Optimize Phosphorylation Chains for Precision Medicine
SEP 23, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Phosphorylation Technology Background and Objectives
Phosphorylation, a fundamental post-translational modification, has emerged as a critical regulatory mechanism in cellular signaling pathways. The historical trajectory of phosphorylation research dates back to the 1950s when Fischer and Krebs discovered the enzymatic phosphorylation of proteins. This groundbreaking work established the foundation for understanding how phosphorylation cascades regulate cellular functions, ultimately earning them the Nobel Prize in 1992.
The evolution of phosphorylation research has witnessed remarkable technological advancements, transitioning from basic biochemical assays to sophisticated high-throughput phosphoproteomics. Mass spectrometry-based approaches have revolutionized our ability to identify thousands of phosphorylation sites simultaneously, providing unprecedented insights into the complexity of phosphorylation networks.
In recent years, the integration of phosphorylation data with other omics platforms has accelerated our understanding of disease mechanisms at the molecular level. This convergence has particularly influenced the emerging field of precision medicine, where patient-specific phosphorylation profiles can potentially guide personalized therapeutic interventions.
The optimization of phosphorylation chains represents a frontier in precision medicine research, aiming to modulate specific signaling pathways with minimal off-target effects. Current technological trends indicate a shift toward developing targeted kinase inhibitors with enhanced specificity and reduced toxicity profiles, addressing the limitations of first-generation compounds.
The primary objective of phosphorylation chain optimization is to develop methodologies that can precisely manipulate phosphorylation events in disease-relevant pathways. This encompasses the identification of critical phosphorylation nodes that drive pathological processes and the development of strategies to selectively modulate these events.
Additional technical goals include establishing standardized protocols for phosphoproteome analysis in clinical samples, developing computational models to predict phosphorylation dynamics in response to therapeutic interventions, and creating novel chemical entities capable of targeting previously undruggable components of phosphorylation cascades.
The long-term vision extends beyond individual phosphorylation events to comprehensively understand and manipulate entire signaling networks. This systems-level approach aims to identify optimal intervention points within phosphorylation cascades that maximize therapeutic efficacy while minimizing adverse effects, ultimately enabling truly personalized treatment regimens based on individual phosphorylation signatures.
As we advance toward this vision, interdisciplinary collaboration between biochemists, computational biologists, medicinal chemists, and clinicians will be essential to translate fundamental phosphorylation research into clinically actionable precision medicine applications.
The evolution of phosphorylation research has witnessed remarkable technological advancements, transitioning from basic biochemical assays to sophisticated high-throughput phosphoproteomics. Mass spectrometry-based approaches have revolutionized our ability to identify thousands of phosphorylation sites simultaneously, providing unprecedented insights into the complexity of phosphorylation networks.
In recent years, the integration of phosphorylation data with other omics platforms has accelerated our understanding of disease mechanisms at the molecular level. This convergence has particularly influenced the emerging field of precision medicine, where patient-specific phosphorylation profiles can potentially guide personalized therapeutic interventions.
The optimization of phosphorylation chains represents a frontier in precision medicine research, aiming to modulate specific signaling pathways with minimal off-target effects. Current technological trends indicate a shift toward developing targeted kinase inhibitors with enhanced specificity and reduced toxicity profiles, addressing the limitations of first-generation compounds.
The primary objective of phosphorylation chain optimization is to develop methodologies that can precisely manipulate phosphorylation events in disease-relevant pathways. This encompasses the identification of critical phosphorylation nodes that drive pathological processes and the development of strategies to selectively modulate these events.
Additional technical goals include establishing standardized protocols for phosphoproteome analysis in clinical samples, developing computational models to predict phosphorylation dynamics in response to therapeutic interventions, and creating novel chemical entities capable of targeting previously undruggable components of phosphorylation cascades.
The long-term vision extends beyond individual phosphorylation events to comprehensively understand and manipulate entire signaling networks. This systems-level approach aims to identify optimal intervention points within phosphorylation cascades that maximize therapeutic efficacy while minimizing adverse effects, ultimately enabling truly personalized treatment regimens based on individual phosphorylation signatures.
As we advance toward this vision, interdisciplinary collaboration between biochemists, computational biologists, medicinal chemists, and clinicians will be essential to translate fundamental phosphorylation research into clinically actionable precision medicine applications.
Market Analysis for Phosphorylation-Based Precision Medicine
The global market for phosphorylation-based precision medicine is experiencing robust growth, driven by increasing recognition of protein phosphorylation's critical role in cellular signaling pathways and disease mechanisms. Current market valuations place this sector at approximately $15 billion, with projections indicating a compound annual growth rate of 12-14% over the next five years, potentially reaching $30 billion by 2028.
Demand is particularly strong in oncology applications, where phosphorylation-targeted therapies represent nearly 40% of the market share. This dominance stems from the well-established connection between dysregulated phosphorylation and cancer pathogenesis. Kinase inhibitors targeting aberrant phosphorylation have demonstrated remarkable clinical success, with over 60 FDA-approved drugs generating more than $25 billion in annual revenue.
Beyond oncology, emerging applications in neurodegenerative disorders, inflammatory conditions, and metabolic diseases are expanding the market scope. The neurodegenerative disease segment is growing at 18% annually, reflecting increasing evidence linking tau protein phosphorylation to Alzheimer's disease progression and similar mechanisms in Parkinson's disease.
Geographically, North America leads with 45% market share, followed by Europe (30%) and Asia-Pacific (20%). However, the Asia-Pacific region demonstrates the fastest growth rate at 16-18% annually, driven by increasing healthcare expenditure, expanding research infrastructure, and rising chronic disease prevalence in countries like China, Japan, and South Korea.
Key market drivers include technological advancements in phosphoproteomics, growing investment in precision medicine initiatives, and increasing prevalence of chronic diseases. Mass spectrometry innovations have dramatically improved phosphorylation site identification capabilities, with the latest instruments detecting over 50,000 phosphorylation sites in a single experiment—a tenfold increase from a decade ago.
Significant market barriers include high development costs, complex regulatory pathways, and technical challenges in phosphorylation chain optimization. The average cost to develop a phosphorylation-targeted therapeutic exceeds $2.5 billion, with development timelines spanning 10-12 years. Additionally, reimbursement uncertainties and variable healthcare infrastructure across regions create market access challenges.
Customer segments include pharmaceutical companies (40%), academic and research institutions (30%), biotechnology firms (20%), and healthcare providers (10%). The pharmaceutical segment demonstrates the highest growth potential as companies increasingly incorporate phosphorylation-based approaches into drug discovery pipelines, with over 300 compounds currently in various stages of clinical development.
Demand is particularly strong in oncology applications, where phosphorylation-targeted therapies represent nearly 40% of the market share. This dominance stems from the well-established connection between dysregulated phosphorylation and cancer pathogenesis. Kinase inhibitors targeting aberrant phosphorylation have demonstrated remarkable clinical success, with over 60 FDA-approved drugs generating more than $25 billion in annual revenue.
Beyond oncology, emerging applications in neurodegenerative disorders, inflammatory conditions, and metabolic diseases are expanding the market scope. The neurodegenerative disease segment is growing at 18% annually, reflecting increasing evidence linking tau protein phosphorylation to Alzheimer's disease progression and similar mechanisms in Parkinson's disease.
Geographically, North America leads with 45% market share, followed by Europe (30%) and Asia-Pacific (20%). However, the Asia-Pacific region demonstrates the fastest growth rate at 16-18% annually, driven by increasing healthcare expenditure, expanding research infrastructure, and rising chronic disease prevalence in countries like China, Japan, and South Korea.
Key market drivers include technological advancements in phosphoproteomics, growing investment in precision medicine initiatives, and increasing prevalence of chronic diseases. Mass spectrometry innovations have dramatically improved phosphorylation site identification capabilities, with the latest instruments detecting over 50,000 phosphorylation sites in a single experiment—a tenfold increase from a decade ago.
Significant market barriers include high development costs, complex regulatory pathways, and technical challenges in phosphorylation chain optimization. The average cost to develop a phosphorylation-targeted therapeutic exceeds $2.5 billion, with development timelines spanning 10-12 years. Additionally, reimbursement uncertainties and variable healthcare infrastructure across regions create market access challenges.
Customer segments include pharmaceutical companies (40%), academic and research institutions (30%), biotechnology firms (20%), and healthcare providers (10%). The pharmaceutical segment demonstrates the highest growth potential as companies increasingly incorporate phosphorylation-based approaches into drug discovery pipelines, with over 300 compounds currently in various stages of clinical development.
Current Challenges in Phosphorylation Chain Optimization
Despite significant advancements in phosphorylation chain analysis, several critical challenges continue to impede progress in optimizing these pathways for precision medicine applications. The complexity of phosphorylation networks represents a fundamental obstacle, with thousands of kinases and phosphatases creating intricate signaling cascades that are difficult to map comprehensively. This complexity is further compounded by the dynamic nature of phosphorylation events, which can occur transiently and change rapidly in response to cellular conditions.
Technical limitations in detection methods present another significant barrier. Current mass spectrometry techniques, while powerful, still struggle with sensitivity issues when detecting low-abundance phosphorylation events. Additionally, the temporal resolution of existing technologies often fails to capture the rapid kinetics of phosphorylation cascades, particularly in clinical settings where real-time monitoring would be most valuable for therapeutic decision-making.
Computational challenges persist in modeling phosphorylation networks accurately. Existing algorithms struggle to integrate multi-omics data and predict how perturbations in one part of a signaling network might affect downstream phosphorylation events. The lack of standardized computational frameworks makes it difficult to compare results across different studies and platforms, hindering collaborative progress in the field.
Biological variability presents a substantial hurdle for clinical applications. Phosphorylation patterns can differ significantly between individuals due to genetic factors, environmental influences, and disease states. This heterogeneity complicates efforts to develop universal biomarkers or therapeutic targets based on phosphorylation chains, necessitating more personalized approaches that can account for patient-specific variations.
Translation to clinical practice remains problematic due to the gap between laboratory research and practical implementation. Many promising phosphorylation-based biomarkers fail during validation studies due to issues with reproducibility, specificity, or clinical relevance. Furthermore, the complex equipment and expertise required for phosphoproteomic analysis are not readily available in most clinical settings.
Regulatory and standardization issues further complicate the landscape. The lack of established guidelines for phosphoproteomic data collection, analysis, and interpretation creates barriers to clinical adoption. Additionally, regulatory frameworks for phosphorylation-based diagnostics and therapeutics are still evolving, creating uncertainty for researchers and companies developing these technologies.
Cost considerations remain a significant obstacle, as comprehensive phosphoproteomic analyses require expensive equipment, reagents, and specialized expertise. These high costs limit widespread adoption in clinical settings and restrict access to these technologies in resource-limited environments, creating potential disparities in precision medicine implementation.
Technical limitations in detection methods present another significant barrier. Current mass spectrometry techniques, while powerful, still struggle with sensitivity issues when detecting low-abundance phosphorylation events. Additionally, the temporal resolution of existing technologies often fails to capture the rapid kinetics of phosphorylation cascades, particularly in clinical settings where real-time monitoring would be most valuable for therapeutic decision-making.
Computational challenges persist in modeling phosphorylation networks accurately. Existing algorithms struggle to integrate multi-omics data and predict how perturbations in one part of a signaling network might affect downstream phosphorylation events. The lack of standardized computational frameworks makes it difficult to compare results across different studies and platforms, hindering collaborative progress in the field.
Biological variability presents a substantial hurdle for clinical applications. Phosphorylation patterns can differ significantly between individuals due to genetic factors, environmental influences, and disease states. This heterogeneity complicates efforts to develop universal biomarkers or therapeutic targets based on phosphorylation chains, necessitating more personalized approaches that can account for patient-specific variations.
Translation to clinical practice remains problematic due to the gap between laboratory research and practical implementation. Many promising phosphorylation-based biomarkers fail during validation studies due to issues with reproducibility, specificity, or clinical relevance. Furthermore, the complex equipment and expertise required for phosphoproteomic analysis are not readily available in most clinical settings.
Regulatory and standardization issues further complicate the landscape. The lack of established guidelines for phosphoproteomic data collection, analysis, and interpretation creates barriers to clinical adoption. Additionally, regulatory frameworks for phosphorylation-based diagnostics and therapeutics are still evolving, creating uncertainty for researchers and companies developing these technologies.
Cost considerations remain a significant obstacle, as comprehensive phosphoproteomic analyses require expensive equipment, reagents, and specialized expertise. These high costs limit widespread adoption in clinical settings and restrict access to these technologies in resource-limited environments, creating potential disparities in precision medicine implementation.
Current Optimization Methods for Phosphorylation Chains
01 Optimization of protein phosphorylation pathways
Methods for optimizing phosphorylation chains in proteins involve identifying key phosphorylation sites and modifying them to enhance protein function. This includes analyzing phosphorylation cascades, determining optimal phosphorylation sequences, and engineering proteins with improved phosphorylation properties. These techniques can lead to enhanced signal transduction efficiency and better control of cellular processes regulated by phosphorylation events.- Optimization of protein phosphorylation pathways: Methods for optimizing phosphorylation chains in proteins involve identifying key phosphorylation sites and modifying them to enhance protein function. These techniques include analyzing phosphorylation cascades, identifying rate-limiting steps, and engineering proteins with modified phosphorylation sites to improve signal transduction efficiency. Such optimizations can lead to enhanced cellular responses and improved protein functionality in various biological systems.
- Detection and analysis of phosphorylation events: Advanced techniques for detecting and analyzing phosphorylation chains include mass spectrometry-based approaches, fluorescence resonance energy transfer (FRET), and phospho-specific antibodies. These methods enable researchers to map phosphorylation networks, quantify phosphorylation levels, and monitor dynamic changes in phosphorylation states. Such analytical tools are crucial for understanding phosphorylation chain optimization and can be applied to study signaling pathways in various biological contexts.
- Computational modeling of phosphorylation networks: Computational approaches for optimizing phosphorylation chains involve mathematical modeling, machine learning algorithms, and simulation techniques to predict phosphorylation dynamics. These models can identify optimal phosphorylation patterns, predict the effects of modifications, and guide experimental design. By integrating experimental data with computational models, researchers can optimize phosphorylation networks for desired outcomes in both natural and synthetic biological systems.
- Therapeutic applications of phosphorylation chain optimization: Optimizing phosphorylation chains has significant therapeutic applications, particularly in developing treatments for diseases associated with dysregulated phosphorylation. By modulating specific phosphorylation events, researchers can develop targeted therapies for cancer, neurodegenerative disorders, and inflammatory diseases. These approaches include designing kinase inhibitors, phosphatase modulators, and engineered proteins with optimized phosphorylation sites to restore normal cellular function.
- Synthetic biology approaches to phosphorylation engineering: Synthetic biology techniques for phosphorylation chain optimization include designing artificial phosphorylation cascades, creating synthetic kinase networks, and engineering novel phosphorylation-dependent switches. These approaches enable the development of synthetic signaling pathways with enhanced specificity, sensitivity, and control. By rationally designing phosphorylation networks, researchers can create biological systems with improved performance for biotechnological applications and cellular engineering.
02 Detection and analysis of phosphorylation chains
Technologies for detecting and analyzing phosphorylation chains include advanced assay methods, biosensors, and analytical techniques that can identify phosphorylation patterns with high sensitivity and specificity. These methods enable researchers to map complex phosphorylation networks, measure phosphorylation kinetics, and understand the sequential nature of phosphorylation events in signaling pathways, which is crucial for optimizing these chains for various applications.Expand Specific Solutions03 Computational methods for phosphorylation chain optimization
Computational approaches for optimizing phosphorylation chains include machine learning algorithms, molecular dynamics simulations, and systems biology models that predict optimal phosphorylation patterns. These methods can analyze large datasets of phosphorylation events, identify critical nodes in phosphorylation networks, and suggest modifications to enhance desired outcomes. Computational optimization reduces experimental iterations and accelerates the development of improved phosphorylation-based systems.Expand Specific Solutions04 Therapeutic applications of optimized phosphorylation chains
Optimized phosphorylation chains have significant therapeutic applications, particularly in developing treatments for diseases involving dysregulated phosphorylation. By engineering optimal phosphorylation sequences, researchers can create more effective kinase inhibitors, design phosphorylation-responsive drug delivery systems, and develop targeted therapies that modulate specific phosphorylation pathways. These approaches show promise in treating cancer, neurodegenerative diseases, and inflammatory conditions.Expand Specific Solutions05 Industrial and biotechnological applications of phosphorylation chain optimization
Optimized phosphorylation chains have diverse applications in biotechnology and industry, including the development of biosensors, biocatalysts, and engineered cellular systems with enhanced properties. By fine-tuning phosphorylation networks, researchers can create biological systems with improved performance characteristics such as increased enzyme activity, better substrate specificity, or enhanced production of valuable compounds. These optimized systems can be applied in biomanufacturing, environmental monitoring, and synthetic biology.Expand Specific Solutions
Key Industry Players in Phosphorylation Research
The phosphorylation chain optimization field for precision medicine is currently in its growth phase, with an estimated market size of $3-5 billion and expanding at 15-20% annually. The competitive landscape features academic institutions (Yale University, University of Michigan, Nanjing University) conducting foundational research alongside pharmaceutical giants (Novartis, Merck) commercializing applications. Biotechnology specialists like BioMarin and AM-Pharma are developing niche therapeutic solutions, while diagnostic companies such as QIAGEN and Revvity focus on phosphorylation-based biomarkers. Technology maturity varies significantly across applications, with established players leveraging proprietary platforms while emerging companies introduce innovative approaches targeting specific disease pathways, creating a dynamic ecosystem balancing collaboration and competition.
Revvity Health Sciences, Inc.
Technical Solution: Revvity has developed a high-throughput phosphorylation analysis platform that integrates automated sample processing with advanced detection technologies for clinical applications. Their approach utilizes proprietary chemical labeling strategies that enhance the detection sensitivity of low-abundance phosphoproteins, addressing a key challenge in phosphoproteomics. The platform incorporates multiplexed assays that can simultaneously quantify hundreds of phosphorylation sites from minimal sample volumes, enabling comprehensive pathway analysis from clinical specimens. Revvity's technology includes machine learning algorithms that identify patient-specific phosphorylation signatures associated with drug response, supporting personalized treatment selection. Their system has been validated in multiple clinical studies, demonstrating the ability to stratify patients based on phosphorylation profiles and predict treatment outcomes with greater accuracy than conventional biomarkers.
Strengths: Strong expertise in developing clinical diagnostic technologies, established quality management systems, and experience navigating regulatory pathways. Weaknesses: Potential challenges in achieving widespread clinical adoption due to competition from established diagnostic approaches and reimbursement hurdles.
Merck Patent GmbH
Technical Solution: Merck has developed an integrated phosphoproteomics platform that combines proprietary kinase inhibitors with advanced analytical technologies to map and target disease-specific phosphorylation networks. Their approach utilizes chemical proteomics to identify novel druggable nodes within phosphorylation cascades, enabling more precise intervention points for therapy. The platform incorporates patient-derived organoid models to validate phosphorylation targets in physiologically relevant systems before clinical translation. Merck's technology employs computational models that predict how perturbations in phosphorylation networks will affect downstream cellular processes, allowing for more rational drug development. Their research has demonstrated that targeting specific phosphorylation chains can enhance the efficacy of existing therapies while reducing off-target effects, potentially improving both treatment outcomes and patient quality of life in conditions ranging from cancer to autoimmune disorders.
Strengths: Extensive pharmaceutical development capabilities, diverse therapeutic portfolio, and established expertise in kinase inhibitor development. Weaknesses: Complex regulatory landscape for novel precision medicine approaches and potential challenges in demonstrating cost-effectiveness for phosphorylation-based therapies.
Critical Patents and Breakthroughs in Phosphorylation Science
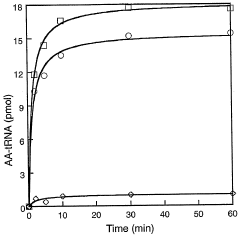
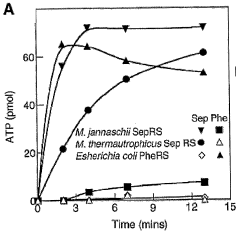
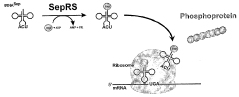
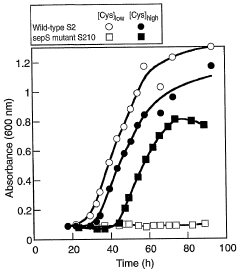
Site specific incorporation of phosphoserine into polypeptides using phosphoseryl-trna synthetase
PatentWO2006107813A2
Innovation
- The use of nucleic acids encoding genes with SepRS and tRNASep activity, specifically the cysteinyl-tRNA from Methanocaldococcus jannaschii and the class II-type O-phosphoseryl-tRNA synthetase, to preferentially aminoacylate tRNASep with O-phosphoserine, allowing for the targeted incorporation of phosphoserine into proteins during translation, even at stop codons or unconventional codons.
Method for the enrichment of phosphorylated and/or glycosylated analytes
PatentWO2009152893A2
Innovation
- The use of titanium dioxide particles with a magnetite core and an unannealed titanium dioxide surface, having an irregular shape and structure, for selective enrichment of phosphorylated and glycosylated analytes in an acidic binding buffer, followed by elution with a suitable buffer, enhances the yield and selectivity of the enrichment process.
Clinical Implementation Strategies and Validation Methods
The implementation of phosphorylation chain optimization in clinical settings requires systematic approaches that balance scientific rigor with practical considerations. Healthcare institutions adopting these technologies must develop standardized protocols that integrate phosphorylation profiling into existing clinical workflows. These protocols should specify sample collection procedures, processing timelines, and quality control measures to ensure consistent results across different patient populations.
Validation of phosphorylation-based precision medicine approaches necessitates multi-phase clinical trials designed specifically for molecular pathway interventions. Initial validation should focus on analytical performance metrics, including sensitivity, specificity, reproducibility, and robustness across diverse sample types. This technical validation must be followed by clinical validation studies that correlate phosphorylation patterns with treatment outcomes in retrospective patient cohorts.
Prospective interventional trials represent the gold standard for validating phosphorylation chain optimization approaches. These trials should incorporate adaptive designs that allow for treatment modifications based on dynamic changes in phosphorylation profiles during therapy. Implementing such trials requires specialized bioinformatics infrastructure capable of real-time data processing and interpretation to guide clinical decision-making.
Healthcare provider training constitutes a critical component of successful implementation. Clinicians must develop competency in interpreting phosphorylation data and translating findings into actionable treatment decisions. This necessitates the development of educational programs that bridge the knowledge gap between molecular biology and clinical practice, potentially through simulation-based learning and case studies.
Regulatory considerations present significant challenges for clinical implementation. Developers must navigate complex approval pathways for phosphorylation-based diagnostic and therapeutic approaches. This requires comprehensive documentation of analytical validation studies, clinical performance data, and risk management protocols. Engagement with regulatory bodies early in development can help identify potential hurdles and streamline the approval process.
Cost-effectiveness analysis represents another crucial validation dimension. Healthcare systems must evaluate the economic impact of implementing phosphorylation-based precision medicine approaches compared to standard care. Such analyses should consider not only direct costs of testing and targeted therapies but also potential savings from avoided ineffective treatments and improved patient outcomes.
Validation of phosphorylation-based precision medicine approaches necessitates multi-phase clinical trials designed specifically for molecular pathway interventions. Initial validation should focus on analytical performance metrics, including sensitivity, specificity, reproducibility, and robustness across diverse sample types. This technical validation must be followed by clinical validation studies that correlate phosphorylation patterns with treatment outcomes in retrospective patient cohorts.
Prospective interventional trials represent the gold standard for validating phosphorylation chain optimization approaches. These trials should incorporate adaptive designs that allow for treatment modifications based on dynamic changes in phosphorylation profiles during therapy. Implementing such trials requires specialized bioinformatics infrastructure capable of real-time data processing and interpretation to guide clinical decision-making.
Healthcare provider training constitutes a critical component of successful implementation. Clinicians must develop competency in interpreting phosphorylation data and translating findings into actionable treatment decisions. This necessitates the development of educational programs that bridge the knowledge gap between molecular biology and clinical practice, potentially through simulation-based learning and case studies.
Regulatory considerations present significant challenges for clinical implementation. Developers must navigate complex approval pathways for phosphorylation-based diagnostic and therapeutic approaches. This requires comprehensive documentation of analytical validation studies, clinical performance data, and risk management protocols. Engagement with regulatory bodies early in development can help identify potential hurdles and streamline the approval process.
Cost-effectiveness analysis represents another crucial validation dimension. Healthcare systems must evaluate the economic impact of implementing phosphorylation-based precision medicine approaches compared to standard care. Such analyses should consider not only direct costs of testing and targeted therapies but also potential savings from avoided ineffective treatments and improved patient outcomes.
Regulatory Considerations for Phosphorylation-Based Therapies
The regulatory landscape for phosphorylation-based therapies presents a complex framework that developers must navigate carefully. The FDA and EMA have established specific guidelines for precision medicine approaches that target post-translational modifications, with phosphorylation pathways requiring particular attention due to their widespread cellular impacts. These regulatory bodies demand robust evidence of specificity, with minimal off-target effects being a critical consideration for approval processes.
Clinical trial designs for phosphorylation-targeted therapies must incorporate biomarker validation strategies that demonstrate the correlation between phosphorylation status and clinical outcomes. Regulatory agencies increasingly require companion diagnostics that can accurately measure phosphorylation levels in patient samples, creating an additional layer of regulatory complexity for developers in this space.
Safety monitoring requirements are particularly stringent for phosphorylation modulators due to the potential for cascade effects throughout multiple signaling pathways. Long-term safety data collection is typically mandated, with post-market surveillance plans becoming standard components of regulatory submissions for these advanced therapeutic approaches.
International harmonization efforts are underway to standardize regulatory approaches to phosphorylation-based therapies, though significant regional differences persist. The International Council for Harmonisation (ICH) has begun developing specific guidance documents addressing the unique challenges of kinase inhibitors and phosphatase modulators, which may eventually streamline global development pathways.
Accelerated approval pathways may be available for phosphorylation-targeted therapies addressing serious conditions with unmet medical needs. However, these pathways typically require clearly defined surrogate endpoints that reliably predict clinical benefit, which can be challenging to establish for complex phosphorylation networks with multiple downstream effects.
Intellectual property considerations intersect with regulatory requirements, as patent protection strategies must account for regulatory exclusivity periods. The specificity of phosphorylation targeting mechanisms often allows for strong patent positions, though regulatory agencies may require additional data to support claims of novelty and non-obviousness in therapeutic applications.
Ethical considerations and patient consent frameworks are evolving to address the implications of phosphorylation-based precision medicine approaches, particularly regarding genetic testing that may predict response to these therapies. Regulatory bodies increasingly expect developers to address these ethical dimensions in their submission packages.
Clinical trial designs for phosphorylation-targeted therapies must incorporate biomarker validation strategies that demonstrate the correlation between phosphorylation status and clinical outcomes. Regulatory agencies increasingly require companion diagnostics that can accurately measure phosphorylation levels in patient samples, creating an additional layer of regulatory complexity for developers in this space.
Safety monitoring requirements are particularly stringent for phosphorylation modulators due to the potential for cascade effects throughout multiple signaling pathways. Long-term safety data collection is typically mandated, with post-market surveillance plans becoming standard components of regulatory submissions for these advanced therapeutic approaches.
International harmonization efforts are underway to standardize regulatory approaches to phosphorylation-based therapies, though significant regional differences persist. The International Council for Harmonisation (ICH) has begun developing specific guidance documents addressing the unique challenges of kinase inhibitors and phosphatase modulators, which may eventually streamline global development pathways.
Accelerated approval pathways may be available for phosphorylation-targeted therapies addressing serious conditions with unmet medical needs. However, these pathways typically require clearly defined surrogate endpoints that reliably predict clinical benefit, which can be challenging to establish for complex phosphorylation networks with multiple downstream effects.
Intellectual property considerations intersect with regulatory requirements, as patent protection strategies must account for regulatory exclusivity periods. The specificity of phosphorylation targeting mechanisms often allows for strong patent positions, though regulatory agencies may require additional data to support claims of novelty and non-obviousness in therapeutic applications.
Ethical considerations and patient consent frameworks are evolving to address the implications of phosphorylation-based precision medicine approaches, particularly regarding genetic testing that may predict response to these therapies. Regulatory bodies increasingly expect developers to address these ethical dimensions in their submission packages.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!