Measure Phosphorylation Kinetics in Metabolic Studies
SEP 23, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Phosphorylation Kinetics Background and Research Objectives
Phosphorylation represents one of the most critical post-translational modifications in biological systems, playing a pivotal role in cellular signaling, metabolic regulation, and energy transfer. Since its discovery in the early 20th century, the study of phosphorylation has evolved from basic biochemical characterization to sophisticated real-time kinetic analyses that reveal the dynamic nature of metabolic processes. This evolution has been driven by technological advancements in analytical chemistry, molecular biology, and computational methods.
The field has witnessed significant milestones, including the Nobel Prize-winning work on reversible protein phosphorylation by Edmond Fischer and Edwin Krebs in the 1950s, which established phosphorylation as a fundamental regulatory mechanism. Subsequent decades saw the identification of hundreds of kinases and phosphatases that orchestrate these processes, culminating in our current understanding of complex phosphorylation networks that govern cellular metabolism.
Recent technological breakthroughs, particularly in mass spectrometry, fluorescence-based techniques, and isotope labeling methods, have revolutionized our ability to measure phosphorylation events with unprecedented temporal and spatial resolution. These advances have shifted the research paradigm from static snapshots to dynamic profiles of phosphorylation cascades, enabling a systems-level understanding of metabolic regulation.
The current trajectory of phosphorylation research is moving toward integrating multi-omics data to create comprehensive models of metabolic networks. This trend is supported by machine learning algorithms that can predict phosphorylation patterns and their metabolic consequences, offering new insights into disease mechanisms and potential therapeutic targets.
The primary objective of this technical research is to evaluate cutting-edge methodologies for measuring phosphorylation kinetics in metabolic studies, with particular emphasis on techniques that offer high temporal resolution, specificity, and sensitivity. We aim to identify approaches that can capture the dynamic nature of phosphorylation events in complex biological systems, from single cells to tissues and organisms.
Additionally, we seek to assess the feasibility of integrating these measurements into broader metabolic profiling efforts, creating a more holistic view of cellular metabolism. This integration would enable researchers to correlate phosphorylation events with metabolite fluxes, providing deeper insights into how phosphorylation regulates metabolic pathways under various physiological and pathological conditions.
The ultimate goal is to establish a technological framework that supports the development of predictive models for metabolic regulation, potentially leading to novel therapeutic strategies for metabolic disorders, cancer, and other diseases characterized by dysregulated phosphorylation.
The field has witnessed significant milestones, including the Nobel Prize-winning work on reversible protein phosphorylation by Edmond Fischer and Edwin Krebs in the 1950s, which established phosphorylation as a fundamental regulatory mechanism. Subsequent decades saw the identification of hundreds of kinases and phosphatases that orchestrate these processes, culminating in our current understanding of complex phosphorylation networks that govern cellular metabolism.
Recent technological breakthroughs, particularly in mass spectrometry, fluorescence-based techniques, and isotope labeling methods, have revolutionized our ability to measure phosphorylation events with unprecedented temporal and spatial resolution. These advances have shifted the research paradigm from static snapshots to dynamic profiles of phosphorylation cascades, enabling a systems-level understanding of metabolic regulation.
The current trajectory of phosphorylation research is moving toward integrating multi-omics data to create comprehensive models of metabolic networks. This trend is supported by machine learning algorithms that can predict phosphorylation patterns and their metabolic consequences, offering new insights into disease mechanisms and potential therapeutic targets.
The primary objective of this technical research is to evaluate cutting-edge methodologies for measuring phosphorylation kinetics in metabolic studies, with particular emphasis on techniques that offer high temporal resolution, specificity, and sensitivity. We aim to identify approaches that can capture the dynamic nature of phosphorylation events in complex biological systems, from single cells to tissues and organisms.
Additionally, we seek to assess the feasibility of integrating these measurements into broader metabolic profiling efforts, creating a more holistic view of cellular metabolism. This integration would enable researchers to correlate phosphorylation events with metabolite fluxes, providing deeper insights into how phosphorylation regulates metabolic pathways under various physiological and pathological conditions.
The ultimate goal is to establish a technological framework that supports the development of predictive models for metabolic regulation, potentially leading to novel therapeutic strategies for metabolic disorders, cancer, and other diseases characterized by dysregulated phosphorylation.
Market Applications for Metabolic Phosphorylation Analysis
The phosphorylation analysis market in metabolic studies spans multiple sectors with significant growth potential. The healthcare sector represents the largest application area, where phosphorylation kinetics measurements are crucial for understanding metabolic disorders such as diabetes, obesity, and cardiovascular diseases. These analyses enable precise monitoring of insulin signaling pathways and energy metabolism regulation, directly supporting diagnostic development and personalized medicine approaches.
Pharmaceutical research and development constitutes another major market segment, with companies investing heavily in phosphorylation analysis technologies to develop metabolic disorder treatments. The ability to measure phosphorylation kinetics in real-time provides critical data for drug discovery processes, allowing researchers to evaluate compound efficacy and mechanism of action at the molecular level. This application has become essential in the development pipeline for metabolic therapeutics.
The academic research market continues to expand as institutions worldwide investigate fundamental metabolic processes. Phosphorylation analysis tools support basic science investigations that ultimately feed into translational research. This sector drives innovation in methodology and contributes significantly to technology advancement despite having more limited budgets than commercial entities.
Biotechnology companies represent a rapidly growing market segment, particularly those focused on metabolic engineering and synthetic biology. These firms utilize phosphorylation kinetics measurements to optimize cellular metabolic pathways for bioproduction of chemicals, fuels, and pharmaceuticals. The ability to monitor phosphorylation events in engineered organisms provides crucial feedback for strain improvement and process optimization.
The agricultural sector has emerged as an unexpected growth area for metabolic phosphorylation analysis. Researchers apply these technologies to understand plant metabolism under various environmental conditions, supporting the development of more resilient and productive crop varieties. This application addresses critical challenges in food security and sustainable agriculture.
Clinical diagnostics represents perhaps the most promising future market, with phosphorylation-based biomarkers showing potential for early disease detection and treatment monitoring. Several diagnostic companies are developing platforms that can measure specific phosphorylation signatures associated with metabolic dysfunction, creating opportunities for preventive healthcare approaches and more effective disease management.
The sports science and nutrition industry has also begun adopting phosphorylation analysis technologies to optimize athletic performance and recovery through precise metabolic monitoring. This niche market demonstrates how the technology continues to find novel applications beyond traditional research settings.
Pharmaceutical research and development constitutes another major market segment, with companies investing heavily in phosphorylation analysis technologies to develop metabolic disorder treatments. The ability to measure phosphorylation kinetics in real-time provides critical data for drug discovery processes, allowing researchers to evaluate compound efficacy and mechanism of action at the molecular level. This application has become essential in the development pipeline for metabolic therapeutics.
The academic research market continues to expand as institutions worldwide investigate fundamental metabolic processes. Phosphorylation analysis tools support basic science investigations that ultimately feed into translational research. This sector drives innovation in methodology and contributes significantly to technology advancement despite having more limited budgets than commercial entities.
Biotechnology companies represent a rapidly growing market segment, particularly those focused on metabolic engineering and synthetic biology. These firms utilize phosphorylation kinetics measurements to optimize cellular metabolic pathways for bioproduction of chemicals, fuels, and pharmaceuticals. The ability to monitor phosphorylation events in engineered organisms provides crucial feedback for strain improvement and process optimization.
The agricultural sector has emerged as an unexpected growth area for metabolic phosphorylation analysis. Researchers apply these technologies to understand plant metabolism under various environmental conditions, supporting the development of more resilient and productive crop varieties. This application addresses critical challenges in food security and sustainable agriculture.
Clinical diagnostics represents perhaps the most promising future market, with phosphorylation-based biomarkers showing potential for early disease detection and treatment monitoring. Several diagnostic companies are developing platforms that can measure specific phosphorylation signatures associated with metabolic dysfunction, creating opportunities for preventive healthcare approaches and more effective disease management.
The sports science and nutrition industry has also begun adopting phosphorylation analysis technologies to optimize athletic performance and recovery through precise metabolic monitoring. This niche market demonstrates how the technology continues to find novel applications beyond traditional research settings.
Current Challenges in Phosphorylation Measurement Techniques
Despite significant advancements in phosphorylation measurement techniques, researchers face several persistent challenges when attempting to accurately measure phosphorylation kinetics in metabolic studies. The temporal resolution of current methods remains a significant limitation, as many cellular phosphorylation events occur within milliseconds to seconds, while most analytical techniques require minutes to hours for sample preparation and analysis, creating a fundamental mismatch between biological processes and measurement capabilities.
Sample preparation introduces considerable variability and potential artifacts. The rapid nature of phosphorylation/dephosphorylation cycles means that phosphorylation states can change during sample collection and processing. Even with rapid quenching techniques, maintaining the native phosphorylation state throughout the analytical workflow remains problematic, particularly in complex biological matrices like tissue samples or blood.
Sensitivity thresholds present another major challenge. Many physiologically relevant phosphorylation events involve low-abundance proteins or occur at substoichiometric levels, making detection difficult with conventional methods. While mass spectrometry has improved detection limits, quantifying low-abundance phosphorylation events in complex biological samples still requires significant enrichment steps that can introduce bias.
Multiplexing capabilities are currently insufficient for comprehensive phosphorylation network analysis. Metabolic processes involve complex signaling networks with numerous phosphorylation sites acting in concert. Most techniques can only measure a limited number of phosphorylation events simultaneously, preventing researchers from capturing the full complexity of phosphorylation networks in real-time.
Quantitative accuracy remains problematic across different measurement platforms. Variations in antibody specificity for immunoassays, ionization efficiency in mass spectrometry, and signal-to-noise ratios in fluorescence-based methods all contribute to quantification challenges. Absolute quantification of phosphorylation stoichiometry (the proportion of a protein that is phosphorylated) is particularly difficult to achieve reliably.
In vivo measurement capabilities are severely limited. Most current techniques require cell lysis or tissue homogenization, preventing continuous monitoring of phosphorylation dynamics in living systems. While some fluorescence-based biosensors allow in vivo measurements, they typically cover only a small subset of phosphorylation events and may interfere with normal cellular processes.
Data integration and interpretation present significant computational challenges. The high-dimensional data generated from phosphorylation studies requires sophisticated computational approaches to extract meaningful biological insights. Current analytical pipelines struggle to integrate phosphorylation data with other omics datasets to provide a comprehensive view of metabolic regulation.
Sample preparation introduces considerable variability and potential artifacts. The rapid nature of phosphorylation/dephosphorylation cycles means that phosphorylation states can change during sample collection and processing. Even with rapid quenching techniques, maintaining the native phosphorylation state throughout the analytical workflow remains problematic, particularly in complex biological matrices like tissue samples or blood.
Sensitivity thresholds present another major challenge. Many physiologically relevant phosphorylation events involve low-abundance proteins or occur at substoichiometric levels, making detection difficult with conventional methods. While mass spectrometry has improved detection limits, quantifying low-abundance phosphorylation events in complex biological samples still requires significant enrichment steps that can introduce bias.
Multiplexing capabilities are currently insufficient for comprehensive phosphorylation network analysis. Metabolic processes involve complex signaling networks with numerous phosphorylation sites acting in concert. Most techniques can only measure a limited number of phosphorylation events simultaneously, preventing researchers from capturing the full complexity of phosphorylation networks in real-time.
Quantitative accuracy remains problematic across different measurement platforms. Variations in antibody specificity for immunoassays, ionization efficiency in mass spectrometry, and signal-to-noise ratios in fluorescence-based methods all contribute to quantification challenges. Absolute quantification of phosphorylation stoichiometry (the proportion of a protein that is phosphorylated) is particularly difficult to achieve reliably.
In vivo measurement capabilities are severely limited. Most current techniques require cell lysis or tissue homogenization, preventing continuous monitoring of phosphorylation dynamics in living systems. While some fluorescence-based biosensors allow in vivo measurements, they typically cover only a small subset of phosphorylation events and may interfere with normal cellular processes.
Data integration and interpretation present significant computational challenges. The high-dimensional data generated from phosphorylation studies requires sophisticated computational approaches to extract meaningful biological insights. Current analytical pipelines struggle to integrate phosphorylation data with other omics datasets to provide a comprehensive view of metabolic regulation.
Established Protocols for Phosphorylation Kinetics Analysis
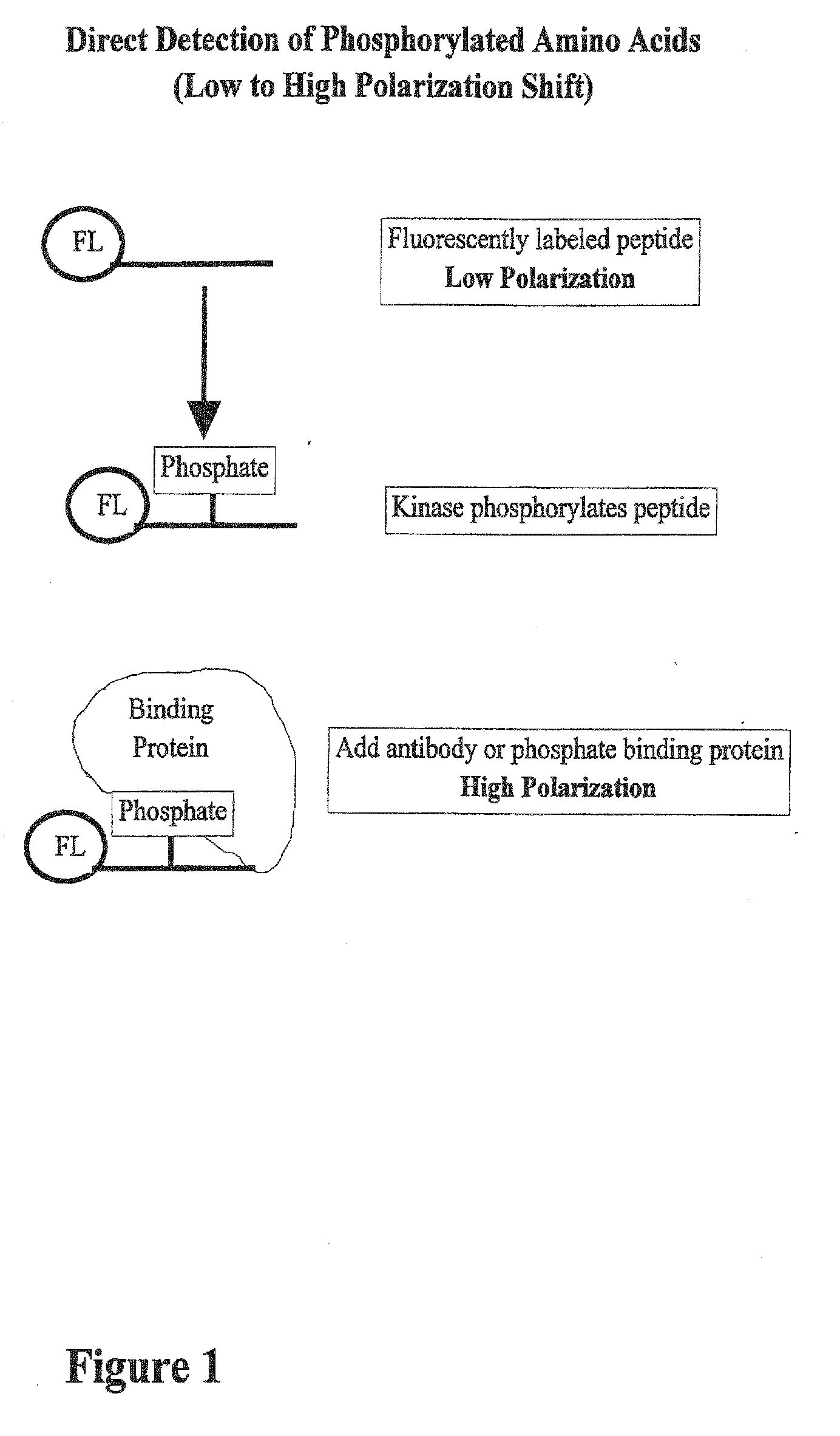
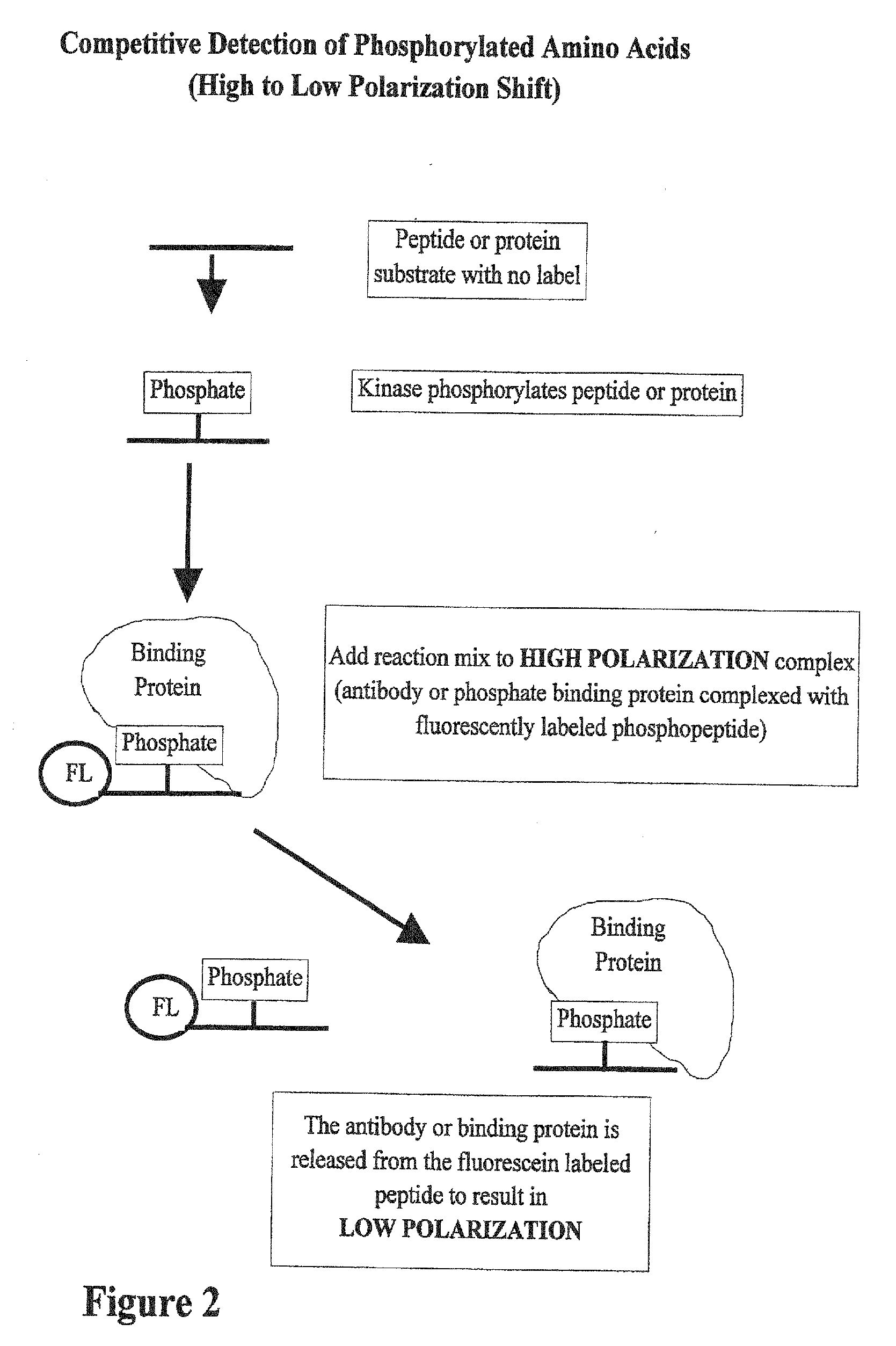
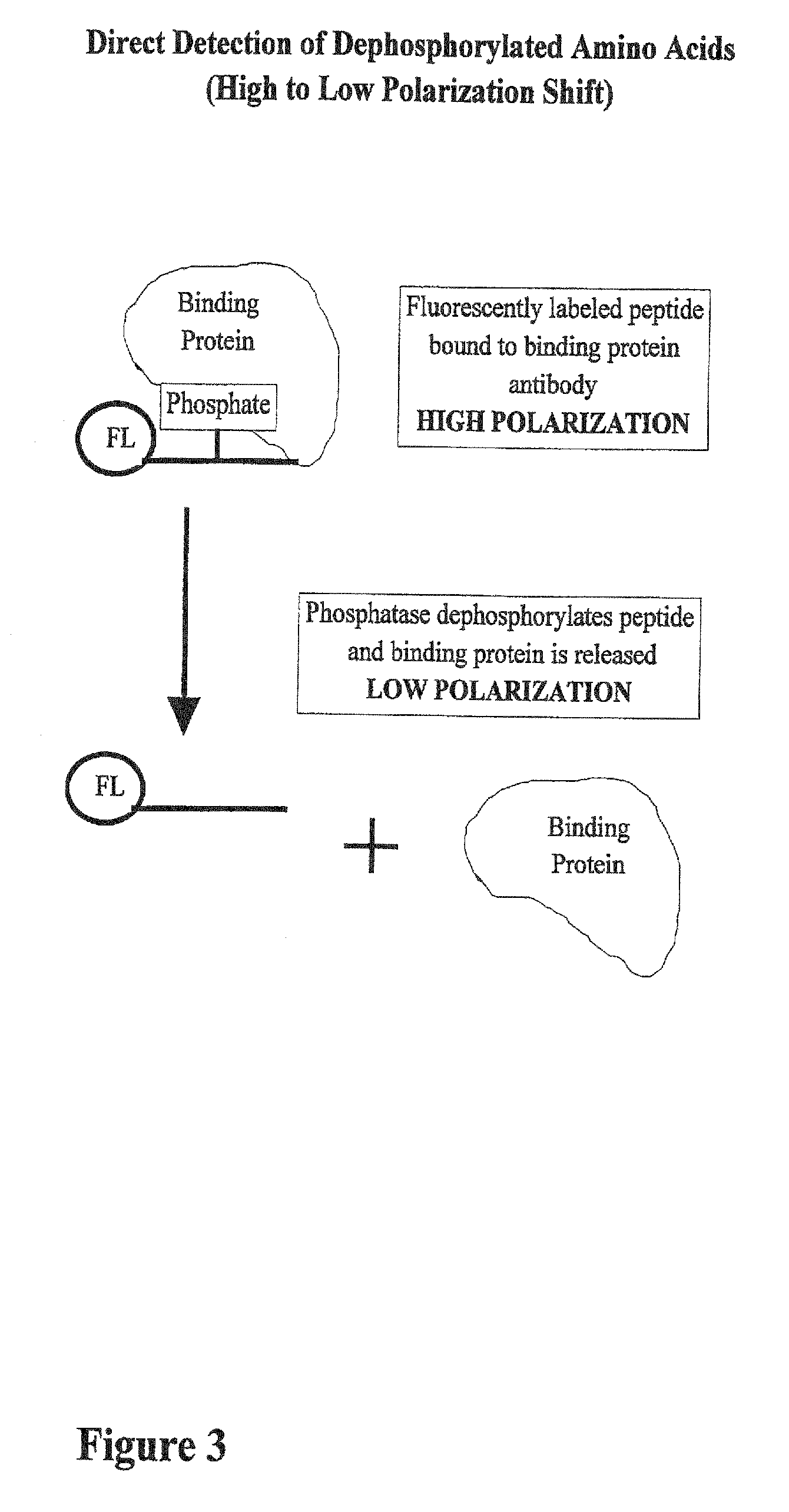
01 Fluorescence-based phosphorylation detection methods
Fluorescence-based techniques are widely used for measuring phosphorylation kinetics. These methods utilize fluorescent probes or tags that bind specifically to phosphorylated proteins or peptides, allowing real-time monitoring of phosphorylation events. The intensity of fluorescence signals correlates with the degree of phosphorylation, enabling quantitative analysis of kinase activity and phosphorylation rates. These techniques offer high sensitivity and can be adapted for high-throughput screening applications.- Methods for measuring protein phosphorylation kinetics: Various techniques and methods have been developed to measure the kinetics of protein phosphorylation reactions. These methods involve monitoring the rate at which phosphate groups are added to proteins by kinases or removed by phosphatases. Advanced analytical techniques such as mass spectrometry, fluorescence-based assays, and radiometric methods are commonly employed to track phosphorylation events in real-time or at specific time intervals, providing valuable data on reaction rates, enzyme efficiency, and substrate specificity.
- High-throughput screening platforms for phosphorylation analysis: High-throughput platforms have been developed to analyze phosphorylation kinetics across multiple samples simultaneously. These systems often incorporate automated liquid handling, microarray technology, and integrated detection systems to rapidly measure phosphorylation events. Such platforms enable efficient screening of kinase inhibitors, substrate specificity profiling, and comprehensive phosphorylation network analysis, significantly accelerating research in drug discovery and systems biology.
- Biosensors and imaging techniques for real-time phosphorylation monitoring: Advanced biosensors and imaging techniques have been developed to monitor phosphorylation kinetics in real-time and in living cells. These approaches often utilize fluorescent or luminescent reporters that change their signal properties upon phosphorylation. Techniques such as FRET (Förster Resonance Energy Transfer), bioluminescence, and phospho-specific antibody-based sensors allow researchers to visualize phosphorylation events with high temporal and spatial resolution, providing insights into the dynamics of signaling pathways in their native cellular context.
- Computational models and algorithms for phosphorylation kinetics analysis: Computational approaches have been developed to analyze and model phosphorylation kinetics data. These include mathematical models, machine learning algorithms, and statistical methods that can process complex phosphorylation datasets to extract kinetic parameters, identify patterns, and predict system behaviors. Such computational tools are essential for interpreting experimental data, simulating phosphorylation networks, and understanding the quantitative aspects of signal transduction pathways in normal and disease states.
- Applications of phosphorylation kinetics in drug discovery and disease diagnosis: Phosphorylation kinetics measurements have important applications in drug discovery and disease diagnosis. By understanding the rates and mechanisms of phosphorylation in disease-relevant pathways, researchers can identify potential therapeutic targets and develop kinase inhibitors with optimal properties. Additionally, abnormal phosphorylation kinetics can serve as biomarkers for various diseases, including cancer and neurodegenerative disorders, enabling early diagnosis and personalized treatment approaches based on individual phosphorylation profiles.
02 Mass spectrometry-based phosphorylation analysis
Mass spectrometry has emerged as a powerful tool for measuring phosphorylation kinetics with high precision. This approach allows for the identification and quantification of phosphorylated residues within proteins, enabling detailed kinetic studies of phosphorylation events. Advanced mass spectrometry techniques can detect multiple phosphorylation sites simultaneously and track changes in phosphorylation status over time, providing comprehensive insights into signaling pathways and regulatory mechanisms.Expand Specific Solutions03 Microarray and chip-based phosphorylation assays
Microarray and chip-based platforms enable high-throughput measurement of phosphorylation kinetics. These systems typically involve immobilizing substrate proteins or peptides on a solid surface and monitoring phosphorylation reactions using various detection methods. The miniaturized format allows for parallel analysis of multiple samples with minimal reagent consumption. These technologies are particularly valuable for screening kinase inhibitors and studying complex phosphorylation networks in various biological contexts.Expand Specific Solutions04 Computational modeling of phosphorylation kinetics
Computational approaches have been developed to model and predict phosphorylation kinetics based on experimental data. These methods employ mathematical algorithms and statistical analyses to interpret complex phosphorylation patterns and extract kinetic parameters. Computational models can simulate phosphorylation dynamics under various conditions, helping to understand the temporal aspects of signaling pathways and predict system responses to perturbations. These approaches complement experimental techniques and provide deeper insights into phosphorylation mechanisms.Expand Specific Solutions05 Antibody-based phosphorylation detection systems
Antibody-based methods utilize phospho-specific antibodies that selectively recognize phosphorylated proteins or specific phosphorylation sites. These techniques include ELISA, Western blotting, and immunoprecipitation approaches adapted for kinetic measurements. By sampling reactions at different time points, researchers can track phosphorylation progression and determine reaction rates. The high specificity of phospho-antibodies enables precise monitoring of individual phosphorylation events within complex biological samples.Expand Specific Solutions
Leading Research Groups and Commercial Entities
Phosphorylation kinetics measurement in metabolic studies is currently in a growth phase, with the market expanding due to increasing applications in drug development and disease research. The global market size is estimated to exceed $2 billion, driven by rising demand for precision medicine and metabolic disorder treatments. Technologically, the field shows moderate maturity with established techniques, but significant innovation continues. Leading players include Life Technologies Corp. and F. Hoffmann-La Roche Ltd., who offer commercial platforms, while academic institutions like Tsinghua University and University of California contribute fundamental research advances. Vertex Pharmaceuticals and Biogen are leveraging phosphorylation analysis for drug development, while specialized companies like PamGene BV provide niche solutions with proprietary microarray technologies for kinase activity profiling.
Life Technologies Corp.
Technical Solution: Life Technologies has developed advanced phosphoproteomics platforms for measuring phosphorylation kinetics in metabolic studies. Their SILAC (Stable Isotope Labeling with Amino acids in Cell culture) technology enables quantitative analysis of phosphorylation events over time by incorporating isotopically labeled amino acids into proteins. This approach is complemented by their high-resolution mass spectrometry systems that can detect phosphopeptides with exceptional sensitivity. Their integrated workflow includes phosphopeptide enrichment techniques using titanium dioxide (TiO2) and immobilized metal affinity chromatography (IMAC), which significantly increases the coverage of phosphorylation sites. Life Technologies' real-time kinase activity assays allow researchers to monitor phosphorylation events in living cells, providing dynamic information about metabolic pathways and their regulation.
Strengths: Comprehensive end-to-end solutions from sample preparation to analysis; high sensitivity detection of low-abundance phosphopeptides; ability to quantify thousands of phosphorylation sites in a single experiment. Weaknesses: High cost of instrumentation and reagents; requires significant technical expertise; complex data analysis workflows that may be challenging for non-specialists.
F. Hoffmann-La Roche Ltd.
Technical Solution: Roche has pioneered innovative approaches for measuring phosphorylation kinetics in metabolic studies through their PhosphoFlow technology platform. This system combines flow cytometry with phospho-specific antibodies to analyze phosphorylation events at the single-cell level with temporal resolution. Their technology enables researchers to track multiple phosphorylation sites simultaneously across different cell populations, providing insights into cell-specific metabolic responses. Roche has also developed multiplexed immunoassay platforms that can quantify dozens of phosphoproteins from minimal sample volumes, making it suitable for clinical specimens. Their integrated bioinformatics tools facilitate the interpretation of complex phosphorylation networks in the context of metabolic pathways. Additionally, Roche offers isotope-labeled ATP analogs that can be used to track phosphorylation kinetics in real-time, providing direct measurements of enzyme activity in complex biological systems.
Strengths: Single-cell resolution allows for heterogeneity analysis in mixed cell populations; high throughput capabilities suitable for screening applications; minimal sample requirements making it applicable to precious clinical samples. Weaknesses: Limited to known phosphorylation sites with available antibodies; potential cross-reactivity issues with antibody-based detection; less comprehensive than mass spectrometry-based approaches for discovery applications.
Key Technologies in Phosphorylation Site Identification
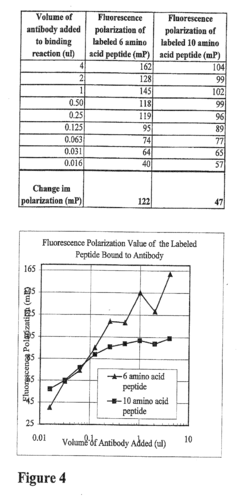
Kinase and phosphatase activity measurement using fluorescent polarization
PatentInactiveUS20070026475A1
Innovation
- A fluorescence polarization assay that measures kinase activity by detecting changes in polarization values of fluorescently labeled molecules, allowing for real-time, homogeneous measurements without the need for separation or immobilization, using a competition assay with a reporter complex and binding molecules to quantify phosphorylated amino acids and enzyme activity.
Assays for measuring phosphate modification enzyme activity
PatentInactiveIN2430MUM2007A
Innovation
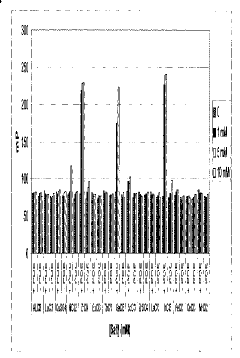
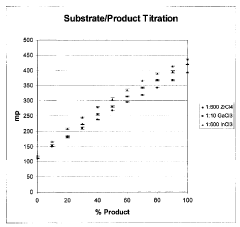
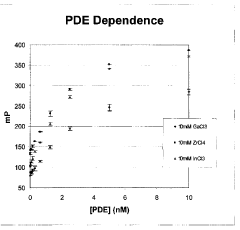
- The use of indium (III) and zirconium (IV) ions, which specifically bind to phosphorylated molecules, allowing for the development of assays that measure enzyme activity and screen for modulating substances by correlating binding extent with enzyme activity in kinases, phosphatases, cyclases, and phosphodiesterases.
Computational Tools for Phosphorylation Data Analysis
The computational landscape for phosphorylation data analysis has evolved significantly in recent years, with specialized tools designed to handle the complex kinetic data generated in metabolic studies. Mass spectrometry-based phosphoproteomics generates enormous datasets that require sophisticated computational approaches for proper interpretation and analysis.
Several categories of computational tools have emerged as essential for phosphorylation kinetics analysis. Data processing tools like MaxQuant, Skyline, and OpenMS provide robust platforms for the initial processing of raw mass spectrometry data, offering capabilities for peak detection, quantification, and identification of phosphopeptides with high accuracy and reproducibility.
Statistical analysis frameworks have been developed specifically for phosphorylation data, including Perseus, MSstats, and LIMMA, which enable researchers to perform normalization, statistical testing, and visualization of phosphorylation dynamics across different experimental conditions and time points. These tools incorporate specialized algorithms that account for the unique characteristics of phosphoproteomics data.
Kinetic modeling software represents another critical category, with tools like KinasePA, PTMapper, and PhosphoSiteAnalyzer designed to model phosphorylation and dephosphorylation rates based on time-series data. These applications implement various mathematical models, from simple first-order kinetics to complex differential equations that capture the nuances of enzymatic reactions in cellular contexts.
Network analysis tools such as Cytoscape with specialized plugins (PhosphoPath, KinomeXplorer) facilitate the visualization and analysis of phosphorylation networks, enabling researchers to identify key regulatory nodes and signaling pathways affected in metabolic studies. These tools often integrate with public databases of known kinase-substrate relationships to provide biological context.
Machine learning approaches are increasingly being applied to phosphorylation data analysis, with algorithms capable of predicting kinase activities from phosphoproteomics data, identifying patterns in phosphorylation dynamics, and classifying phosphorylation events based on their functional significance. Tools like KinCon, NetworKIN, and DeepPhos represent this emerging trend.
Integration platforms that combine multiple analytical approaches are becoming more prevalent, allowing researchers to perform comprehensive analyses within a single computational environment. Examples include PHOSIDA, PhosphoSitePlus, and Phospho.ELM, which integrate data processing, statistical analysis, and biological interpretation capabilities.
Several categories of computational tools have emerged as essential for phosphorylation kinetics analysis. Data processing tools like MaxQuant, Skyline, and OpenMS provide robust platforms for the initial processing of raw mass spectrometry data, offering capabilities for peak detection, quantification, and identification of phosphopeptides with high accuracy and reproducibility.
Statistical analysis frameworks have been developed specifically for phosphorylation data, including Perseus, MSstats, and LIMMA, which enable researchers to perform normalization, statistical testing, and visualization of phosphorylation dynamics across different experimental conditions and time points. These tools incorporate specialized algorithms that account for the unique characteristics of phosphoproteomics data.
Kinetic modeling software represents another critical category, with tools like KinasePA, PTMapper, and PhosphoSiteAnalyzer designed to model phosphorylation and dephosphorylation rates based on time-series data. These applications implement various mathematical models, from simple first-order kinetics to complex differential equations that capture the nuances of enzymatic reactions in cellular contexts.
Network analysis tools such as Cytoscape with specialized plugins (PhosphoPath, KinomeXplorer) facilitate the visualization and analysis of phosphorylation networks, enabling researchers to identify key regulatory nodes and signaling pathways affected in metabolic studies. These tools often integrate with public databases of known kinase-substrate relationships to provide biological context.
Machine learning approaches are increasingly being applied to phosphorylation data analysis, with algorithms capable of predicting kinase activities from phosphoproteomics data, identifying patterns in phosphorylation dynamics, and classifying phosphorylation events based on their functional significance. Tools like KinCon, NetworKIN, and DeepPhos represent this emerging trend.
Integration platforms that combine multiple analytical approaches are becoming more prevalent, allowing researchers to perform comprehensive analyses within a single computational environment. Examples include PHOSIDA, PhosphoSitePlus, and Phospho.ELM, which integrate data processing, statistical analysis, and biological interpretation capabilities.
Standardization and Reproducibility Challenges
The standardization and reproducibility of phosphorylation kinetics measurements in metabolic studies face significant challenges that impact research quality and cross-laboratory comparisons. Current methodologies exhibit considerable variability in sample preparation protocols, with differences in cell lysis buffers, phosphatase inhibitor concentrations, and handling times introducing substantial experimental noise. These variations can dramatically alter phosphorylation states before measurement even begins, creating artificial differences unrelated to biological phenomena.
Instrument calibration represents another critical challenge, as mass spectrometry platforms, Western blotting systems, and flow cytometry equipment require different calibration standards and procedures. The absence of universally accepted reference materials for phosphorylated proteins compounds this issue, making absolute quantification particularly problematic. Laboratories often develop internal standards that lack broader validation, limiting data comparability across research groups.
Data normalization approaches further complicate reproducibility efforts. The field employs diverse normalization strategies—from total protein normalization to housekeeping protein references and isotope labeling techniques—each introducing its own biases. This methodological heterogeneity makes meta-analyses challenging and hinders the establishment of reliable kinetic parameters for phosphorylation events in metabolic pathways.
Environmental factors introduce additional variability, as temperature fluctuations, humidity levels, and even circadian rhythms can influence enzyme kinetics and phosphorylation rates. These factors are rarely controlled consistently across laboratories, creating another layer of complexity when attempting to reproduce published findings.
Statistical analysis practices vary widely as well, with inconsistent approaches to outlier identification, curve fitting for kinetic data, and significance testing. The field lacks consensus on minimum reporting standards for experimental conditions and analytical parameters, making it difficult to evaluate methodological rigor or replicate published protocols with precision.
Recent collaborative efforts have begun addressing these challenges through initiatives like the Phosphorylation Assay Standardization Consortium (PASC), which aims to develop standardized protocols and reference materials. Additionally, journals increasingly require detailed methodological reporting and raw data availability. Technology developers are also contributing by creating automated systems with built-in quality controls and standardized workflows to reduce operator-dependent variability. Despite these advances, achieving robust reproducibility in phosphorylation kinetics measurements remains an ongoing challenge requiring coordinated effort across the metabolic research community.
Instrument calibration represents another critical challenge, as mass spectrometry platforms, Western blotting systems, and flow cytometry equipment require different calibration standards and procedures. The absence of universally accepted reference materials for phosphorylated proteins compounds this issue, making absolute quantification particularly problematic. Laboratories often develop internal standards that lack broader validation, limiting data comparability across research groups.
Data normalization approaches further complicate reproducibility efforts. The field employs diverse normalization strategies—from total protein normalization to housekeeping protein references and isotope labeling techniques—each introducing its own biases. This methodological heterogeneity makes meta-analyses challenging and hinders the establishment of reliable kinetic parameters for phosphorylation events in metabolic pathways.
Environmental factors introduce additional variability, as temperature fluctuations, humidity levels, and even circadian rhythms can influence enzyme kinetics and phosphorylation rates. These factors are rarely controlled consistently across laboratories, creating another layer of complexity when attempting to reproduce published findings.
Statistical analysis practices vary widely as well, with inconsistent approaches to outlier identification, curve fitting for kinetic data, and significance testing. The field lacks consensus on minimum reporting standards for experimental conditions and analytical parameters, making it difficult to evaluate methodological rigor or replicate published protocols with precision.
Recent collaborative efforts have begun addressing these challenges through initiatives like the Phosphorylation Assay Standardization Consortium (PASC), which aims to develop standardized protocols and reference materials. Additionally, journals increasingly require detailed methodological reporting and raw data availability. Technology developers are also contributing by creating automated systems with built-in quality controls and standardized workflows to reduce operator-dependent variability. Despite these advances, achieving robust reproducibility in phosphorylation kinetics measurements remains an ongoing challenge requiring coordinated effort across the metabolic research community.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!