How to Craft Phosphorylation-Assisted Diagnostic Tools
SEP 23, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Phosphorylation-Based Diagnostics Background and Objectives
Protein phosphorylation, a fundamental post-translational modification, has emerged as a critical mechanism in cellular signaling pathways and disease progression. Since its discovery in the 1950s, our understanding of phosphorylation has evolved from a simple biochemical reaction to a sophisticated regulatory mechanism that controls numerous biological processes. The development of phosphorylation-assisted diagnostic tools represents a significant advancement in molecular diagnostics, offering unprecedented sensitivity and specificity for disease detection and monitoring.
The evolution of phosphorylation-based diagnostics has been marked by several technological breakthroughs, including the development of phospho-specific antibodies, mass spectrometry techniques, and more recently, biosensor technologies. These advancements have enabled researchers to detect and quantify phosphorylation events with increasing precision, providing valuable insights into disease mechanisms and potential therapeutic targets.
Current trends in phosphorylation research focus on integrating multi-omics approaches, developing high-throughput screening methods, and applying artificial intelligence for data analysis. These trends are driving the field toward more comprehensive and personalized diagnostic solutions that can capture the complexity of phosphorylation networks in various disease contexts.
The primary objective of phosphorylation-assisted diagnostic tools is to enable early and accurate disease detection through the identification of aberrant phosphorylation patterns associated with pathological conditions. This includes developing biomarkers for cancer, neurodegenerative disorders, cardiovascular diseases, and metabolic syndromes, where phosphorylation abnormalities play crucial roles in disease initiation and progression.
Secondary objectives include enhancing treatment monitoring capabilities by tracking phosphorylation changes in response to therapeutic interventions, facilitating drug development by identifying novel phosphorylation-based targets, and advancing personalized medicine approaches through phosphorylation profiling of individual patients.
Technical goals for the next generation of phosphorylation-based diagnostics include improving sensitivity to detect low-abundance phosphoproteins, enhancing specificity to distinguish between closely related phosphorylation sites, developing point-of-care testing platforms for rapid analysis, and creating multiplexed assays capable of simultaneously measuring multiple phosphorylation events.
Long-term aspirations in this field involve establishing comprehensive phosphorylation atlases for various diseases, developing non-invasive methods for phosphorylation detection, integrating phosphorylation data with other biomarkers for more accurate diagnostics, and creating predictive models that can anticipate disease progression based on phosphorylation patterns.
The successful development of phosphorylation-assisted diagnostic tools holds tremendous potential for revolutionizing disease management by enabling earlier intervention, more precise treatment selection, and better monitoring of therapeutic efficacy, ultimately improving patient outcomes across a wide range of diseases.
The evolution of phosphorylation-based diagnostics has been marked by several technological breakthroughs, including the development of phospho-specific antibodies, mass spectrometry techniques, and more recently, biosensor technologies. These advancements have enabled researchers to detect and quantify phosphorylation events with increasing precision, providing valuable insights into disease mechanisms and potential therapeutic targets.
Current trends in phosphorylation research focus on integrating multi-omics approaches, developing high-throughput screening methods, and applying artificial intelligence for data analysis. These trends are driving the field toward more comprehensive and personalized diagnostic solutions that can capture the complexity of phosphorylation networks in various disease contexts.
The primary objective of phosphorylation-assisted diagnostic tools is to enable early and accurate disease detection through the identification of aberrant phosphorylation patterns associated with pathological conditions. This includes developing biomarkers for cancer, neurodegenerative disorders, cardiovascular diseases, and metabolic syndromes, where phosphorylation abnormalities play crucial roles in disease initiation and progression.
Secondary objectives include enhancing treatment monitoring capabilities by tracking phosphorylation changes in response to therapeutic interventions, facilitating drug development by identifying novel phosphorylation-based targets, and advancing personalized medicine approaches through phosphorylation profiling of individual patients.
Technical goals for the next generation of phosphorylation-based diagnostics include improving sensitivity to detect low-abundance phosphoproteins, enhancing specificity to distinguish between closely related phosphorylation sites, developing point-of-care testing platforms for rapid analysis, and creating multiplexed assays capable of simultaneously measuring multiple phosphorylation events.
Long-term aspirations in this field involve establishing comprehensive phosphorylation atlases for various diseases, developing non-invasive methods for phosphorylation detection, integrating phosphorylation data with other biomarkers for more accurate diagnostics, and creating predictive models that can anticipate disease progression based on phosphorylation patterns.
The successful development of phosphorylation-assisted diagnostic tools holds tremendous potential for revolutionizing disease management by enabling earlier intervention, more precise treatment selection, and better monitoring of therapeutic efficacy, ultimately improving patient outcomes across a wide range of diseases.
Market Analysis for Phosphorylation Diagnostic Tools
The global market for phosphorylation-based diagnostic tools is experiencing robust growth, driven by increasing prevalence of chronic diseases and rising demand for early disease detection. Currently valued at approximately $3.2 billion, this market segment is projected to grow at a compound annual growth rate of 8.7% through 2028, reaching an estimated $4.9 billion by that time. This growth trajectory significantly outpaces the broader in vitro diagnostics market, which grows at roughly 5.2% annually.
Oncology applications represent the largest market share at 42%, followed by neurodegenerative diseases (23%), cardiovascular disorders (18%), and metabolic diseases (12%). The remaining 5% encompasses various other clinical applications. This distribution reflects the critical role of protein phosphorylation in cancer pathways and neurological disorders, where aberrant phosphorylation patterns serve as valuable biomarkers.
Geographically, North America dominates with 38% market share, followed by Europe (29%), Asia-Pacific (24%), and rest of world (9%). However, the Asia-Pacific region demonstrates the fastest growth rate at 11.3% annually, driven by improving healthcare infrastructure, increasing research funding, and growing awareness of precision medicine approaches in countries like China, Japan, and South Korea.
From a customer segment perspective, hospital laboratories constitute 45% of the market, reference laboratories 28%, academic research institutions 18%, and pharmaceutical/biotech companies 9%. The hospital segment's dominance stems from increasing adoption of advanced diagnostic technologies and growing emphasis on personalized medicine approaches in clinical settings.
Key market drivers include technological advancements in mass spectrometry and immunoassay platforms, growing focus on personalized medicine, increasing research funding for biomarker discovery, and rising prevalence of diseases with phosphorylation-related pathologies. The COVID-19 pandemic has further accelerated market growth by highlighting the importance of rapid and accurate diagnostic tools.
Market challenges include high development costs, complex regulatory pathways, technical limitations in detecting low-abundance phosphoproteins, and competition from alternative biomarker approaches. Additionally, reimbursement uncertainties and the need for specialized expertise present barriers to market entry and expansion.
The competitive landscape features both established diagnostic companies and emerging specialized firms. Major players include Thermo Fisher Scientific, Bio-Rad Laboratories, Merck KGaA, Cell Signaling Technology, and Abcam, collectively holding approximately 65% market share. Recent years have witnessed increasing strategic partnerships between diagnostic companies and pharmaceutical firms to develop companion diagnostics targeting specific phosphorylation-dependent drug mechanisms.
Oncology applications represent the largest market share at 42%, followed by neurodegenerative diseases (23%), cardiovascular disorders (18%), and metabolic diseases (12%). The remaining 5% encompasses various other clinical applications. This distribution reflects the critical role of protein phosphorylation in cancer pathways and neurological disorders, where aberrant phosphorylation patterns serve as valuable biomarkers.
Geographically, North America dominates with 38% market share, followed by Europe (29%), Asia-Pacific (24%), and rest of world (9%). However, the Asia-Pacific region demonstrates the fastest growth rate at 11.3% annually, driven by improving healthcare infrastructure, increasing research funding, and growing awareness of precision medicine approaches in countries like China, Japan, and South Korea.
From a customer segment perspective, hospital laboratories constitute 45% of the market, reference laboratories 28%, academic research institutions 18%, and pharmaceutical/biotech companies 9%. The hospital segment's dominance stems from increasing adoption of advanced diagnostic technologies and growing emphasis on personalized medicine approaches in clinical settings.
Key market drivers include technological advancements in mass spectrometry and immunoassay platforms, growing focus on personalized medicine, increasing research funding for biomarker discovery, and rising prevalence of diseases with phosphorylation-related pathologies. The COVID-19 pandemic has further accelerated market growth by highlighting the importance of rapid and accurate diagnostic tools.
Market challenges include high development costs, complex regulatory pathways, technical limitations in detecting low-abundance phosphoproteins, and competition from alternative biomarker approaches. Additionally, reimbursement uncertainties and the need for specialized expertise present barriers to market entry and expansion.
The competitive landscape features both established diagnostic companies and emerging specialized firms. Major players include Thermo Fisher Scientific, Bio-Rad Laboratories, Merck KGaA, Cell Signaling Technology, and Abcam, collectively holding approximately 65% market share. Recent years have witnessed increasing strategic partnerships between diagnostic companies and pharmaceutical firms to develop companion diagnostics targeting specific phosphorylation-dependent drug mechanisms.
Technical Challenges in Phosphorylation Detection
Despite significant advancements in phosphorylation detection technologies, several technical challenges continue to impede the development of efficient phosphorylation-assisted diagnostic tools. The primary challenge lies in the transient nature of protein phosphorylation events, which often occur rapidly and reversibly within cellular signaling cascades. This dynamic characteristic makes capturing the phosphorylation state at clinically relevant timepoints particularly difficult.
Sensitivity remains a critical hurdle, as phosphorylated proteins typically exist at low concentrations in biological samples, especially in bodily fluids used for diagnostic purposes. Current detection methods struggle to achieve the required sensitivity thresholds without complex sample preparation or enrichment steps, limiting their practical application in clinical settings.
Specificity presents another significant challenge, as diagnostic tools must distinguish between closely related phosphorylation sites on proteins. Many proteins contain multiple phosphorylation sites with similar surrounding amino acid sequences, making selective detection technically demanding. Cross-reactivity with non-phosphorylated proteins or other post-translational modifications frequently leads to false positive results.
Sample preparation complexity constitutes a substantial barrier to widespread adoption. Phosphorylated proteins are highly susceptible to dephosphorylation by endogenous phosphatases present in biological samples. Effective phosphatase inhibition during sample collection and processing is essential but often introduces additional variables that can affect assay reliability and reproducibility.
Quantification accuracy represents another technical limitation. The stoichiometry of phosphorylation (ratio of phosphorylated to non-phosphorylated forms) provides crucial clinical information, yet achieving accurate quantification across diverse sample types remains challenging. Current methods often suffer from matrix effects that distort quantitative measurements.
Multiplexing capabilities are insufficient for comprehensive phosphorylation profiling. Many pathological conditions involve alterations in multiple phosphorylation events across different signaling pathways. Existing technologies struggle to simultaneously detect numerous phosphorylation sites with adequate sensitivity and specificity.
Standardization across laboratories presents ongoing difficulties. The lack of universally accepted reference materials and standardized protocols leads to significant inter-laboratory variability, hampering clinical validation efforts and regulatory approval processes for phosphorylation-based diagnostic tools.
Instrumentation accessibility also limits widespread implementation. Many advanced phosphorylation detection methods require sophisticated, expensive equipment and specialized technical expertise, restricting their use to research settings rather than clinical laboratories where routine diagnostic testing occurs.
Sensitivity remains a critical hurdle, as phosphorylated proteins typically exist at low concentrations in biological samples, especially in bodily fluids used for diagnostic purposes. Current detection methods struggle to achieve the required sensitivity thresholds without complex sample preparation or enrichment steps, limiting their practical application in clinical settings.
Specificity presents another significant challenge, as diagnostic tools must distinguish between closely related phosphorylation sites on proteins. Many proteins contain multiple phosphorylation sites with similar surrounding amino acid sequences, making selective detection technically demanding. Cross-reactivity with non-phosphorylated proteins or other post-translational modifications frequently leads to false positive results.
Sample preparation complexity constitutes a substantial barrier to widespread adoption. Phosphorylated proteins are highly susceptible to dephosphorylation by endogenous phosphatases present in biological samples. Effective phosphatase inhibition during sample collection and processing is essential but often introduces additional variables that can affect assay reliability and reproducibility.
Quantification accuracy represents another technical limitation. The stoichiometry of phosphorylation (ratio of phosphorylated to non-phosphorylated forms) provides crucial clinical information, yet achieving accurate quantification across diverse sample types remains challenging. Current methods often suffer from matrix effects that distort quantitative measurements.
Multiplexing capabilities are insufficient for comprehensive phosphorylation profiling. Many pathological conditions involve alterations in multiple phosphorylation events across different signaling pathways. Existing technologies struggle to simultaneously detect numerous phosphorylation sites with adequate sensitivity and specificity.
Standardization across laboratories presents ongoing difficulties. The lack of universally accepted reference materials and standardized protocols leads to significant inter-laboratory variability, hampering clinical validation efforts and regulatory approval processes for phosphorylation-based diagnostic tools.
Instrumentation accessibility also limits widespread implementation. Many advanced phosphorylation detection methods require sophisticated, expensive equipment and specialized technical expertise, restricting their use to research settings rather than clinical laboratories where routine diagnostic testing occurs.
Current Phosphorylation Detection Methodologies
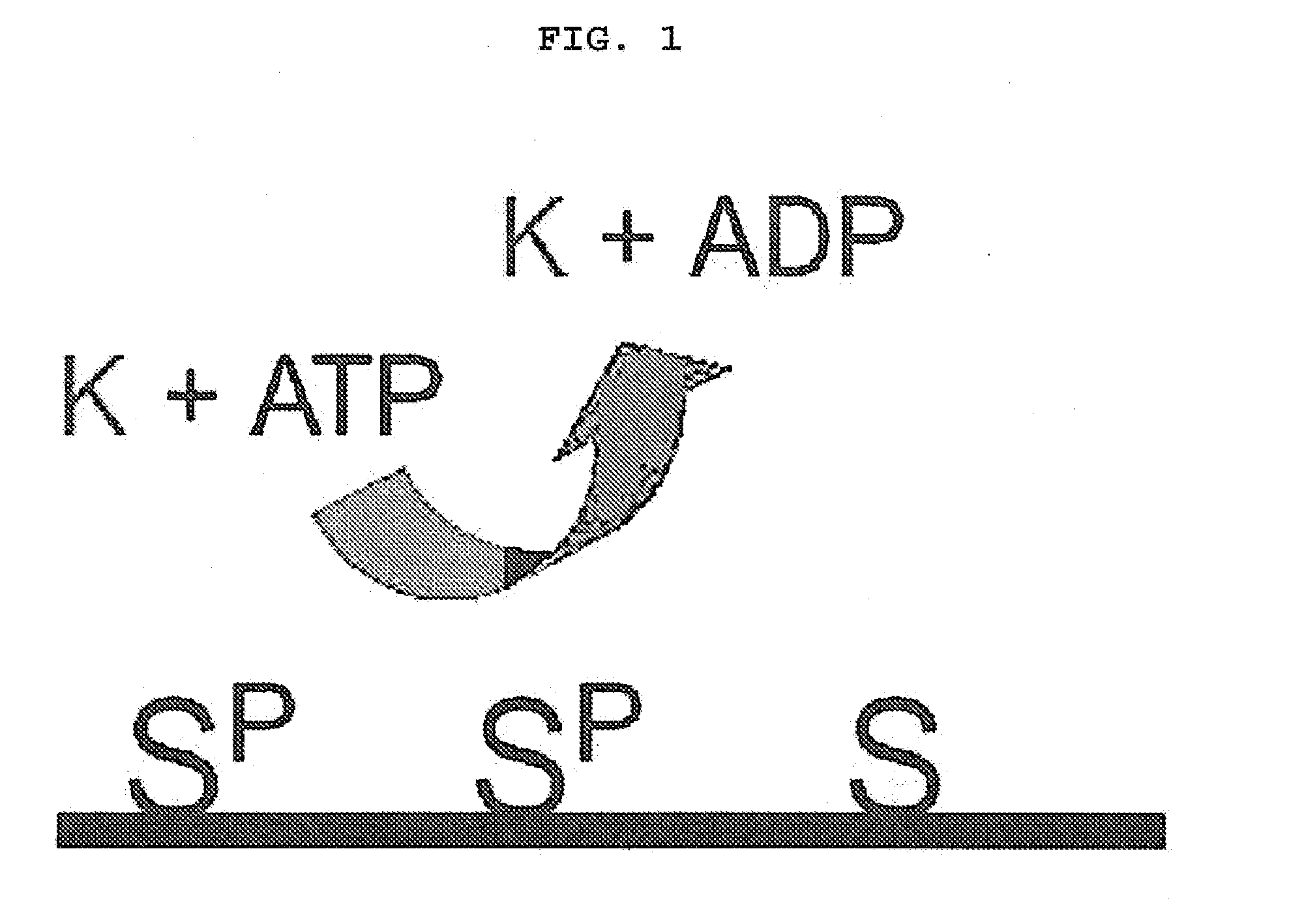
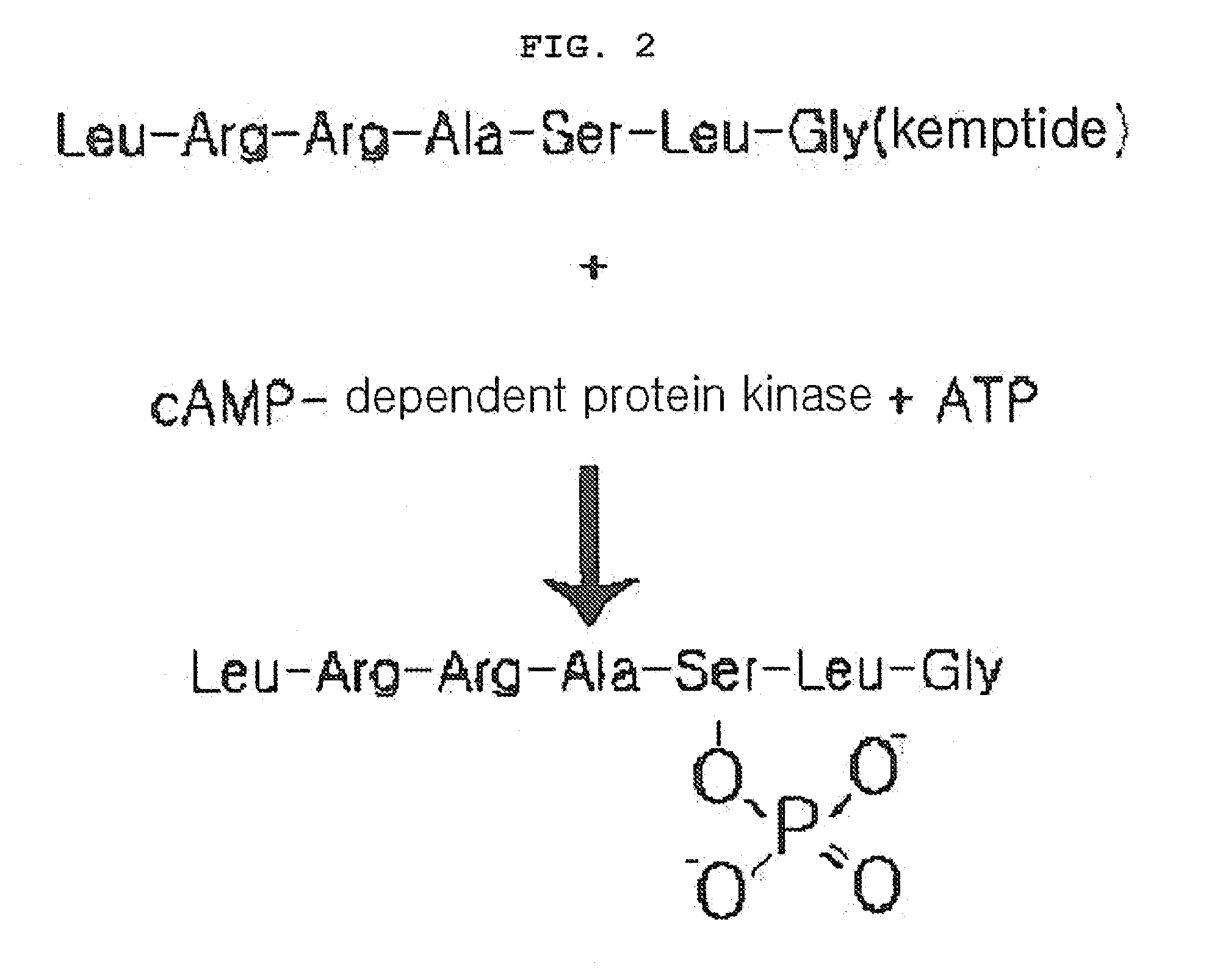
01 Phosphorylation-based biomarkers for diagnostic accuracy
Phosphorylation of specific proteins can serve as biomarkers for various diseases, enhancing diagnostic accuracy. These biomarkers can be detected using specialized assays that measure phosphorylation states of target proteins. The phosphorylation patterns can indicate disease progression or treatment response, providing valuable diagnostic information with improved sensitivity and specificity compared to conventional methods.- Phosphorylation biomarkers for diagnostic accuracy: Phosphorylation-based biomarkers can be utilized in diagnostic tools to improve accuracy in disease detection. These biomarkers involve the measurement of phosphorylated proteins that change during disease states, providing specific molecular signatures. The detection of these phosphorylation patterns enables earlier and more precise diagnosis of various conditions, particularly in cancer and neurological disorders, leading to improved clinical outcomes through targeted therapeutic interventions.
- Machine learning algorithms for phosphorylation data analysis: Advanced machine learning and artificial intelligence algorithms are employed to analyze complex phosphorylation data patterns, significantly enhancing diagnostic accuracy. These computational approaches can identify subtle phosphorylation signatures that might be missed by conventional methods, enabling more precise patient stratification and personalized medicine approaches. The integration of machine learning with phosphoproteomics creates powerful diagnostic tools that can process large datasets and identify clinically relevant phosphorylation patterns.
- Multiplexed phosphorylation detection systems: Multiplexed detection systems allow for simultaneous measurement of multiple phosphorylation events, increasing the diagnostic power and accuracy of these tools. These systems utilize various technologies such as mass spectrometry, antibody arrays, and fluorescence-based detection methods to analyze multiple phosphorylation sites concurrently. This comprehensive approach provides a more complete picture of cellular signaling states, improving diagnostic sensitivity and specificity while reducing the time and sample volume required for analysis.
- Quality control and validation methods for phosphorylation assays: Robust quality control and validation methods are essential for ensuring the reliability and accuracy of phosphorylation-based diagnostic tools. These methods include standardization protocols, reference materials, and statistical approaches to assess assay performance. Implementation of these quality measures helps minimize variability, reduce false results, and ensure consistent performance across different laboratories and testing conditions, ultimately improving the clinical utility of phosphorylation-based diagnostics.
- Point-of-care phosphorylation diagnostic devices: Point-of-care devices for phosphorylation-based diagnostics bring advanced molecular testing closer to patients, enabling rapid results and improved clinical decision-making. These portable systems incorporate miniaturized detection technologies and simplified workflows to measure phosphorylation biomarkers outside of traditional laboratory settings. The development of these accessible diagnostic tools increases the practical utility of phosphorylation analysis in various healthcare settings, from emergency departments to resource-limited environments.
02 Automated analysis systems for phosphorylation detection
Advanced automated systems have been developed to analyze phosphorylation patterns in biological samples. These systems incorporate machine learning algorithms and image processing techniques to quantify phosphorylation levels with high precision. The automation reduces human error and increases throughput, allowing for more reliable and faster diagnostic results in clinical settings.Expand Specific Solutions03 Quality control methods for phosphorylation-based diagnostics
Quality control protocols are essential for ensuring the reliability of phosphorylation-based diagnostic tools. These methods include standardization of sample preparation, calibration of detection instruments, and validation of results against known controls. Implementation of these quality control measures significantly improves the accuracy and reproducibility of phosphorylation-based diagnostic tests across different laboratories.Expand Specific Solutions04 Integration of phosphorylation data with clinical parameters
Combining phosphorylation data with other clinical parameters enhances diagnostic accuracy. Integrated diagnostic approaches incorporate patient history, conventional laboratory tests, and phosphorylation profiles to create comprehensive diagnostic algorithms. This multi-parameter approach provides more context for interpretation and improves the overall accuracy of disease diagnosis and prognosis prediction.Expand Specific Solutions05 Novel phosphorylation detection technologies
Innovative technologies for detecting protein phosphorylation states have been developed to improve diagnostic accuracy. These include fluorescence-based assays, mass spectrometry techniques, and biosensor platforms that can detect phosphorylation events with high sensitivity. These advanced detection methods enable earlier disease detection and more precise monitoring of treatment responses, significantly improving patient outcomes.Expand Specific Solutions
Leading Companies in Phosphoprotein Diagnostics
The phosphorylation-assisted diagnostic tools market is in a growth phase, characterized by increasing adoption of molecular diagnostics and personalized medicine approaches. The global market size is expanding rapidly, driven by rising prevalence of chronic diseases and demand for early detection technologies. Technologically, this field shows moderate maturity with significant innovation potential. Key players include established healthcare giants like Siemens AG and DuPont de Nemours, alongside specialized biotech companies such as Verastem and ARKRAY. Academic institutions including Tsinghua University and Xiamen University contribute significant research advancements. Konica Minolta and Toshiba are leveraging their imaging expertise to develop novel phosphorylation detection platforms, while pharmaceutical companies like Kurita Water Industries are exploring applications in environmental monitoring. The competitive landscape reflects a blend of established corporations and emerging innovators focused on improving sensitivity, specificity, and clinical utility of phosphorylation-based diagnostics.
Verastem, Inc.
Technical Solution: Verastem has developed a comprehensive phosphorylation-based diagnostic platform focusing on cancer detection and treatment monitoring. Their technology leverages phosphoproteomic analysis to identify specific phosphorylation signatures in tumor cells, particularly targeting the focal adhesion kinase (FAK) and PI3K/mTOR signaling pathways. The company's diagnostic tools incorporate proprietary antibodies that recognize specific phosphorylation sites on key proteins involved in cancer progression. Their VS-6766 (RAF/MEK inhibitor) development program includes companion diagnostics that monitor phosphorylation status of downstream targets to predict treatment response and guide personalized therapy decisions. Verastem's platform combines mass spectrometry-based phosphoprotein detection with machine learning algorithms to interpret complex phosphorylation patterns, enabling early detection of treatment resistance mechanisms.
Strengths: Highly specific for cancer-related phosphorylation events with demonstrated clinical validation in detecting treatment resistance before conventional methods. Weaknesses: Limited to specific cancer pathways rather than broad phosphorylation profiling, and requires specialized equipment for analysis that may limit point-of-care applications.
ARKRAY, Inc.
Technical Solution: ARKRAY has pioneered phosphorylation-assisted diagnostic tools primarily focused on diabetes management and metabolic disorders. Their technology platform incorporates phosphorylation-specific biosensors that detect phosphorylated proteins associated with insulin signaling pathways. ARKRAY's diagnostic approach utilizes proprietary enzyme-linked immunosorbent assays (ELISAs) with phospho-specific antibodies to quantify the phosphorylation status of key proteins like insulin receptor substrate (IRS) and protein kinase B (Akt). The company has developed point-of-care testing devices that can rapidly measure phosphorylation levels in minimal blood samples, providing clinicians with immediate information about a patient's metabolic status. Their systems incorporate microfluidic technology to enable multiplexed detection of several phosphorylation sites simultaneously, increasing diagnostic accuracy while maintaining ease of use. ARKRAY has also developed reagent kits that preserve phosphorylation status during sample collection and processing, addressing a critical challenge in phosphoprotein analysis.
Strengths: Excellent sensitivity for detecting subtle changes in phosphorylation status with rapid turnaround time suitable for clinical settings. Weaknesses: Current applications primarily limited to diabetes and metabolic disorders, with less development in other disease areas requiring phosphorylation analysis.
Key Patents in Phosphorylation-Based Biomarkers
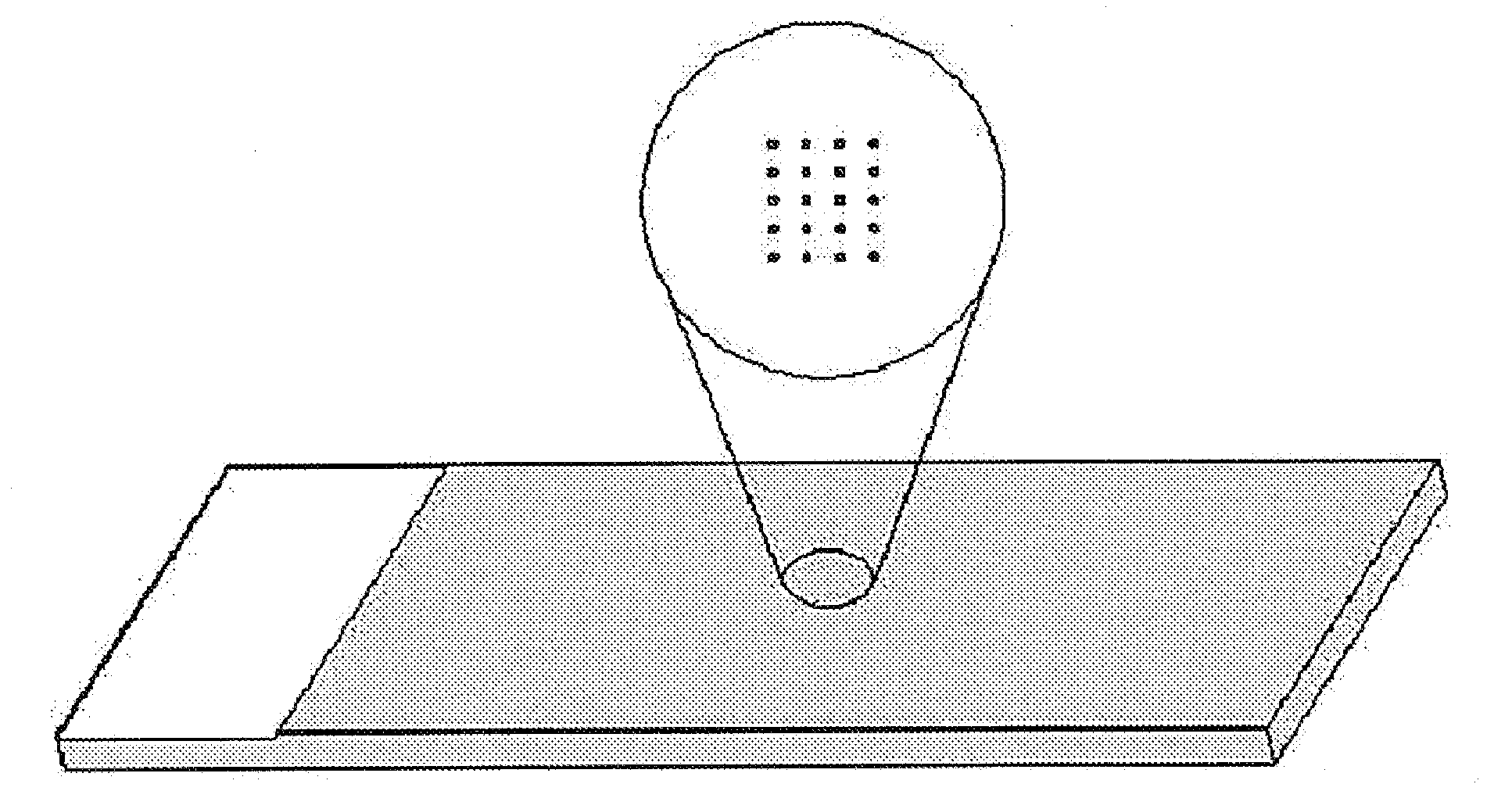
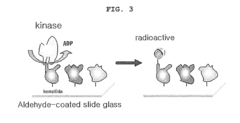
The biochip for the detection of phosphorylation and the detection method using the same
PatentInactiveUS20100029507A1
Innovation
- A biochip integrated with a kinase substrate and radioisotope-labeled cofactor ([γ-32P]ATP) that allows for faster and more accurate phosphorylation detection using a smaller sample size, eliminating the need for blocking materials and simplifying the analysis process.
Electrochemical attachment of phosphonic acids to metallic substrates and osteoconductive medical devices containing same
PatentActiveUS20210207282A1
Innovation
- A method involving the use of organic solvents like alcohols and tetrahydrofuran to solubilize longer chain phosphonic acids, combined with electrochemical attachment in anodization-like conditions, allowing for the attachment of phosphonic acids with chain lengths greater than three carbon atoms to metals such as titanium and aluminum, enhancing surface modification and integration.
Clinical Validation Requirements
Clinical validation represents a critical phase in the development pathway for phosphorylation-assisted diagnostic tools, requiring rigorous testing to ensure accuracy, reliability, and clinical utility before implementation in healthcare settings. Regulatory bodies such as the FDA in the United States and the EMA in Europe mandate comprehensive validation studies that demonstrate both analytical and clinical performance metrics.
The validation process typically begins with analytical validation, establishing the test's precision, accuracy, sensitivity, and specificity under controlled laboratory conditions. For phosphorylation-based diagnostics, this includes verification of phosphorylation site detection limits and reproducibility across different sample types and processing conditions.
Clinical validation subsequently requires prospective studies with patient populations that represent the intended use case. These studies must demonstrate the diagnostic tool's ability to accurately identify disease states or predict treatment responses based on phosphorylation patterns. Minimum sample sizes are typically determined through power calculations, with most regulatory agencies requiring hundreds of samples across diverse demographic groups.
Performance metrics that must be established include positive and negative predictive values, sensitivity, specificity, and area under the ROC curve. For phosphorylation-based diagnostics, additional considerations include the stability of phosphorylation markers during sample collection, transport, and storage, as these post-translational modifications can be highly labile.
Comparison against current gold standard diagnostic methods is essential, with statistical analyses demonstrating non-inferiority or superiority. For novel phosphorylation biomarkers without established comparators, validation must include correlation with clinical outcomes and long-term follow-up data.
Regulatory submissions require detailed documentation of validation protocols, statistical analysis plans, and quality control measures. For phosphorylation-assisted diagnostics, this includes validation of antibody specificity for phospho-epitopes, mass spectrometry calibration protocols, or other detection methodologies specific to phosphorylation status assessment.
Multi-center validation studies are increasingly required to demonstrate reproducibility across different clinical settings and laboratory environments. This is particularly important for phosphorylation-based diagnostics, as sample handling procedures can significantly impact phosphorylation status detection.
Cost-effectiveness analyses are becoming standard components of validation requirements, particularly for novel diagnostic approaches. These analyses must demonstrate the economic value of implementing phosphorylation-assisted diagnostic tools compared to conventional methods, considering both direct costs and downstream healthcare utilization impacts.
The validation process typically begins with analytical validation, establishing the test's precision, accuracy, sensitivity, and specificity under controlled laboratory conditions. For phosphorylation-based diagnostics, this includes verification of phosphorylation site detection limits and reproducibility across different sample types and processing conditions.
Clinical validation subsequently requires prospective studies with patient populations that represent the intended use case. These studies must demonstrate the diagnostic tool's ability to accurately identify disease states or predict treatment responses based on phosphorylation patterns. Minimum sample sizes are typically determined through power calculations, with most regulatory agencies requiring hundreds of samples across diverse demographic groups.
Performance metrics that must be established include positive and negative predictive values, sensitivity, specificity, and area under the ROC curve. For phosphorylation-based diagnostics, additional considerations include the stability of phosphorylation markers during sample collection, transport, and storage, as these post-translational modifications can be highly labile.
Comparison against current gold standard diagnostic methods is essential, with statistical analyses demonstrating non-inferiority or superiority. For novel phosphorylation biomarkers without established comparators, validation must include correlation with clinical outcomes and long-term follow-up data.
Regulatory submissions require detailed documentation of validation protocols, statistical analysis plans, and quality control measures. For phosphorylation-assisted diagnostics, this includes validation of antibody specificity for phospho-epitopes, mass spectrometry calibration protocols, or other detection methodologies specific to phosphorylation status assessment.
Multi-center validation studies are increasingly required to demonstrate reproducibility across different clinical settings and laboratory environments. This is particularly important for phosphorylation-based diagnostics, as sample handling procedures can significantly impact phosphorylation status detection.
Cost-effectiveness analyses are becoming standard components of validation requirements, particularly for novel diagnostic approaches. These analyses must demonstrate the economic value of implementing phosphorylation-assisted diagnostic tools compared to conventional methods, considering both direct costs and downstream healthcare utilization impacts.
Regulatory Pathway for Phosphorylation Diagnostics
The regulatory landscape for phosphorylation-based diagnostic tools involves navigating complex approval processes across different global jurisdictions. In the United States, the FDA categorizes these tools under in vitro diagnostic devices (IVDs), with specific pathways depending on risk classification. Novel phosphorylation assays typically require premarket approval (PMA), while modifications to existing technologies may qualify for the less stringent 510(k) clearance process. The FDA's breakthrough device designation offers accelerated review for diagnostics demonstrating substantial improvement over existing alternatives.
European regulatory frameworks under the In Vitro Diagnostic Regulation (IVDR) implemented in 2022 have significantly increased requirements for clinical evidence and post-market surveillance. Phosphorylation-based diagnostics typically fall under Class C or D, requiring notified body assessment and conformity evaluation before receiving CE marking. Manufacturers must demonstrate analytical performance, clinical performance, and scientific validity through comprehensive technical documentation.
In Asia, Japan's PMDA and China's NMPA maintain distinct regulatory pathways with country-specific requirements for clinical validation studies. These markets increasingly require local clinical data, presenting additional hurdles for global deployment of phosphorylation diagnostic technologies.
Regulatory considerations specifically for phosphorylation-based diagnostics include demonstrating reproducibility of phosphorylation detection methods, validation of phosphorylation site specificity, and establishing clinical utility thresholds. Standardization remains challenging due to pre-analytical variables affecting phosphorylation status, requiring robust protocols for sample collection, handling, and storage.
Reimbursement pathways present another critical regulatory consideration. In the US, obtaining specific CPT codes for novel phosphorylation assays requires demonstrating clinical utility through outcomes studies. The Centers for Medicare & Medicaid Services (CMS) evaluates diagnostic tests through the Clinical Laboratory Fee Schedule, with coverage determinations significantly impacting commercial adoption.
Emerging regulatory trends include the FDA's increased focus on companion diagnostics, particularly relevant for phosphorylation-based tests that guide targeted therapy selection. Additionally, regulatory bodies are developing frameworks for artificial intelligence integration in diagnostic interpretation, potentially benefiting complex phosphoproteomic data analysis. Companies developing phosphorylation diagnostics should engage early with regulatory authorities through pre-submission consultations to establish appropriate validation requirements and expedite the approval process.
European regulatory frameworks under the In Vitro Diagnostic Regulation (IVDR) implemented in 2022 have significantly increased requirements for clinical evidence and post-market surveillance. Phosphorylation-based diagnostics typically fall under Class C or D, requiring notified body assessment and conformity evaluation before receiving CE marking. Manufacturers must demonstrate analytical performance, clinical performance, and scientific validity through comprehensive technical documentation.
In Asia, Japan's PMDA and China's NMPA maintain distinct regulatory pathways with country-specific requirements for clinical validation studies. These markets increasingly require local clinical data, presenting additional hurdles for global deployment of phosphorylation diagnostic technologies.
Regulatory considerations specifically for phosphorylation-based diagnostics include demonstrating reproducibility of phosphorylation detection methods, validation of phosphorylation site specificity, and establishing clinical utility thresholds. Standardization remains challenging due to pre-analytical variables affecting phosphorylation status, requiring robust protocols for sample collection, handling, and storage.
Reimbursement pathways present another critical regulatory consideration. In the US, obtaining specific CPT codes for novel phosphorylation assays requires demonstrating clinical utility through outcomes studies. The Centers for Medicare & Medicaid Services (CMS) evaluates diagnostic tests through the Clinical Laboratory Fee Schedule, with coverage determinations significantly impacting commercial adoption.
Emerging regulatory trends include the FDA's increased focus on companion diagnostics, particularly relevant for phosphorylation-based tests that guide targeted therapy selection. Additionally, regulatory bodies are developing frameworks for artificial intelligence integration in diagnostic interpretation, potentially benefiting complex phosphoproteomic data analysis. Companies developing phosphorylation diagnostics should engage early with regulatory authorities through pre-submission consultations to establish appropriate validation requirements and expedite the approval process.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!