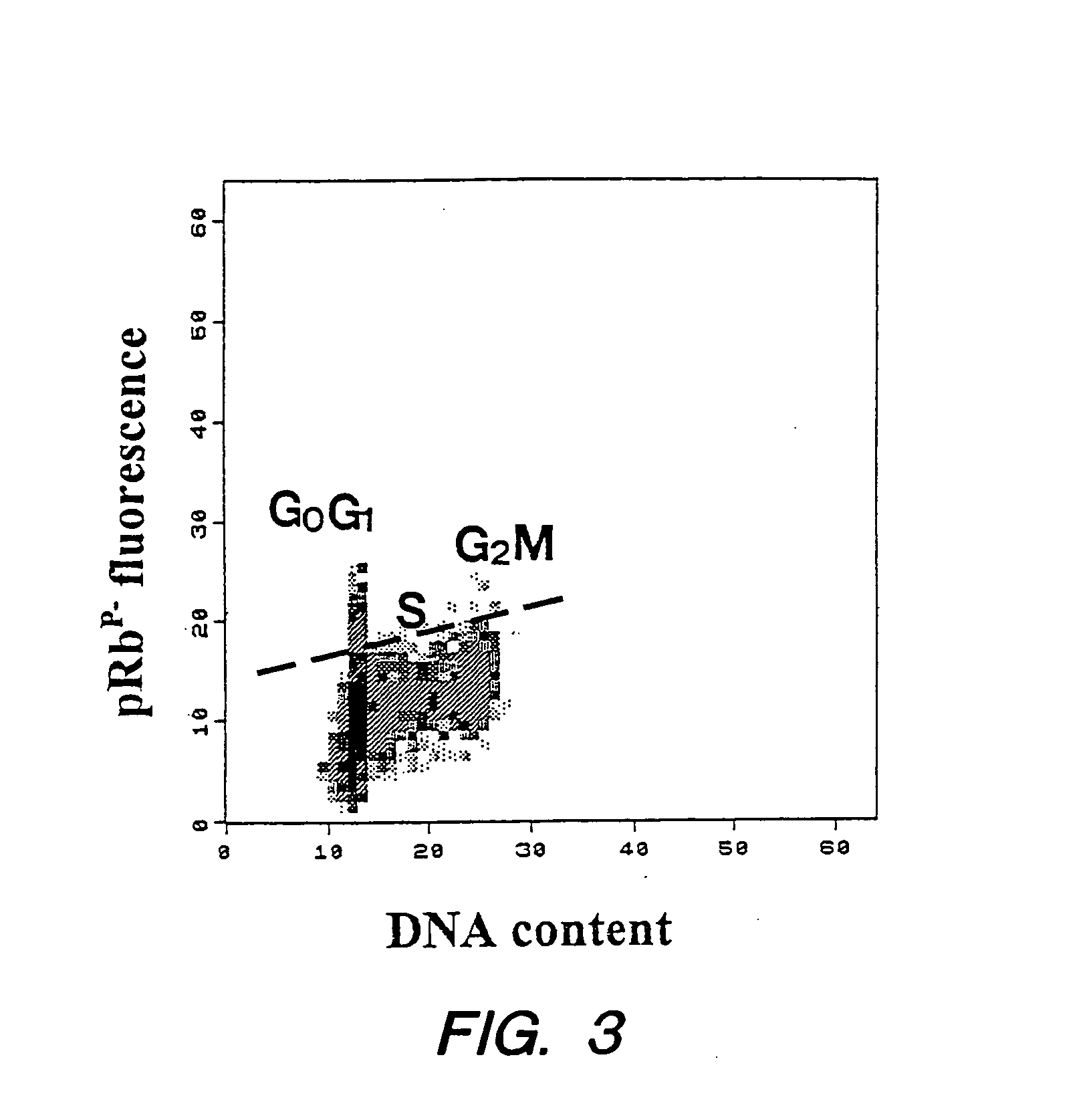
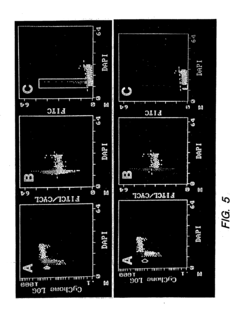
Detection of protein conformations in single cells
PatentInactiveUS20050042694A1
Innovation
- Development of methods and reagents that allow for the concurrent and discriminable detection of discrete functional conformations of pRB in individual cells using fluorophore-conjugated antibodies and flow cytometry, enabling the assessment of pRB phosphorylation states without artificial synchronization of the cell cycle.
Method and device for measuring multiple physiological properties of cells
PatentInactiveUS7638321B2
Innovation
- A method and apparatus that temporarily create a closed sample chamber within a larger vessel, allowing for high cell density measurements while maintaining low cell density for health, using sensors to analyze analytes without active perfusion, enabling rapid and quantitative flux rate determination of extracellular analytes.
Regulatory Considerations for Cell-Based Assay Technologies
Cell-based assay technologies used for measuring phosphorylation turnover in living cells must navigate a complex regulatory landscape. In the United States, the FDA's Center for Devices and Radiological Health (CDRH) oversees the regulation of diagnostic assays, with specific guidelines for cell-based technologies under 21 CFR Part 864. These regulations establish requirements for validation, quality control, and performance characteristics that must be demonstrated before commercial deployment.
European regulatory frameworks, primarily through the In Vitro Diagnostic Regulation (IVDR 2017/746), impose stringent requirements on cell-based assays, particularly those used in clinical settings. The IVDR's risk-based classification system places most phosphorylation measurement technologies in Class C or D, necessitating conformity assessment by notified bodies and comprehensive technical documentation.
Laboratories implementing these technologies must also consider compliance with CLIA (Clinical Laboratory Improvement Amendments) in the US or equivalent laboratory standards internationally. These regulations mandate specific quality management systems, personnel qualifications, and proficiency testing requirements that directly impact the implementation of phosphorylation turnover assays.
Data privacy considerations present another regulatory dimension, especially when patient samples are involved. HIPAA in the US and GDPR in Europe establish strict protocols for handling, storing, and transmitting data generated from cell-based assays, requiring robust data management systems and security protocols.
Intellectual property protection represents a significant regulatory consideration, with many phosphorylation measurement technologies protected by patents. Organizations must conduct thorough freedom-to-operate analyses before implementing these technologies to avoid infringement issues.
Emerging regulatory trends indicate increasing scrutiny of artificial intelligence and machine learning components often integrated into advanced cell analysis platforms. Regulatory bodies are developing frameworks to address the validation of algorithms used in image analysis and data interpretation for phosphorylation turnover measurements.
International harmonization efforts, such as those by the International Medical Device Regulators Forum (IMDRF), are working to standardize requirements across jurisdictions, potentially simplifying the regulatory pathway for novel cell-based assay technologies while maintaining rigorous safety and efficacy standards.
European regulatory frameworks, primarily through the In Vitro Diagnostic Regulation (IVDR 2017/746), impose stringent requirements on cell-based assays, particularly those used in clinical settings. The IVDR's risk-based classification system places most phosphorylation measurement technologies in Class C or D, necessitating conformity assessment by notified bodies and comprehensive technical documentation.
Laboratories implementing these technologies must also consider compliance with CLIA (Clinical Laboratory Improvement Amendments) in the US or equivalent laboratory standards internationally. These regulations mandate specific quality management systems, personnel qualifications, and proficiency testing requirements that directly impact the implementation of phosphorylation turnover assays.
Data privacy considerations present another regulatory dimension, especially when patient samples are involved. HIPAA in the US and GDPR in Europe establish strict protocols for handling, storing, and transmitting data generated from cell-based assays, requiring robust data management systems and security protocols.
Intellectual property protection represents a significant regulatory consideration, with many phosphorylation measurement technologies protected by patents. Organizations must conduct thorough freedom-to-operate analyses before implementing these technologies to avoid infringement issues.
Emerging regulatory trends indicate increasing scrutiny of artificial intelligence and machine learning components often integrated into advanced cell analysis platforms. Regulatory bodies are developing frameworks to address the validation of algorithms used in image analysis and data interpretation for phosphorylation turnover measurements.
International harmonization efforts, such as those by the International Medical Device Regulators Forum (IMDRF), are working to standardize requirements across jurisdictions, potentially simplifying the regulatory pathway for novel cell-based assay technologies while maintaining rigorous safety and efficacy standards.
Translational Applications in Drug Discovery and Development
Phosphorylation turnover measurement technologies have significant translational applications in drug discovery and development processes. The ability to monitor protein phosphorylation dynamics in living cells provides crucial insights for pharmaceutical companies developing kinase inhibitors and other drugs targeting phosphorylation-dependent pathways.
In early-stage drug discovery, these measurement techniques enable high-throughput screening of compound libraries to identify molecules that modulate specific phosphorylation events. This application has revolutionized the identification of lead compounds, particularly for diseases where aberrant phosphorylation plays a pathological role, such as cancer, neurodegenerative disorders, and inflammatory conditions.
For lead optimization, real-time phosphorylation turnover measurements allow medicinal chemists to rapidly assess structure-activity relationships and optimize compound efficacy. The ability to observe immediate effects on phosphorylation dynamics provides valuable feedback for iterative compound design, significantly accelerating the optimization process compared to traditional endpoint assays.
These technologies also enhance target validation by confirming that observed phenotypic changes correlate with modulation of specific phosphorylation events. This application is particularly valuable for novel drug targets where the relationship between phosphorylation status and disease pathology requires validation before significant investment in drug development.
In preclinical development, phosphorylation turnover measurements facilitate mechanism-of-action studies, helping researchers understand how candidate drugs affect complex signaling networks. This knowledge is essential for predicting potential off-target effects and toxicity issues before advancing to clinical trials, potentially saving substantial resources by identifying problematic compounds earlier.
The technology also supports biomarker development by identifying phosphorylation events that reliably indicate drug activity. These pharmacodynamic biomarkers can subsequently be used in clinical trials to confirm target engagement and biological activity at different dosages, supporting dose selection and providing early indications of efficacy.
Furthermore, phosphorylation turnover measurement techniques are increasingly employed in patient stratification strategies, identifying individuals whose disease exhibits specific phosphorylation patterns that may predict responsiveness to targeted therapies. This application aligns with the growing emphasis on precision medicine approaches in drug development, potentially improving clinical trial success rates by focusing on patient populations most likely to benefit.
In early-stage drug discovery, these measurement techniques enable high-throughput screening of compound libraries to identify molecules that modulate specific phosphorylation events. This application has revolutionized the identification of lead compounds, particularly for diseases where aberrant phosphorylation plays a pathological role, such as cancer, neurodegenerative disorders, and inflammatory conditions.
For lead optimization, real-time phosphorylation turnover measurements allow medicinal chemists to rapidly assess structure-activity relationships and optimize compound efficacy. The ability to observe immediate effects on phosphorylation dynamics provides valuable feedback for iterative compound design, significantly accelerating the optimization process compared to traditional endpoint assays.
These technologies also enhance target validation by confirming that observed phenotypic changes correlate with modulation of specific phosphorylation events. This application is particularly valuable for novel drug targets where the relationship between phosphorylation status and disease pathology requires validation before significant investment in drug development.
In preclinical development, phosphorylation turnover measurements facilitate mechanism-of-action studies, helping researchers understand how candidate drugs affect complex signaling networks. This knowledge is essential for predicting potential off-target effects and toxicity issues before advancing to clinical trials, potentially saving substantial resources by identifying problematic compounds earlier.
The technology also supports biomarker development by identifying phosphorylation events that reliably indicate drug activity. These pharmacodynamic biomarkers can subsequently be used in clinical trials to confirm target engagement and biological activity at different dosages, supporting dose selection and providing early indications of efficacy.
Furthermore, phosphorylation turnover measurement techniques are increasingly employed in patient stratification strategies, identifying individuals whose disease exhibits specific phosphorylation patterns that may predict responsiveness to targeted therapies. This application aligns with the growing emphasis on precision medicine approaches in drug development, potentially improving clinical trial success rates by focusing on patient populations most likely to benefit.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!