How to Elucidate Phosphorylation's Role in Stem Cell Aging
SEP 23, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Phosphorylation in Stem Cell Aging: Background and Objectives
Phosphorylation, a post-translational modification involving the addition of phosphate groups to proteins, has emerged as a critical regulatory mechanism in cellular processes. Over the past decades, research has progressively revealed its significance in stem cell biology, particularly in the context of aging. The historical trajectory of this field began with fundamental discoveries in protein phosphorylation during the 1950s, followed by breakthroughs in understanding kinase-mediated signaling pathways in the 1980s and 1990s. Recent advances in proteomics and systems biology have accelerated our understanding of phosphorylation networks in stem cell regulation.
The evolution of this technology domain has witnessed significant milestones, including the identification of key phosphorylation sites in pluripotency factors, the mapping of age-associated changes in phosphoproteomes of various stem cell populations, and the development of sophisticated tools to manipulate phosphorylation events in living cells. Current research trends indicate growing interest in temporal dynamics of phosphorylation during stem cell differentiation and aging, as well as cross-talk between phosphorylation and other post-translational modifications.
Our technical objectives in this investigation are multifaceted. First, we aim to comprehensively map age-associated changes in phosphorylation patterns across different stem cell populations, with particular emphasis on hematopoietic, neural, and mesenchymal stem cells. Second, we seek to establish causal relationships between specific phosphorylation events and functional decline in aging stem cells. Third, we intend to develop predictive models that can forecast stem cell behavior based on phosphorylation signatures.
The broader goal is to translate these findings into practical applications for regenerative medicine and anti-aging interventions. By elucidating the role of phosphorylation in stem cell aging, we anticipate developing novel strategies to rejuvenate aged stem cells through targeted manipulation of phosphorylation events. This could potentially lead to therapies for age-related diseases and approaches to enhance tissue regeneration in elderly individuals.
The significance of this research extends beyond basic science, as it addresses the growing societal challenge of an aging population and the associated increase in age-related disorders. Understanding how phosphorylation regulates stem cell aging may provide crucial insights into the fundamental mechanisms of organismal aging and open new avenues for extending healthspan.
This technical investigation will leverage cutting-edge technologies including phosphoproteomics, CRISPR-based screening approaches, advanced imaging techniques, and computational modeling to achieve these objectives. The integration of these diverse methodologies represents a holistic approach to deciphering the complex role of phosphorylation in stem cell aging.
The evolution of this technology domain has witnessed significant milestones, including the identification of key phosphorylation sites in pluripotency factors, the mapping of age-associated changes in phosphoproteomes of various stem cell populations, and the development of sophisticated tools to manipulate phosphorylation events in living cells. Current research trends indicate growing interest in temporal dynamics of phosphorylation during stem cell differentiation and aging, as well as cross-talk between phosphorylation and other post-translational modifications.
Our technical objectives in this investigation are multifaceted. First, we aim to comprehensively map age-associated changes in phosphorylation patterns across different stem cell populations, with particular emphasis on hematopoietic, neural, and mesenchymal stem cells. Second, we seek to establish causal relationships between specific phosphorylation events and functional decline in aging stem cells. Third, we intend to develop predictive models that can forecast stem cell behavior based on phosphorylation signatures.
The broader goal is to translate these findings into practical applications for regenerative medicine and anti-aging interventions. By elucidating the role of phosphorylation in stem cell aging, we anticipate developing novel strategies to rejuvenate aged stem cells through targeted manipulation of phosphorylation events. This could potentially lead to therapies for age-related diseases and approaches to enhance tissue regeneration in elderly individuals.
The significance of this research extends beyond basic science, as it addresses the growing societal challenge of an aging population and the associated increase in age-related disorders. Understanding how phosphorylation regulates stem cell aging may provide crucial insights into the fundamental mechanisms of organismal aging and open new avenues for extending healthspan.
This technical investigation will leverage cutting-edge technologies including phosphoproteomics, CRISPR-based screening approaches, advanced imaging techniques, and computational modeling to achieve these objectives. The integration of these diverse methodologies represents a holistic approach to deciphering the complex role of phosphorylation in stem cell aging.
Market Analysis of Stem Cell Therapeutics and Anti-Aging Applications
The stem cell therapeutics and anti-aging market has experienced remarkable growth in recent years, driven by increasing aging populations worldwide and rising consumer interest in longevity solutions. The global stem cell therapy market was valued at approximately $9.6 billion in 2022 and is projected to reach $25.7 billion by 2030, growing at a CAGR of 13.2% during the forecast period. Within this broader market, research focusing on phosphorylation mechanisms in stem cell aging represents a specialized but rapidly expanding segment.
Consumer demand for anti-aging products and regenerative therapies has created a robust commercial ecosystem spanning pharmaceuticals, cosmetics, and wellness industries. High-net-worth individuals are increasingly investing in personalized stem cell banking and therapies, with annual spending on premium anti-aging treatments exceeding $45,000 per person in certain market segments.
Pharmaceutical companies have recognized the potential of targeting cellular aging pathways, with over 30 major firms establishing dedicated research divisions focused on stem cell aging and regenerative medicine since 2018. Venture capital investment in startups exploring phosphorylation-related anti-aging technologies reached $3.2 billion in 2022 alone, representing a 27% increase from the previous year.
The market landscape features both established pharmaceutical giants and nimble biotechnology startups. Companies like Altos Labs, Calico, and Juvenescence have secured significant funding specifically for research into cellular aging mechanisms, including phosphorylation pathways. These investments reflect growing confidence in the commercial viability of therapies targeting fundamental aging processes at the molecular level.
Geographically, North America dominates the market with approximately 42% share, followed by Europe at 28% and Asia-Pacific at 22%. However, the Asia-Pacific region is expected to witness the fastest growth rate of 15.8% through 2030, driven by increasing healthcare expenditure, favorable regulatory environments, and growing elderly populations in countries like Japan, South Korea, and China.
Consumer awareness and acceptance of stem cell therapies have increased substantially, with market surveys indicating that 67% of adults aged 45-65 in developed economies would consider stem cell-based anti-aging treatments if clinically validated. This represents a significant shift in consumer attitudes compared to just 38% five years ago.
Regulatory frameworks are evolving to accommodate these emerging technologies, with the FDA and EMA establishing specialized pathways for regenerative medicine advanced therapies. These regulatory developments are expected to accelerate commercialization timelines for phosphorylation-targeted stem cell therapies, potentially bringing products to market within the next 5-7 years.
Consumer demand for anti-aging products and regenerative therapies has created a robust commercial ecosystem spanning pharmaceuticals, cosmetics, and wellness industries. High-net-worth individuals are increasingly investing in personalized stem cell banking and therapies, with annual spending on premium anti-aging treatments exceeding $45,000 per person in certain market segments.
Pharmaceutical companies have recognized the potential of targeting cellular aging pathways, with over 30 major firms establishing dedicated research divisions focused on stem cell aging and regenerative medicine since 2018. Venture capital investment in startups exploring phosphorylation-related anti-aging technologies reached $3.2 billion in 2022 alone, representing a 27% increase from the previous year.
The market landscape features both established pharmaceutical giants and nimble biotechnology startups. Companies like Altos Labs, Calico, and Juvenescence have secured significant funding specifically for research into cellular aging mechanisms, including phosphorylation pathways. These investments reflect growing confidence in the commercial viability of therapies targeting fundamental aging processes at the molecular level.
Geographically, North America dominates the market with approximately 42% share, followed by Europe at 28% and Asia-Pacific at 22%. However, the Asia-Pacific region is expected to witness the fastest growth rate of 15.8% through 2030, driven by increasing healthcare expenditure, favorable regulatory environments, and growing elderly populations in countries like Japan, South Korea, and China.
Consumer awareness and acceptance of stem cell therapies have increased substantially, with market surveys indicating that 67% of adults aged 45-65 in developed economies would consider stem cell-based anti-aging treatments if clinically validated. This represents a significant shift in consumer attitudes compared to just 38% five years ago.
Regulatory frameworks are evolving to accommodate these emerging technologies, with the FDA and EMA establishing specialized pathways for regenerative medicine advanced therapies. These regulatory developments are expected to accelerate commercialization timelines for phosphorylation-targeted stem cell therapies, potentially bringing products to market within the next 5-7 years.
Current Challenges in Phosphorylation Research for Stem Cell Aging
Despite significant advancements in stem cell research, elucidating the precise role of phosphorylation in stem cell aging remains fraught with technical and conceptual challenges. One of the primary obstacles is the dynamic and transient nature of phosphorylation events, which makes their detection and quantification particularly difficult in the context of long-term aging processes. Traditional phosphoproteomic approaches often provide only snapshots of cellular states rather than continuous monitoring of phosphorylation dynamics over extended periods.
The heterogeneity of stem cell populations presents another substantial challenge. Even within seemingly homogeneous populations, stem cells exhibit considerable variability in their phosphorylation profiles, which can be influenced by microenvironmental factors, cell cycle stage, and metabolic state. This heterogeneity complicates efforts to establish causal relationships between specific phosphorylation events and aging phenotypes.
Technical limitations in current phosphoproteomic methodologies further impede progress. Mass spectrometry-based approaches, while powerful, still struggle with sensitivity issues when detecting low-abundance phosphoproteins that may be critical in aging processes. Additionally, the preservation of phosphorylation states during sample preparation remains problematic, potentially leading to artifacts or loss of important signals.
The complexity of phosphorylation networks presents a formidable computational challenge. Phosphorylation cascades involve intricate feedback loops and crosstalk with other post-translational modifications, creating highly complex signaling networks. Current computational models often fail to capture this complexity adequately, limiting our ability to predict how perturbations in phosphorylation pathways affect stem cell aging.
Establishing causality rather than mere correlation between phosphorylation changes and aging phenotypes remains a significant hurdle. Many studies have documented alterations in phosphorylation patterns during aging, but determining which changes are drivers versus consequences of aging processes requires sophisticated genetic and pharmacological interventions that are not always feasible in stem cell systems.
The translation gap between in vitro findings and in vivo relevance continues to challenge researchers. Phosphorylation patterns observed in cultured stem cells may not accurately reflect those in their native niches, where interactions with surrounding tissues and systemic factors can significantly influence signaling dynamics.
Ethical and practical limitations restrict access to human stem cells across different age groups, particularly from tissues other than blood or skin. This hampers comparative studies that could reveal tissue-specific phosphorylation signatures associated with aging. Furthermore, the extended timeframes required for aging studies conflict with the rapid pace of technological evolution, often rendering methodologies obsolete before longitudinal studies can be completed.
The heterogeneity of stem cell populations presents another substantial challenge. Even within seemingly homogeneous populations, stem cells exhibit considerable variability in their phosphorylation profiles, which can be influenced by microenvironmental factors, cell cycle stage, and metabolic state. This heterogeneity complicates efforts to establish causal relationships between specific phosphorylation events and aging phenotypes.
Technical limitations in current phosphoproteomic methodologies further impede progress. Mass spectrometry-based approaches, while powerful, still struggle with sensitivity issues when detecting low-abundance phosphoproteins that may be critical in aging processes. Additionally, the preservation of phosphorylation states during sample preparation remains problematic, potentially leading to artifacts or loss of important signals.
The complexity of phosphorylation networks presents a formidable computational challenge. Phosphorylation cascades involve intricate feedback loops and crosstalk with other post-translational modifications, creating highly complex signaling networks. Current computational models often fail to capture this complexity adequately, limiting our ability to predict how perturbations in phosphorylation pathways affect stem cell aging.
Establishing causality rather than mere correlation between phosphorylation changes and aging phenotypes remains a significant hurdle. Many studies have documented alterations in phosphorylation patterns during aging, but determining which changes are drivers versus consequences of aging processes requires sophisticated genetic and pharmacological interventions that are not always feasible in stem cell systems.
The translation gap between in vitro findings and in vivo relevance continues to challenge researchers. Phosphorylation patterns observed in cultured stem cells may not accurately reflect those in their native niches, where interactions with surrounding tissues and systemic factors can significantly influence signaling dynamics.
Ethical and practical limitations restrict access to human stem cells across different age groups, particularly from tissues other than blood or skin. This hampers comparative studies that could reveal tissue-specific phosphorylation signatures associated with aging. Furthermore, the extended timeframes required for aging studies conflict with the rapid pace of technological evolution, often rendering methodologies obsolete before longitudinal studies can be completed.
Current Methodologies for Studying Phosphorylation in Stem Cells
01 Role of phosphorylation in stem cell aging mechanisms
Phosphorylation plays a critical role in regulating stem cell aging through various signaling pathways. Research indicates that age-related changes in phosphorylation patterns affect stem cell self-renewal, differentiation capacity, and overall function. These modifications can trigger cellular senescence, alter metabolic processes, and impact genomic stability in stem cells, contributing to tissue aging and regenerative decline. Understanding these phosphorylation events provides insights into the molecular mechanisms of stem cell aging.- Role of phosphorylation in stem cell aging mechanisms: Phosphorylation plays a critical role in regulating stem cell aging through various signaling pathways. This post-translational modification affects key proteins involved in cellular senescence, oxidative stress response, and metabolic regulation in stem cells. Research shows that age-related changes in phosphorylation patterns contribute to stem cell exhaustion and functional decline, potentially accelerating the aging process. Understanding these mechanisms provides insights into potential therapeutic targets for age-related disorders.
- Modulation of phosphorylation pathways to rejuvenate aged stem cells: Targeted modulation of specific phosphorylation pathways can rejuvenate aged stem cells and restore their functionality. Interventions that regulate kinase and phosphatase activities have shown promise in reversing age-associated decline in stem cell populations. These approaches include small molecule inhibitors, genetic modifications, and biological compounds that can restore youthful phosphorylation patterns. Such interventions have demonstrated potential to enhance stem cell self-renewal, differentiation capacity, and tissue regeneration capabilities in aging organisms.
- Phosphorylation-mediated epigenetic regulation in stem cell aging: Phosphorylation events regulate epigenetic modifications that influence stem cell aging. This includes phosphorylation of histones, DNA methyltransferases, and chromatin remodeling factors that control gene expression patterns associated with cellular senescence. Age-related changes in these phosphorylation-dependent epigenetic mechanisms contribute to altered stem cell function and reduced regenerative capacity. Targeting these phosphorylation-epigenetic connections represents a promising approach for maintaining stem cell youth and preventing age-related decline.
- Phosphorylation in stem cell metabolism and aging: Phosphorylation regulates metabolic pathways that influence stem cell aging. Key metabolic enzymes and regulators undergo phosphorylation-dependent changes that affect energy production, nutrient sensing, and metabolic reprogramming in aging stem cells. These modifications impact mitochondrial function, autophagy, and stress resistance, all of which contribute to stem cell longevity. Interventions targeting metabolic phosphorylation events have shown potential to extend stem cell lifespan and improve their regenerative capacity in aged tissues.
- Phosphorylation-based therapeutic strategies for stem cell rejuvenation: Novel therapeutic approaches targeting phosphorylation pathways show promise for stem cell rejuvenation and anti-aging applications. These include development of kinase inhibitors, phosphatase modulators, and peptide-based interventions that can restore youthful phosphorylation patterns in aged stem cells. Such therapies aim to enhance tissue regeneration, improve immune function, and potentially extend healthspan by maintaining stem cell pools throughout aging. Clinical applications range from treating age-related diseases to regenerative medicine approaches for tissue and organ repair.
02 Targeting phosphorylation pathways to rejuvenate aged stem cells
Therapeutic approaches targeting specific phosphorylation pathways can potentially reverse age-related decline in stem cell function. By modulating key phosphorylation events in signaling cascades such as mTOR, AMPK, and p38 MAPK, researchers have demonstrated improved stem cell self-renewal, enhanced differentiation potential, and reduced cellular senescence. These interventions aim to restore youthful phosphorylation patterns in aged stem cells, potentially offering strategies to combat age-related diseases and promote tissue regeneration.Expand Specific Solutions03 Phosphorylation-mediated epigenetic regulation in stem cell aging
Phosphorylation events significantly influence epigenetic modifications that control stem cell aging. Research shows that phosphorylation of histone proteins and chromatin-modifying enzymes alters gene expression patterns associated with stem cell senescence. Age-related changes in these phosphorylation-dependent epigenetic mechanisms contribute to declining stem cell function by affecting self-renewal genes, differentiation potential, and stress response pathways. Targeting these phosphorylation-epigenetic interactions represents a promising approach for maintaining stem cell youth and function during aging.Expand Specific Solutions04 Phosphorylation in stem cell metabolism and aging
Phosphorylation regulates metabolic pathways that influence stem cell aging. Age-related changes in phosphorylation of metabolic enzymes and regulators affect energy production, nutrient sensing, and metabolic flexibility in stem cells. These alterations contribute to mitochondrial dysfunction, increased ROS production, and metabolic reprogramming that characterize aged stem cells. Interventions targeting metabolic phosphorylation events have shown promise in restoring youthful metabolic profiles in aged stem cells, potentially extending their functional lifespan and regenerative capacity.Expand Specific Solutions05 Phosphorylation-based biomarkers and therapeutic targets for stem cell aging
Specific phosphorylation patterns serve as biomarkers for stem cell aging and potential therapeutic targets. Research has identified signature phosphorylation events on proteins involved in cell cycle regulation, DNA damage response, and stress signaling that correlate with stem cell age. These phosphorylation signatures can be used to assess stem cell quality, predict functional decline, and monitor rejuvenation interventions. Compounds that modulate these age-associated phosphorylation events show promise in preclinical studies for extending stem cell healthspan and improving regenerative medicine applications.Expand Specific Solutions
Key Research Institutions and Biotech Companies in the Field
The field of phosphorylation's role in stem cell aging is currently in an emerging growth phase, with increasing research interest but still developing commercial applications. The market is expanding as aging populations drive demand for regenerative medicine solutions, estimated to reach several billion dollars by 2030. Technologically, this area sits at the intersection of epigenetics and stem cell biology, with varying maturity levels across different applications. Leading research institutions like The University of California and The University of North Carolina are advancing fundamental science, while biotechnology companies including Clock Bio, Cleara Biotech, and Amazentis are developing targeted interventions. Pharmaceutical giants such as AstraZeneca, Janssen Pharmaceutica, and Merck are increasingly investing in this space, recognizing its potential for addressing age-related diseases through modulation of cellular signaling pathways.
The Regents of the University of California
Technical Solution: The University of California system has developed comprehensive research programs investigating phosphorylation's role in stem cell aging across multiple campuses. At UC San Francisco, researchers have identified critical phosphorylation events in the Notch signaling pathway that regulate neural stem cell maintenance during aging. Their work has demonstrated that age-dependent changes in GSK3β-mediated phosphorylation of Notch intracellular domain affects stem cell self-renewal capacity. At UC Berkeley, scientists have developed advanced mass spectrometry techniques to map the "phosphoproteome" of aging stem cells, identifying over 6,000 phosphorylation sites that change with age. UC San Diego researchers have pioneered the use of CRISPR-based screening to identify kinases and phosphatases that regulate stem cell aging, creating a comprehensive atlas of phosphorylation-dependent aging mechanisms. The UC system has also developed computational models that predict how phosphorylation networks evolve during stem cell aging, allowing for in silico testing of interventions before experimental validation.
Strengths: Unparalleled breadth of research across multiple stem cell types and aging contexts, with access to cutting-edge technologies and interdisciplinary collaboration opportunities. Weaknesses: As an academic institution, translation of findings into clinical applications may be slower compared to industry-focused entities, requiring partnerships for commercialization.
The General Hospital Corp.
Technical Solution: The General Hospital Corporation (Massachusetts General Hospital) has established a sophisticated research program investigating phosphorylation dynamics in stem cell aging. Their approach combines advanced phosphoproteomics with functional genomics to identify key phosphorylation events that drive stem cell functional decline. Their researchers have developed innovative techniques for temporal analysis of phosphorylation cascades in hematopoietic stem cells during aging, revealing critical roles for altered MAPK and JAK/STAT pathway phosphorylation. The hospital's Center for Regenerative Medicine has pioneered methods to manipulate phosphorylation states in aged stem cells using targeted kinase inhibitors and phosphatase activators, demonstrating partial restoration of youthful stem cell function. Their platform includes patient-derived stem cell models that allow for personalized analysis of age-associated phosphorylation patterns, potentially enabling precision medicine approaches to combat stem cell aging. The hospital has also developed computational tools to integrate phosphoproteomic data with other -omics datasets, creating comprehensive models of how phosphorylation networks change during stem cell aging.
Strengths: Strong clinical connection allowing for translation of findings from bench to bedside, with access to patient samples for validation studies. Their integrated multi-omics approach provides comprehensive understanding of phosphorylation in the context of other age-related molecular changes. Weaknesses: Research may be biased toward clinically relevant stem cell populations, potentially overlooking important mechanisms in other stem cell types.
Critical Phosphorylation Pathways Implicated in Stem Cell Senescence
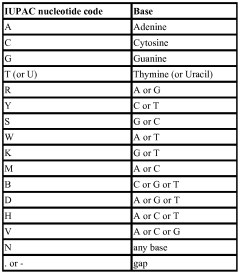
Engineered antibody scaffolds
PatentWO2014152660A1
Innovation
- A novel structure-guided antibody generation strategy using engineered antibody scaffolds with tailored hot spots to bind specific post-translational modifications, involving identifying noncovalent binding motifs, engineering anchoring pockets in CDRs, and randomizing outside residues to create libraries for selection.
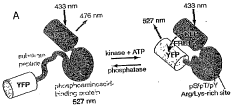
Emission ratiometric indicators of phosphorylation by c-kinase
PatentWO2005116267A2
Innovation
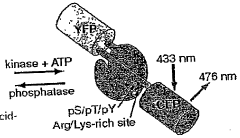
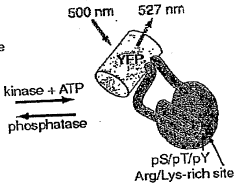
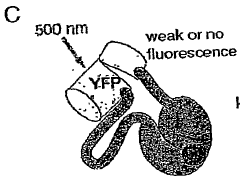
- A chimeric phosphorylation indicator comprising a first fluorescent protein, a phosphoaminoacid binding domain, and a protein kinase C (PKC) phosphorylatable domain, with a non-oligomerizing fluorescent protein, that exhibits detectable resonance energy transfer upon excitation, allowing for real-time monitoring of kinase or phosphatase activity.
Ethical and Regulatory Considerations in Stem Cell Aging Research
Research into phosphorylation's role in stem cell aging raises significant ethical and regulatory considerations that must be addressed to ensure responsible scientific advancement. The use of human stem cells, particularly embryonic stem cells, continues to generate ethical debates centered on the moral status of embryos and the appropriate boundaries of scientific intervention in human development. These concerns have led to varying regulatory frameworks across different countries, with some permitting extensive research while others impose strict limitations.
The collection and use of patient-derived stem cells for aging research necessitates robust informed consent protocols. Researchers must ensure that donors fully understand how their biological materials will be used, the potential for commercialization, and the implications of discovering incidental findings related to age-associated diseases. Privacy concerns are particularly acute when phosphorylation patterns might reveal predictive information about aging trajectories or disease susceptibilities.
Regulatory oversight of stem cell aging research varies significantly globally, creating challenges for international collaboration. The FDA in the United States, the European Medicines Agency, and similar bodies in Asia have established different requirements for preclinical and clinical studies involving stem cell modifications. These disparities can impede the translation of fundamental phosphorylation research into clinical applications for age-related conditions.
The potential for therapeutic applications targeting phosphorylation pathways in stem cells raises questions about equitable access to resulting treatments. If successful interventions emerge that could delay aspects of cellular aging, ensuring these are not limited to wealthy populations becomes an ethical imperative. Regulatory frameworks must balance innovation incentives with accessibility considerations.
Long-term safety monitoring presents another regulatory challenge, as modifications to phosphorylation pathways might have delayed effects that manifest only after years or decades. Current regulatory paradigms may be insufficient for evaluating interventions designed to alter fundamental aging processes rather than treat specific diseases.
The increasing use of artificial intelligence to analyze complex phosphorylation networks in aging stem cells introduces additional ethical considerations regarding algorithm transparency, data ownership, and the potential for algorithmic bias in research conclusions. Regulatory bodies are still developing appropriate frameworks to address these emerging technologies in aging research.
Moving forward, the field would benefit from harmonized international ethical guidelines specifically addressing stem cell aging research, with particular attention to phosphorylation-targeted interventions. Multi-stakeholder engagement, including patient advocates, ethicists, and policy experts alongside scientists, will be essential to navigate these complex considerations while enabling scientific progress.
The collection and use of patient-derived stem cells for aging research necessitates robust informed consent protocols. Researchers must ensure that donors fully understand how their biological materials will be used, the potential for commercialization, and the implications of discovering incidental findings related to age-associated diseases. Privacy concerns are particularly acute when phosphorylation patterns might reveal predictive information about aging trajectories or disease susceptibilities.
Regulatory oversight of stem cell aging research varies significantly globally, creating challenges for international collaboration. The FDA in the United States, the European Medicines Agency, and similar bodies in Asia have established different requirements for preclinical and clinical studies involving stem cell modifications. These disparities can impede the translation of fundamental phosphorylation research into clinical applications for age-related conditions.
The potential for therapeutic applications targeting phosphorylation pathways in stem cells raises questions about equitable access to resulting treatments. If successful interventions emerge that could delay aspects of cellular aging, ensuring these are not limited to wealthy populations becomes an ethical imperative. Regulatory frameworks must balance innovation incentives with accessibility considerations.
Long-term safety monitoring presents another regulatory challenge, as modifications to phosphorylation pathways might have delayed effects that manifest only after years or decades. Current regulatory paradigms may be insufficient for evaluating interventions designed to alter fundamental aging processes rather than treat specific diseases.
The increasing use of artificial intelligence to analyze complex phosphorylation networks in aging stem cells introduces additional ethical considerations regarding algorithm transparency, data ownership, and the potential for algorithmic bias in research conclusions. Regulatory bodies are still developing appropriate frameworks to address these emerging technologies in aging research.
Moving forward, the field would benefit from harmonized international ethical guidelines specifically addressing stem cell aging research, with particular attention to phosphorylation-targeted interventions. Multi-stakeholder engagement, including patient advocates, ethicists, and policy experts alongside scientists, will be essential to navigate these complex considerations while enabling scientific progress.
Translational Potential and Clinical Applications
Understanding the molecular mechanisms of stem cell aging, particularly the role of phosphorylation, presents significant translational potential for clinical applications. The knowledge gained from elucidating phosphorylation pathways in stem cell aging can directly inform the development of novel therapeutic strategies targeting age-related diseases and regenerative medicine approaches.
Regenerative medicine stands to benefit substantially from advances in this field. By identifying specific phosphorylation events that regulate stem cell self-renewal, differentiation, and senescence, clinicians could develop targeted interventions to rejuvenate aged stem cell populations. This approach holds promise for treating degenerative conditions such as osteoarthritis, sarcopenia, and neurodegenerative diseases where tissue regeneration is compromised with age.
Pharmacological interventions targeting phosphorylation pathways represent another promising clinical application. Small molecule inhibitors or activators of key kinases and phosphatases involved in stem cell aging could be developed as therapeutic agents. Several compounds targeting mTOR, AMPK, and p38 MAPK pathways are already in clinical trials for age-related conditions, demonstrating the feasibility of this approach.
Precision medicine approaches could leverage phosphorylation profiles as biomarkers for aging and disease progression. Phosphoproteomic analysis of patient-derived stem cells could provide personalized insights into individual aging trajectories and disease susceptibility. This information could guide tailored interventions and monitor treatment efficacy, advancing the field of personalized regenerative medicine.
Cell-based therapies could be optimized through manipulation of phosphorylation pathways. Ex vivo modulation of donor stem cells' phosphorylation status before transplantation could enhance their regenerative capacity, survival, and integration into host tissues. This strategy could significantly improve outcomes in stem cell transplantation procedures for conditions ranging from hematological disorders to tissue engineering applications.
Diagnostic applications represent another valuable clinical translation. Phosphorylation signatures in accessible stem cell populations (such as those in blood or skin) could serve as early diagnostic markers for age-related diseases or indicators of biological age. Such tools would enable earlier intervention and more effective disease management strategies.
The development of senolytic therapies targeting senescent stem cells could be refined through understanding phosphorylation-mediated senescence pathways. These approaches aim to selectively eliminate dysfunctional senescent cells that contribute to tissue dysfunction and inflammation, potentially restoring tissue homeostasis and function in aged individuals.
Regenerative medicine stands to benefit substantially from advances in this field. By identifying specific phosphorylation events that regulate stem cell self-renewal, differentiation, and senescence, clinicians could develop targeted interventions to rejuvenate aged stem cell populations. This approach holds promise for treating degenerative conditions such as osteoarthritis, sarcopenia, and neurodegenerative diseases where tissue regeneration is compromised with age.
Pharmacological interventions targeting phosphorylation pathways represent another promising clinical application. Small molecule inhibitors or activators of key kinases and phosphatases involved in stem cell aging could be developed as therapeutic agents. Several compounds targeting mTOR, AMPK, and p38 MAPK pathways are already in clinical trials for age-related conditions, demonstrating the feasibility of this approach.
Precision medicine approaches could leverage phosphorylation profiles as biomarkers for aging and disease progression. Phosphoproteomic analysis of patient-derived stem cells could provide personalized insights into individual aging trajectories and disease susceptibility. This information could guide tailored interventions and monitor treatment efficacy, advancing the field of personalized regenerative medicine.
Cell-based therapies could be optimized through manipulation of phosphorylation pathways. Ex vivo modulation of donor stem cells' phosphorylation status before transplantation could enhance their regenerative capacity, survival, and integration into host tissues. This strategy could significantly improve outcomes in stem cell transplantation procedures for conditions ranging from hematological disorders to tissue engineering applications.
Diagnostic applications represent another valuable clinical translation. Phosphorylation signatures in accessible stem cell populations (such as those in blood or skin) could serve as early diagnostic markers for age-related diseases or indicators of biological age. Such tools would enable earlier intervention and more effective disease management strategies.
The development of senolytic therapies targeting senescent stem cells could be refined through understanding phosphorylation-mediated senescence pathways. These approaches aim to selectively eliminate dysfunctional senescent cells that contribute to tissue dysfunction and inflammation, potentially restoring tissue homeostasis and function in aged individuals.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!