Measure Linkages Between Phosphorylation and Disease States
SEP 23, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Phosphorylation-Disease Linkage Background and Objectives
Protein phosphorylation represents one of the most fundamental post-translational modifications in cellular biology, playing a critical role in signal transduction pathways and regulatory mechanisms. Since its discovery in the 1950s, our understanding of phosphorylation has evolved from recognizing it as a simple biochemical process to appreciating its complex role as a master regulator of cellular function. This evolution in understanding has paralleled technological advancements that have enabled increasingly sophisticated analyses of phosphorylation events.
The historical trajectory of phosphorylation research reveals a progressive deepening of knowledge, from initial identification of phosphorylated proteins to current high-throughput phosphoproteomics approaches that can identify thousands of phosphorylation sites simultaneously. This technological progression has enabled researchers to move beyond studying isolated phosphorylation events to examining comprehensive phosphorylation networks and their dynamic changes in response to various stimuli.
Recent years have witnessed a paradigm shift in how we conceptualize the relationship between phosphorylation and disease states. Aberrant phosphorylation patterns have been implicated in numerous pathological conditions, including cancer, neurodegenerative disorders, metabolic diseases, and inflammatory conditions. The recognition that phosphorylation dysregulation represents not merely a consequence but often a driver of disease has elevated its importance as both a diagnostic biomarker and therapeutic target.
The primary objective of measuring linkages between phosphorylation and disease states is to establish causal relationships that can inform both diagnostic and therapeutic strategies. This involves developing methodologies that can accurately quantify phosphorylation events in clinical samples, correlate specific phosphorylation signatures with disease phenotypes, and distinguish pathological phosphorylation patterns from normal biological variation.
A secondary but equally important objective is to elucidate the temporal dynamics of phosphorylation changes during disease progression. Understanding how phosphorylation networks evolve from early to advanced disease stages could potentially identify critical intervention points and enable earlier diagnosis before clinical symptoms manifest.
The ultimate goal of this technological pursuit extends beyond basic scientific understanding to practical clinical applications. These include developing phosphorylation-based diagnostic tests with improved sensitivity and specificity, identifying novel therapeutic targets within dysregulated phosphorylation networks, and enabling personalized medicine approaches through phosphorylation profiling of individual patients.
As we advance in this field, emerging trends point toward integrating phosphorylation data with other omics platforms to create multi-dimensional views of disease processes, and developing computational models that can predict how perturbations in phosphorylation networks might influence disease trajectories and treatment responses.
The historical trajectory of phosphorylation research reveals a progressive deepening of knowledge, from initial identification of phosphorylated proteins to current high-throughput phosphoproteomics approaches that can identify thousands of phosphorylation sites simultaneously. This technological progression has enabled researchers to move beyond studying isolated phosphorylation events to examining comprehensive phosphorylation networks and their dynamic changes in response to various stimuli.
Recent years have witnessed a paradigm shift in how we conceptualize the relationship between phosphorylation and disease states. Aberrant phosphorylation patterns have been implicated in numerous pathological conditions, including cancer, neurodegenerative disorders, metabolic diseases, and inflammatory conditions. The recognition that phosphorylation dysregulation represents not merely a consequence but often a driver of disease has elevated its importance as both a diagnostic biomarker and therapeutic target.
The primary objective of measuring linkages between phosphorylation and disease states is to establish causal relationships that can inform both diagnostic and therapeutic strategies. This involves developing methodologies that can accurately quantify phosphorylation events in clinical samples, correlate specific phosphorylation signatures with disease phenotypes, and distinguish pathological phosphorylation patterns from normal biological variation.
A secondary but equally important objective is to elucidate the temporal dynamics of phosphorylation changes during disease progression. Understanding how phosphorylation networks evolve from early to advanced disease stages could potentially identify critical intervention points and enable earlier diagnosis before clinical symptoms manifest.
The ultimate goal of this technological pursuit extends beyond basic scientific understanding to practical clinical applications. These include developing phosphorylation-based diagnostic tests with improved sensitivity and specificity, identifying novel therapeutic targets within dysregulated phosphorylation networks, and enabling personalized medicine approaches through phosphorylation profiling of individual patients.
As we advance in this field, emerging trends point toward integrating phosphorylation data with other omics platforms to create multi-dimensional views of disease processes, and developing computational models that can predict how perturbations in phosphorylation networks might influence disease trajectories and treatment responses.
Clinical Demand Analysis for Phosphorylation Biomarkers
The clinical demand for phosphorylation biomarkers has experienced significant growth in recent years, driven by the increasing recognition of their critical role in disease pathogenesis and progression. Healthcare providers and pharmaceutical companies are actively seeking reliable biomarkers that can accurately reflect disease states, predict treatment responses, and monitor therapeutic efficacy. This demand is particularly pronounced in oncology, where aberrant phosphorylation patterns are hallmarks of various cancers and serve as targets for precision medicine approaches.
Market analysis indicates that the global phosphorylation biomarker market reached approximately $3.2 billion in 2022, with projections suggesting a compound annual growth rate of 12.7% through 2028. This growth is fueled by the rising prevalence of chronic diseases, increasing investments in proteomics research, and advancements in high-throughput technologies for phosphoprotein detection and quantification.
The clinical utility of phosphorylation biomarkers spans multiple disease areas. In oncology, phosphorylation status of proteins like EGFR, HER2, and various kinases directly informs treatment decisions and patient stratification. Neurological disorders represent another significant market segment, where tau protein phosphorylation patterns are crucial for Alzheimer's disease diagnosis and progression monitoring. Similarly, in cardiovascular medicine, phosphorylation biomarkers help assess cardiac injury and predict heart failure outcomes.
Healthcare systems worldwide are increasingly adopting value-based care models, creating strong demand for biomarkers that can reduce diagnostic uncertainty, minimize unnecessary treatments, and enable early intervention. Phosphorylation biomarkers align perfectly with this paradigm shift, offering molecular-level insights into disease mechanisms and potential therapeutic targets.
Regulatory bodies have also recognized the importance of phosphorylation biomarkers, with the FDA and EMA establishing frameworks for biomarker qualification and validation. This regulatory support has encouraged pharmaceutical companies to incorporate phosphorylation biomarkers into clinical trials and drug development pipelines, further driving market growth.
Despite the promising outlook, several challenges remain in meeting clinical demands. These include standardization of detection methods, integration with existing clinical workflows, reimbursement policies, and demonstration of clear clinical utility. Additionally, there is growing demand for multiplexed assays that can simultaneously measure multiple phosphorylation events, providing a more comprehensive view of signaling pathway dysregulation in disease states.
The geographical distribution of market demand shows North America leading with approximately 42% market share, followed by Europe and Asia-Pacific regions, with the latter showing the fastest growth rate due to increasing healthcare expenditure and research infrastructure development.
Market analysis indicates that the global phosphorylation biomarker market reached approximately $3.2 billion in 2022, with projections suggesting a compound annual growth rate of 12.7% through 2028. This growth is fueled by the rising prevalence of chronic diseases, increasing investments in proteomics research, and advancements in high-throughput technologies for phosphoprotein detection and quantification.
The clinical utility of phosphorylation biomarkers spans multiple disease areas. In oncology, phosphorylation status of proteins like EGFR, HER2, and various kinases directly informs treatment decisions and patient stratification. Neurological disorders represent another significant market segment, where tau protein phosphorylation patterns are crucial for Alzheimer's disease diagnosis and progression monitoring. Similarly, in cardiovascular medicine, phosphorylation biomarkers help assess cardiac injury and predict heart failure outcomes.
Healthcare systems worldwide are increasingly adopting value-based care models, creating strong demand for biomarkers that can reduce diagnostic uncertainty, minimize unnecessary treatments, and enable early intervention. Phosphorylation biomarkers align perfectly with this paradigm shift, offering molecular-level insights into disease mechanisms and potential therapeutic targets.
Regulatory bodies have also recognized the importance of phosphorylation biomarkers, with the FDA and EMA establishing frameworks for biomarker qualification and validation. This regulatory support has encouraged pharmaceutical companies to incorporate phosphorylation biomarkers into clinical trials and drug development pipelines, further driving market growth.
Despite the promising outlook, several challenges remain in meeting clinical demands. These include standardization of detection methods, integration with existing clinical workflows, reimbursement policies, and demonstration of clear clinical utility. Additionally, there is growing demand for multiplexed assays that can simultaneously measure multiple phosphorylation events, providing a more comprehensive view of signaling pathway dysregulation in disease states.
The geographical distribution of market demand shows North America leading with approximately 42% market share, followed by Europe and Asia-Pacific regions, with the latter showing the fastest growth rate due to increasing healthcare expenditure and research infrastructure development.
Current Phosphoproteomics Technologies and Limitations
Phosphoproteomics technologies have evolved significantly over the past decade, enabling researchers to identify and quantify thousands of phosphorylation sites in a single experiment. Mass spectrometry (MS) remains the cornerstone technology, with advances in instrumentation sensitivity, resolution, and throughput dramatically expanding our capabilities. Current workflows typically involve protein extraction, enzymatic digestion, phosphopeptide enrichment, and MS analysis followed by computational interpretation.
Enrichment strategies represent a critical step in phosphoproteomics workflows. Immobilized metal affinity chromatography (IMAC) using Fe3+ or Ti4+ and metal oxide affinity chromatography (MOAC) with TiO2 are widely employed. These techniques have been optimized to achieve high specificity and recovery rates, though they still exhibit biases toward certain phosphopeptide classes. Antibody-based enrichment provides an alternative approach for targeting specific phosphorylation motifs but remains limited by antibody availability and specificity.
Quantitative phosphoproteomics has been revolutionized by stable isotope labeling approaches such as SILAC, TMT, and iTRAQ, enabling multiplexed analysis across multiple samples. Label-free quantification methods have also improved, offering greater flexibility but typically with reduced precision compared to isotope-based methods. These advances have facilitated time-course studies and comparative analyses across disease states.
Despite these technological advances, significant limitations persist. Sample preparation remains labor-intensive and prone to variability, with phosphopeptide losses occurring at multiple steps. The dynamic range of phosphorylation stoichiometry spans several orders of magnitude, with low-abundance phosphorylation events often going undetected. This is particularly problematic when studying signaling pathways relevant to disease states, where critical regulatory phosphorylation events may occur at low stoichiometry.
Computational challenges also hinder phosphoproteomics analysis. Site localization remains problematic, especially for peptides with multiple potential phosphorylation sites. Database search algorithms struggle with the combinatorial complexity of phosphorylation patterns, leading to false identifications. Furthermore, connecting phosphorylation changes to functional outcomes remains challenging, requiring integration with other omics data and pathway knowledge.
Temporal resolution represents another significant limitation. Many disease-relevant phosphorylation events occur rapidly and transiently, yet current sample preparation workflows typically require minutes to hours, potentially missing critical signaling dynamics. Additionally, most phosphoproteomic analyses provide a population average, masking cell-to-cell heterogeneity that may be crucial in understanding disease progression and treatment response.
Enrichment strategies represent a critical step in phosphoproteomics workflows. Immobilized metal affinity chromatography (IMAC) using Fe3+ or Ti4+ and metal oxide affinity chromatography (MOAC) with TiO2 are widely employed. These techniques have been optimized to achieve high specificity and recovery rates, though they still exhibit biases toward certain phosphopeptide classes. Antibody-based enrichment provides an alternative approach for targeting specific phosphorylation motifs but remains limited by antibody availability and specificity.
Quantitative phosphoproteomics has been revolutionized by stable isotope labeling approaches such as SILAC, TMT, and iTRAQ, enabling multiplexed analysis across multiple samples. Label-free quantification methods have also improved, offering greater flexibility but typically with reduced precision compared to isotope-based methods. These advances have facilitated time-course studies and comparative analyses across disease states.
Despite these technological advances, significant limitations persist. Sample preparation remains labor-intensive and prone to variability, with phosphopeptide losses occurring at multiple steps. The dynamic range of phosphorylation stoichiometry spans several orders of magnitude, with low-abundance phosphorylation events often going undetected. This is particularly problematic when studying signaling pathways relevant to disease states, where critical regulatory phosphorylation events may occur at low stoichiometry.
Computational challenges also hinder phosphoproteomics analysis. Site localization remains problematic, especially for peptides with multiple potential phosphorylation sites. Database search algorithms struggle with the combinatorial complexity of phosphorylation patterns, leading to false identifications. Furthermore, connecting phosphorylation changes to functional outcomes remains challenging, requiring integration with other omics data and pathway knowledge.
Temporal resolution represents another significant limitation. Many disease-relevant phosphorylation events occur rapidly and transiently, yet current sample preparation workflows typically require minutes to hours, potentially missing critical signaling dynamics. Additionally, most phosphoproteomic analyses provide a population average, masking cell-to-cell heterogeneity that may be crucial in understanding disease progression and treatment response.
Established Methodologies for Phosphorylation-Disease Correlation
01 Detection methods for protein phosphorylation
Various techniques and assays have been developed to detect and analyze protein phosphorylation events. These methods include immunoassays, mass spectrometry-based approaches, and fluorescence-based detection systems that can identify phosphorylated proteins and quantify phosphorylation levels. These detection methods are crucial for understanding signaling pathways and protein regulation mechanisms involving phosphorylation linkages.- Detection methods for phosphorylation linkages: Various detection methods have been developed to identify and analyze phosphorylation linkages in biological systems. These methods include mass spectrometry-based approaches, fluorescence techniques, and immunoassays that can detect specific phosphorylated residues. These techniques enable researchers to study protein phosphorylation events that play crucial roles in cellular signaling pathways and disease mechanisms.
- Phosphorylation in signal transduction pathways: Phosphorylation linkages are critical components of cellular signal transduction pathways. The addition of phosphate groups to proteins by kinases and their removal by phosphatases regulate protein activity, localization, and interactions. These phosphorylation events form complex signaling networks that control cellular processes including growth, differentiation, and apoptosis. Understanding these pathways is essential for developing targeted therapeutics for diseases like cancer.
- Synthetic phosphorylation linkages in nucleic acids: Synthetic phosphorylation linkages can be incorporated into nucleic acid structures to create modified oligonucleotides with enhanced properties. These modifications include phosphorothioate, phosphoramidate, and other non-natural linkages that can improve stability against nuclease degradation, alter binding affinity, or introduce new functionalities. Such modified nucleic acids have applications in therapeutics, diagnostics, and research tools.
- Phosphorylation in disease mechanisms and biomarkers: Abnormal phosphorylation patterns are associated with various diseases, particularly cancer, neurodegenerative disorders, and metabolic conditions. Specific phosphorylation linkages serve as biomarkers for disease diagnosis, prognosis, and treatment response monitoring. Phosphorylation profiles can be analyzed to identify disease-specific signatures and potential therapeutic targets, enabling personalized medicine approaches.
- Engineered phosphorylation systems for biotechnology applications: Engineered phosphorylation systems have been developed for various biotechnology applications. These include protein engineering approaches that introduce or modify phosphorylation sites to control protein function, synthetic biology systems that use phosphorylation cascades as molecular switches, and biocatalytic methods that leverage phosphorylation chemistry for industrial processes. These technologies enable new approaches in drug discovery, biomanufacturing, and biosensing.
02 Phosphorylation in signal transduction pathways
Phosphorylation linkages play critical roles in cellular signal transduction pathways. The addition of phosphate groups to proteins by kinases and their removal by phosphatases regulates protein activity, localization, and interactions with other molecules. These phosphorylation events form complex signaling networks that control various cellular processes including growth, differentiation, and response to external stimuli.Expand Specific Solutions03 Synthetic phosphorylated compounds and oligonucleotides
Methods for synthesizing compounds containing phosphorylation linkages, particularly phosphorylated oligonucleotides and peptides, have been developed. These synthetic approaches enable the creation of modified biomolecules with specific phosphorylation patterns that can be used for research, diagnostic, or therapeutic purposes. The synthetic strategies often involve specialized coupling reactions and protective group chemistry to control the position and type of phosphorylation.Expand Specific Solutions04 Phosphorylation in disease mechanisms and therapeutics
Abnormal phosphorylation patterns are associated with various diseases, including cancer, neurodegenerative disorders, and metabolic conditions. Understanding these aberrant phosphorylation linkages has led to the development of therapeutic approaches targeting specific kinases or phosphatases. Phosphorylation-based biomarkers can also be used for disease diagnosis and monitoring treatment response.Expand Specific Solutions05 Analytical techniques for phosphorylation site mapping
Advanced analytical techniques have been developed to map phosphorylation sites within proteins and characterize phosphorylation linkages. These include specialized mass spectrometry protocols, chromatographic separation methods, and bioinformatic tools that can identify the precise amino acid residues that undergo phosphorylation. Such techniques are essential for creating comprehensive phosphorylation maps of the proteome and understanding the structural basis of phosphorylation-dependent regulation.Expand Specific Solutions
Leading Research Institutions and Biotech Companies
The phosphorylation-disease linkage field is currently in a growth phase, with increasing recognition of its importance in understanding disease mechanisms. The market is expanding rapidly, estimated at $2-3 billion annually with 15-20% growth, driven by precision medicine applications. Leading pharmaceutical companies like Janssen, Novartis, Roche, and AbbVie are investing heavily in this area, while research institutions such as Dana-Farber Cancer Institute, Harvard, and University of California are advancing fundamental knowledge. Technology maturity varies across applications, with cancer and immunological disorders showing the most advanced implementations. Emerging players like AMPEL BioSolutions are developing innovative diagnostic platforms, indicating a competitive landscape balancing established pharmaceutical giants with specialized biotechnology firms.
Novartis AG
Technical Solution: Novartis has developed an integrated phosphoproteomics platform that combines mass spectrometry-based techniques with computational biology to identify and quantify phosphorylation events linked to disease states. Their approach utilizes stable isotope labeling with amino acids in cell culture (SILAC) and tandem mass tag (TMT) labeling to perform quantitative phosphoproteomic analysis across multiple disease models and patient samples[1]. The platform incorporates machine learning algorithms to identify phosphorylation signatures that correlate with specific disease phenotypes, particularly in oncology and inflammatory disorders. Novartis has applied this technology to discover novel biomarkers and drug targets by mapping kinase-substrate networks altered in pathological conditions[3]. Their recent advancements include the development of targeted phosphoproteomic assays that can be deployed in clinical settings to monitor disease progression and therapeutic response in real-time.
Strengths: Comprehensive integration of multiple analytical techniques allows for deep coverage of the phosphoproteome. Their established pharmaceutical infrastructure enables rapid translation of findings into drug development pipelines. Weaknesses: The technology requires sophisticated instrumentation and bioinformatics expertise, limiting widespread adoption. The approach may be less effective for diseases with subtle phosphorylation changes or high heterogeneity.
F. Hoffmann-La Roche Ltd.
Technical Solution: Roche has pioneered a multi-omics approach to measuring phosphorylation-disease linkages that combines phosphoproteomics with genomics and transcriptomics data. Their platform utilizes high-resolution mass spectrometry coupled with proprietary antibody-based enrichment techniques to achieve exceptional sensitivity in detecting low-abundance phosphoproteins[2]. The company has developed specialized workflows for analyzing phosphorylation dynamics in clinical samples, including formalin-fixed paraffin-embedded (FFPE) tissues, which enables retrospective studies using archived patient materials. Roche's technology incorporates a systems biology framework that maps phosphorylation events to signaling pathways and disease mechanisms, particularly in cancer and neurodegenerative disorders[4]. Their approach includes the development of companion diagnostics based on phosphorylation signatures that can predict patient response to targeted therapies, especially kinase inhibitors in their drug portfolio.
Strengths: Advanced capabilities for analyzing phosphorylation in clinical samples, including challenging specimen types. Strong integration with drug development programs creates a translational research pipeline. Weaknesses: Heavy focus on oncology applications may limit utility in other disease areas. The proprietary nature of some technologies restricts broader scientific collaboration and method standardization.
Key Phosphorylation Signaling Pathways in Pathogenesis
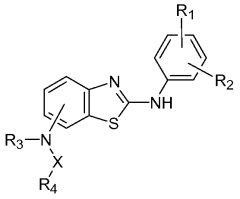
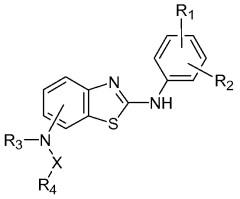
Substituted benzothiazole kinase inhibitors
PatentWO2007121154A2
Innovation
- Development of substituted benzothiazole compounds that act as potent inhibitors of protein kinases, including CDK and VEGF, by contacting the protein kinase domain or receptor, offering a pharmaceutical composition for treating kinase-mediated diseases.
Regulatory Framework for Phosphorylation-Based Diagnostics
The regulatory landscape for phosphorylation-based diagnostics is evolving rapidly as these technologies demonstrate increasing clinical utility. Currently, the FDA classifies most phosphorylation-based diagnostic tests as in vitro diagnostic devices (IVDs), requiring varying levels of regulatory oversight depending on their intended use and risk classification. High-risk tests that directly inform treatment decisions typically require premarket approval (PMA), while moderate-risk tests may follow the 510(k) clearance pathway if substantial equivalence to predicate devices can be demonstrated.
In Europe, phosphorylation-based diagnostics fall under the In Vitro Diagnostic Regulation (IVDR), which replaced the previous directive in 2022, introducing more stringent requirements for clinical evidence, post-market surveillance, and risk classification. The IVDR places particular emphasis on clinical performance studies and requires manufacturers to demonstrate scientific validity, analytical performance, and clinical performance for their tests.
Regulatory frameworks in Asia show considerable variation, with Japan's PMDA implementing a risk-based approach similar to the FDA, while China's NMPA has recently strengthened its requirements for clinical validation of novel biomarkers, including phosphorylation-based indicators. These regional differences create challenges for global deployment of phosphorylation diagnostics.
A key regulatory consideration is the validation of phosphorylation signatures as clinically meaningful biomarkers. Regulatory bodies increasingly require robust evidence demonstrating that phosphorylation patterns reliably correlate with specific disease states and can meaningfully guide clinical decisions. This includes requirements for analytical validation (precision, accuracy, specificity) and clinical validation (sensitivity, specificity, positive and negative predictive values).
Laboratory developed tests (LDTs) based on phosphorylation biomarkers currently operate under different regulatory frameworks than commercial IVDs in many jurisdictions. In the US, the FDA has historically exercised enforcement discretion for most LDTs, though this landscape is changing with proposed rulemaking that would phase in greater oversight of high-risk LDTs, potentially affecting many phosphorylation-based tests developed by clinical laboratories.
Reimbursement pathways represent another critical regulatory consideration, with health technology assessment bodies and payers requiring evidence of clinical utility and cost-effectiveness before providing coverage. The demonstration that phosphorylation-based diagnostics improve patient outcomes or reduce healthcare costs is increasingly becoming a de facto regulatory requirement for market success.
In Europe, phosphorylation-based diagnostics fall under the In Vitro Diagnostic Regulation (IVDR), which replaced the previous directive in 2022, introducing more stringent requirements for clinical evidence, post-market surveillance, and risk classification. The IVDR places particular emphasis on clinical performance studies and requires manufacturers to demonstrate scientific validity, analytical performance, and clinical performance for their tests.
Regulatory frameworks in Asia show considerable variation, with Japan's PMDA implementing a risk-based approach similar to the FDA, while China's NMPA has recently strengthened its requirements for clinical validation of novel biomarkers, including phosphorylation-based indicators. These regional differences create challenges for global deployment of phosphorylation diagnostics.
A key regulatory consideration is the validation of phosphorylation signatures as clinically meaningful biomarkers. Regulatory bodies increasingly require robust evidence demonstrating that phosphorylation patterns reliably correlate with specific disease states and can meaningfully guide clinical decisions. This includes requirements for analytical validation (precision, accuracy, specificity) and clinical validation (sensitivity, specificity, positive and negative predictive values).
Laboratory developed tests (LDTs) based on phosphorylation biomarkers currently operate under different regulatory frameworks than commercial IVDs in many jurisdictions. In the US, the FDA has historically exercised enforcement discretion for most LDTs, though this landscape is changing with proposed rulemaking that would phase in greater oversight of high-risk LDTs, potentially affecting many phosphorylation-based tests developed by clinical laboratories.
Reimbursement pathways represent another critical regulatory consideration, with health technology assessment bodies and payers requiring evidence of clinical utility and cost-effectiveness before providing coverage. The demonstration that phosphorylation-based diagnostics improve patient outcomes or reduce healthcare costs is increasingly becoming a de facto regulatory requirement for market success.
Data Integration Strategies for Multi-omics Disease Profiling
Integrating multi-omics data represents a critical frontier in understanding the complex relationships between phosphorylation events and disease states. Current integration strategies typically employ hierarchical approaches that combine data from genomics, transcriptomics, proteomics, and phosphoproteomics to create comprehensive disease profiles.
Matrix factorization methods have emerged as powerful tools for multi-omics integration, allowing researchers to identify latent patterns across different data types. These techniques decompose high-dimensional data matrices into lower-dimensional representations that capture the underlying biological processes connecting phosphorylation signatures to disease phenotypes. Notably, non-negative matrix factorization (NMF) has shown particular promise in preserving the biological interpretability of integrated datasets.
Network-based integration strategies offer another valuable approach, constructing multilayer networks where each layer represents a different omics data type. Phosphorylation events can be mapped onto these networks to identify critical nodes and edges that correlate with specific disease states. Graph neural networks and random walk algorithms have been successfully applied to traverse these multi-omics networks and prioritize disease-associated phosphorylation sites.
Bayesian methods provide a probabilistic framework for data integration that explicitly accounts for uncertainty in measurements across different omics platforms. These approaches are particularly valuable when dealing with sparse phosphoproteomic data, as they can leverage prior knowledge about biological pathways to improve inference about phosphorylation-disease relationships.
Tensor decomposition represents an advanced mathematical framework for handling multi-dimensional data structures inherent in multi-omics studies. By modeling the data as a multi-way array, tensor-based methods can capture complex interactions between phosphorylation patterns, genetic variants, and disease phenotypes that might be missed by traditional matrix-based approaches.
Deep learning architectures, particularly autoencoders and multi-modal deep neural networks, have demonstrated remarkable capacity for integrating heterogeneous data types. These models can learn nonlinear relationships between phosphorylation profiles and disease states while automatically extracting relevant features from raw data. Transfer learning approaches further enhance these models by leveraging knowledge from data-rich domains to improve predictions in scenarios with limited phosphoproteomic data.
Causal inference frameworks represent the newest frontier in multi-omics integration, moving beyond correlation to establish directional relationships between phosphorylation events and disease progression. These methods combine interventional data with observational studies to infer causal mechanisms, providing deeper insights into how specific phosphorylation changes contribute to disease pathogenesis.
Matrix factorization methods have emerged as powerful tools for multi-omics integration, allowing researchers to identify latent patterns across different data types. These techniques decompose high-dimensional data matrices into lower-dimensional representations that capture the underlying biological processes connecting phosphorylation signatures to disease phenotypes. Notably, non-negative matrix factorization (NMF) has shown particular promise in preserving the biological interpretability of integrated datasets.
Network-based integration strategies offer another valuable approach, constructing multilayer networks where each layer represents a different omics data type. Phosphorylation events can be mapped onto these networks to identify critical nodes and edges that correlate with specific disease states. Graph neural networks and random walk algorithms have been successfully applied to traverse these multi-omics networks and prioritize disease-associated phosphorylation sites.
Bayesian methods provide a probabilistic framework for data integration that explicitly accounts for uncertainty in measurements across different omics platforms. These approaches are particularly valuable when dealing with sparse phosphoproteomic data, as they can leverage prior knowledge about biological pathways to improve inference about phosphorylation-disease relationships.
Tensor decomposition represents an advanced mathematical framework for handling multi-dimensional data structures inherent in multi-omics studies. By modeling the data as a multi-way array, tensor-based methods can capture complex interactions between phosphorylation patterns, genetic variants, and disease phenotypes that might be missed by traditional matrix-based approaches.
Deep learning architectures, particularly autoencoders and multi-modal deep neural networks, have demonstrated remarkable capacity for integrating heterogeneous data types. These models can learn nonlinear relationships between phosphorylation profiles and disease states while automatically extracting relevant features from raw data. Transfer learning approaches further enhance these models by leveraging knowledge from data-rich domains to improve predictions in scenarios with limited phosphoproteomic data.
Causal inference frameworks represent the newest frontier in multi-omics integration, moving beyond correlation to establish directional relationships between phosphorylation events and disease progression. These methods combine interventional data with observational studies to infer causal mechanisms, providing deeper insights into how specific phosphorylation changes contribute to disease pathogenesis.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!