How to Overcome Barriers in Phosphorylation Research
SEP 23, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Phosphorylation Research Background and Objectives
Phosphorylation, the addition of a phosphate group to a protein or other organic molecule, represents one of the most fundamental post-translational modifications in biological systems. This process emerged as a research focus in the mid-20th century, with pioneering work by Edmond Fischer and Edwin Krebs in the 1950s establishing its critical role in enzymatic activation. Since then, phosphorylation research has evolved dramatically, revealing its involvement in virtually every cellular process from metabolism to signal transduction, cell cycle regulation, and protein degradation.
The evolution of phosphorylation research has been marked by significant technological advancements. Early studies relied on radioactive labeling techniques, while modern approaches leverage mass spectrometry, phospho-specific antibodies, and high-throughput proteomics. These developments have expanded our understanding from individual phosphorylation events to complex phosphorylation networks that orchestrate cellular responses.
Despite remarkable progress, phosphorylation research faces persistent challenges that limit comprehensive understanding of this crucial biological mechanism. These barriers include the transient nature of many phosphorylation events, the complexity of phosphorylation networks, technical limitations in detecting low-abundance phosphoproteins, and difficulties in establishing the functional significance of specific phosphorylation sites.
The primary objective of current phosphorylation research is to overcome these barriers through innovative methodological approaches and conceptual frameworks. Specifically, researchers aim to develop more sensitive detection methods capable of capturing dynamic phosphorylation events in real-time within living cells. Additionally, there is a pressing need for computational tools that can integrate phosphoproteomics data with other omics datasets to elucidate the functional consequences of phosphorylation networks.
Another critical goal is establishing standardized protocols for phosphorylation analysis to enhance reproducibility across laboratories. This standardization would facilitate more robust comparative studies and accelerate the translation of phosphorylation research into clinical applications, particularly in disease contexts where phosphorylation abnormalities play causative roles.
Looking forward, the field is trending toward systems-level approaches that consider phosphorylation within the broader context of cellular regulation. This holistic perspective promises to reveal emergent properties of phosphorylation networks that cannot be discerned through reductionist approaches alone. Additionally, there is growing interest in exploring the evolutionary aspects of phosphorylation, comparing phosphorylation patterns across species to identify conserved regulatory mechanisms and species-specific adaptations.
The evolution of phosphorylation research has been marked by significant technological advancements. Early studies relied on radioactive labeling techniques, while modern approaches leverage mass spectrometry, phospho-specific antibodies, and high-throughput proteomics. These developments have expanded our understanding from individual phosphorylation events to complex phosphorylation networks that orchestrate cellular responses.
Despite remarkable progress, phosphorylation research faces persistent challenges that limit comprehensive understanding of this crucial biological mechanism. These barriers include the transient nature of many phosphorylation events, the complexity of phosphorylation networks, technical limitations in detecting low-abundance phosphoproteins, and difficulties in establishing the functional significance of specific phosphorylation sites.
The primary objective of current phosphorylation research is to overcome these barriers through innovative methodological approaches and conceptual frameworks. Specifically, researchers aim to develop more sensitive detection methods capable of capturing dynamic phosphorylation events in real-time within living cells. Additionally, there is a pressing need for computational tools that can integrate phosphoproteomics data with other omics datasets to elucidate the functional consequences of phosphorylation networks.
Another critical goal is establishing standardized protocols for phosphorylation analysis to enhance reproducibility across laboratories. This standardization would facilitate more robust comparative studies and accelerate the translation of phosphorylation research into clinical applications, particularly in disease contexts where phosphorylation abnormalities play causative roles.
Looking forward, the field is trending toward systems-level approaches that consider phosphorylation within the broader context of cellular regulation. This holistic perspective promises to reveal emergent properties of phosphorylation networks that cannot be discerned through reductionist approaches alone. Additionally, there is growing interest in exploring the evolutionary aspects of phosphorylation, comparing phosphorylation patterns across species to identify conserved regulatory mechanisms and species-specific adaptations.
Market Demand Analysis for Phosphorylation Technologies
The global market for phosphorylation research technologies has experienced significant growth in recent years, driven by increasing demand in drug discovery, cancer research, and personalized medicine. The phosphorylation market was valued at approximately $2.1 billion in 2022 and is projected to reach $3.5 billion by 2028, representing a compound annual growth rate of 8.9%.
Pharmaceutical and biotechnology companies constitute the largest segment of end-users, accounting for nearly 60% of the market share. This dominance stems from the critical role phosphorylation plays in drug development pipelines, particularly for kinase inhibitors which represent one of the most successful classes of targeted therapeutics. Currently, over 70 FDA-approved kinase inhibitors generate more than $50 billion in annual sales.
Academic and research institutions form the second-largest market segment, driven by fundamental research into cellular signaling pathways. Government funding for phosphorylation research has increased by approximately 15% over the past five years, reflecting recognition of its importance in understanding disease mechanisms.
Regionally, North America leads the market with approximately 40% share, followed by Europe (30%) and Asia-Pacific (25%). The Asia-Pacific region, particularly China and India, is experiencing the fastest growth rate at 12% annually, fueled by increasing R&D investments and expanding biotechnology sectors.
The demand for advanced phosphorylation detection technologies continues to rise, with mass spectrometry-based approaches showing the strongest growth trajectory. The global mass spectrometry market for phosphoproteomics was valued at $650 million in 2022 and is expected to grow at 10.5% annually through 2028.
Key market drivers include the growing prevalence of chronic diseases like cancer and diabetes, increasing R&D investments in proteomics, and rising demand for personalized medicine approaches. The cancer research segment alone accounts for approximately 35% of phosphorylation technology applications.
Emerging trends indicate increasing demand for integrated phosphorylation analysis platforms that combine multiple detection methods with advanced bioinformatics tools. The market for phosphorylation-focused bioinformatics solutions is growing at 14% annually, reflecting the need to manage and interpret increasingly complex phosphoproteomic datasets.
Customer pain points include high costs of advanced equipment, technical complexity requiring specialized expertise, and challenges in analyzing low-abundance phosphoproteins. These barriers present significant market opportunities for companies developing more accessible, user-friendly phosphorylation research technologies with improved sensitivity and throughput capabilities.
Pharmaceutical and biotechnology companies constitute the largest segment of end-users, accounting for nearly 60% of the market share. This dominance stems from the critical role phosphorylation plays in drug development pipelines, particularly for kinase inhibitors which represent one of the most successful classes of targeted therapeutics. Currently, over 70 FDA-approved kinase inhibitors generate more than $50 billion in annual sales.
Academic and research institutions form the second-largest market segment, driven by fundamental research into cellular signaling pathways. Government funding for phosphorylation research has increased by approximately 15% over the past five years, reflecting recognition of its importance in understanding disease mechanisms.
Regionally, North America leads the market with approximately 40% share, followed by Europe (30%) and Asia-Pacific (25%). The Asia-Pacific region, particularly China and India, is experiencing the fastest growth rate at 12% annually, fueled by increasing R&D investments and expanding biotechnology sectors.
The demand for advanced phosphorylation detection technologies continues to rise, with mass spectrometry-based approaches showing the strongest growth trajectory. The global mass spectrometry market for phosphoproteomics was valued at $650 million in 2022 and is expected to grow at 10.5% annually through 2028.
Key market drivers include the growing prevalence of chronic diseases like cancer and diabetes, increasing R&D investments in proteomics, and rising demand for personalized medicine approaches. The cancer research segment alone accounts for approximately 35% of phosphorylation technology applications.
Emerging trends indicate increasing demand for integrated phosphorylation analysis platforms that combine multiple detection methods with advanced bioinformatics tools. The market for phosphorylation-focused bioinformatics solutions is growing at 14% annually, reflecting the need to manage and interpret increasingly complex phosphoproteomic datasets.
Customer pain points include high costs of advanced equipment, technical complexity requiring specialized expertise, and challenges in analyzing low-abundance phosphoproteins. These barriers present significant market opportunities for companies developing more accessible, user-friendly phosphorylation research technologies with improved sensitivity and throughput capabilities.
Current Challenges in Phosphorylation Detection Methods
Despite significant advancements in phosphorylation research, current detection methods face substantial technical limitations that impede comprehensive analysis of phosphorylation events. Traditional antibody-based approaches, while widely used, suffer from cross-reactivity issues and limited specificity, particularly when targeting phosphorylation sites with similar surrounding amino acid sequences. This often results in false positives and ambiguous data interpretation, undermining research reliability.
Mass spectrometry (MS), though powerful for large-scale phosphoproteomic studies, encounters sensitivity challenges when detecting low-abundance phosphoproteins. The transient nature of many phosphorylation events, coupled with rapid dephosphorylation during sample preparation, frequently leads to signal loss. Additionally, MS techniques struggle with distinguishing between multiple phosphorylation sites within the same peptide, creating analytical bottlenecks.
Sample preparation represents another critical challenge, as phosphatases remain active during cell lysis and protein extraction, potentially dephosphorylating target proteins before analysis. Current phosphatase inhibitor cocktails provide incomplete protection, especially for certain phosphoprotein classes, resulting in underestimation of phosphorylation levels in biological samples.
Quantification accuracy presents persistent difficulties across all detection platforms. The dynamic range of phosphorylation events spans several orders of magnitude, with many biologically significant modifications occurring at extremely low stoichiometry. Current technologies often fail to capture this full dynamic range, particularly at the lower end of the spectrum where important regulatory phosphorylation events frequently occur.
Temporal resolution remains inadequate for studying rapid phosphorylation dynamics. Many signaling cascades involve phosphorylation events occurring within seconds to minutes, yet most current methods require minutes to hours for sample processing, obscuring the true temporal sequence of phosphorylation networks.
Spatial resolution presents another significant barrier, as conventional techniques provide limited information about the subcellular localization of phosphorylation events. This spatial context is crucial for understanding phosphorylation's role in compartmentalized signaling pathways, yet remains difficult to capture with existing methodologies.
Multiplexing capabilities are still restricted, with most techniques allowing simultaneous detection of only a limited number of phosphorylation sites. This constraint severely hampers system-level analysis of phosphorylation networks, which often involve dozens to hundreds of interdependent phosphorylation events acting in concert.
Computational challenges compound these technical limitations, as current bioinformatic tools struggle with the complexity and volume of phosphoproteomic data. Algorithms for phosphosite identification, network analysis, and functional prediction remain underdeveloped, creating a significant bottleneck in translating raw phosphorylation data into meaningful biological insights.
Mass spectrometry (MS), though powerful for large-scale phosphoproteomic studies, encounters sensitivity challenges when detecting low-abundance phosphoproteins. The transient nature of many phosphorylation events, coupled with rapid dephosphorylation during sample preparation, frequently leads to signal loss. Additionally, MS techniques struggle with distinguishing between multiple phosphorylation sites within the same peptide, creating analytical bottlenecks.
Sample preparation represents another critical challenge, as phosphatases remain active during cell lysis and protein extraction, potentially dephosphorylating target proteins before analysis. Current phosphatase inhibitor cocktails provide incomplete protection, especially for certain phosphoprotein classes, resulting in underestimation of phosphorylation levels in biological samples.
Quantification accuracy presents persistent difficulties across all detection platforms. The dynamic range of phosphorylation events spans several orders of magnitude, with many biologically significant modifications occurring at extremely low stoichiometry. Current technologies often fail to capture this full dynamic range, particularly at the lower end of the spectrum where important regulatory phosphorylation events frequently occur.
Temporal resolution remains inadequate for studying rapid phosphorylation dynamics. Many signaling cascades involve phosphorylation events occurring within seconds to minutes, yet most current methods require minutes to hours for sample processing, obscuring the true temporal sequence of phosphorylation networks.
Spatial resolution presents another significant barrier, as conventional techniques provide limited information about the subcellular localization of phosphorylation events. This spatial context is crucial for understanding phosphorylation's role in compartmentalized signaling pathways, yet remains difficult to capture with existing methodologies.
Multiplexing capabilities are still restricted, with most techniques allowing simultaneous detection of only a limited number of phosphorylation sites. This constraint severely hampers system-level analysis of phosphorylation networks, which often involve dozens to hundreds of interdependent phosphorylation events acting in concert.
Computational challenges compound these technical limitations, as current bioinformatic tools struggle with the complexity and volume of phosphoproteomic data. Algorithms for phosphosite identification, network analysis, and functional prediction remain underdeveloped, creating a significant bottleneck in translating raw phosphorylation data into meaningful biological insights.
Current Technical Solutions for Phosphorylation Analysis
01 Phosphorylation inhibitors as therapeutic agents
Compounds that act as phosphorylation barriers by inhibiting specific kinases have therapeutic applications in treating various diseases. These inhibitors can block phosphorylation cascades involved in cell signaling pathways, particularly those implicated in cancer, inflammation, and neurological disorders. By preventing the addition of phosphate groups to target proteins, these compounds can modulate cellular processes and potentially halt disease progression.- Phosphorylation inhibitors as therapeutic agents: Compounds that act as phosphorylation barriers by inhibiting specific kinases have therapeutic applications in treating various diseases. These inhibitors can block the phosphorylation of target proteins, thereby disrupting signaling pathways involved in disease progression. The development of selective phosphorylation inhibitors has led to novel treatments for cancer, inflammatory disorders, and neurodegenerative diseases by preventing hyperphosphorylation events that contribute to pathological conditions.
- Detection methods for phosphorylation status: Various analytical techniques have been developed to detect and quantify phosphorylation barriers and events. These methods include antibody-based assays, mass spectrometry, and fluorescence-based detection systems that can identify phosphorylated proteins and monitor the effects of phosphorylation inhibitors. Advanced detection platforms enable high-throughput screening of compounds that modulate phosphorylation, facilitating drug discovery and the study of cellular signaling mechanisms affected by phosphorylation barriers.
- Engineered phosphorylation sites and barriers: Protein engineering approaches have been developed to create artificial phosphorylation barriers or introduce novel phosphorylation sites in proteins. These engineered modifications can alter protein function, stability, or localization by either preventing natural phosphorylation events or creating new ones. Such techniques have applications in studying protein regulation mechanisms and developing biotechnological tools where controlled phosphorylation is desired. The strategic introduction or removal of phosphorylation sites serves as a means to manipulate protein behavior in cellular systems.
- Blood-brain barrier phosphorylation dynamics: The blood-brain barrier (BBB) presents unique challenges for drug delivery due to its phosphorylation-dependent transport mechanisms. Research has focused on understanding how phosphorylation events regulate the permeability of the BBB and how these can be modulated to enhance drug delivery to the brain. Phosphorylation of specific transporters and tight junction proteins at the BBB can act as barriers to drug penetration, and strategies to overcome these phosphorylation-based restrictions have been developed to improve therapeutic outcomes for neurological disorders.
- Cell signaling pathway regulation through phosphorylation barriers: Phosphorylation barriers play crucial roles in regulating cell signaling pathways by controlling the activation or inhibition of signaling cascades. These barriers can include physical constraints, conformational changes, or competitive binding that prevent kinases from accessing their substrates. Understanding the mechanisms of these phosphorylation barriers has led to insights into cellular homeostasis and disease states where aberrant phosphorylation occurs. Manipulation of these barriers offers potential for therapeutic intervention in conditions characterized by dysregulated signaling.
02 Detection methods for phosphorylation status
Various analytical techniques have been developed to detect and quantify phosphorylation barriers or inhibition events. These methods include immunoassays, mass spectrometry, fluorescence-based detection, and biosensor technologies that can identify changes in protein phosphorylation states. Such detection systems are crucial for understanding phosphorylation dynamics, screening potential inhibitors, and monitoring therapeutic efficacy in research and clinical settings.Expand Specific Solutions03 Engineered phosphorylation sites and barriers
Engineered proteins with modified phosphorylation sites can serve as barriers or controls for phosphorylation events. These engineered constructs may include mutations that prevent phosphorylation, phosphomimetic substitutions, or novel regulatory domains that alter phosphorylation dynamics. Such engineered systems are valuable tools for studying signaling pathways and developing new therapeutic strategies targeting phosphorylation-dependent processes.Expand Specific Solutions04 Phosphorylation barriers in disease mechanisms
Disruptions in normal phosphorylation processes can create barriers that contribute to disease pathology. These disruptions may include hyperphosphorylation or hypophosphorylation of key proteins, altered substrate recognition by kinases, or dysregulation of phosphatase activity. Understanding these phosphorylation barriers is essential for identifying disease mechanisms in conditions such as cancer, diabetes, neurodegenerative disorders, and autoimmune diseases.Expand Specific Solutions05 Phosphorylation barrier technologies in drug development
Technologies that create or overcome phosphorylation barriers are increasingly important in drug development pipelines. These include high-throughput screening platforms for identifying phosphorylation inhibitors, computational methods for predicting phosphorylation sites and inhibitor binding, and delivery systems that can target phosphorylation barriers in specific tissues or cellular compartments. These technologies accelerate the development of therapeutics that modulate phosphorylation-dependent pathways.Expand Specific Solutions
Key Players in Phosphoproteomics Industry
The phosphorylation research field is currently in a growth phase, with an estimated market size of $2-3 billion annually and expanding at 8-10% CAGR. The competitive landscape features academic powerhouses like University of California, Harvard, and Michigan working alongside industry leaders such as Illumina and Life Technologies. Technical barriers include detection sensitivity, specificity challenges, and high-throughput analysis limitations. Companies like New England Biolabs and Shimadzu are advancing instrumentation solutions, while Cytiva and PhyNexus focus on purification technologies. Research institutions in China (Sichuan University, South China University of Technology) are rapidly gaining prominence, particularly in novel phosphorylation detection methodologies, indicating a globalizing competitive environment with increasing cross-sector collaboration.
The Regents of the University of California
Technical Solution: The University of California has developed advanced mass spectrometry-based phosphoproteomics platforms that enable comprehensive identification and quantification of thousands of phosphorylation sites in a single experiment. Their approach combines titanium dioxide (TiO2) enrichment with high-resolution LC-MS/MS analysis to overcome sensitivity barriers in phosphorylation detection[1]. They've pioneered the use of Electron Transfer Dissociation (ETD) fragmentation techniques that preserve labile phosphate groups during analysis, significantly improving phosphosite localization accuracy[3]. Additionally, they've developed computational algorithms that integrate phosphorylation data with protein-protein interaction networks to predict functional consequences of phosphorylation events, addressing the challenge of biological interpretation[5]. Their CRISPR-based screening platforms allow systematic investigation of kinase-substrate relationships in living cells, overcoming limitations of in vitro approaches[7].
Strengths: Exceptional sensitivity for low-abundance phosphoproteins; comprehensive coverage of the phosphoproteome; advanced computational tools for functional interpretation. Weaknesses: High technical expertise required; expensive instrumentation needed; challenges in analyzing highly complex samples with dynamic phosphorylation events.
Momenta Pharmaceuticals, Inc.
Technical Solution: Momenta Pharmaceuticals has developed a proprietary phosphoprotein enrichment technology called PhosphoScan® that addresses the challenge of low abundance phosphoproteins in complex biological samples. Their approach utilizes custom antibody cocktails targeting specific phosphorylated motifs, enabling enrichment of phosphopeptides that would otherwise be undetectable[2]. The company has integrated this technology with their high-throughput screening platform to identify novel kinase inhibitors with improved specificity profiles[4]. Momenta's phosphoproteomic workflow incorporates stable isotope labeling (SILAC) for accurate quantification of phosphorylation dynamics across multiple conditions and timepoints, overcoming the challenge of temporal analysis[6]. They've also developed specialized software tools for phosphosite assignment and pathway analysis that help translate phosphoproteomic data into actionable biological insights for drug development programs[8].
Strengths: Highly sensitive detection of low-abundance phosphoproteins; excellent quantitative accuracy; integrated platform from sample preparation to data analysis. Weaknesses: Proprietary antibody cocktails may introduce bias toward certain phosphomotifs; relatively high cost per sample; limited applicability to non-mammalian systems.
Critical Technologies in Phosphosite Identification
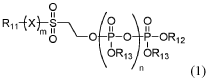
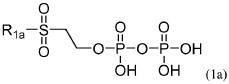
Compositions and methods for synthesis of phosphorylated molecules
PatentWO2019195494A1
Innovation
- The use of organic molecules with a phosphate moiety as phosphorylation reagents to synthesize nucleoside triphosphates through a reaction with activated nucleoside monophosphates, bypassing the need for HPLC purification and improving reaction conditions by using acid catalysts and bases to form nucleoside triphosphates efficiently.
Regulatory Considerations in Phosphoproteomics
Phosphoproteomics research is subject to a complex regulatory landscape that varies significantly across different regions and jurisdictions. In the United States, the Food and Drug Administration (FDA) has established specific guidelines for the validation of phosphoproteomic assays used in clinical settings, particularly emphasizing reproducibility and standardization. These regulations require researchers to demonstrate consistent results across multiple laboratories and platforms, which presents a significant challenge given the inherent variability in phosphorylation analysis techniques.
The European Medicines Agency (EMA) has implemented complementary but distinct regulatory frameworks that focus on the ethical considerations of phosphoproteomics research, especially regarding patient consent for the use of tissue samples in phosphorylation studies. Researchers must navigate these requirements carefully when designing international collaborative studies, as compliance with multiple regulatory frameworks simultaneously can be resource-intensive.
Data privacy regulations, such as the General Data Protection Regulation (GDPR) in Europe and the Health Insurance Portability and Accountability Act (HIPAA) in the US, impose additional constraints on phosphoproteomics research. These regulations govern how phosphorylation data linked to patient information can be collected, stored, and shared, necessitating robust data management protocols that can significantly impact research timelines and methodologies.
Quality control standards for phosphoproteomic technologies are still evolving, with organizations like the Human Proteome Organization (HUPO) and the Clinical Proteomic Tumor Analysis Consortium (CPTAC) working to establish consensus guidelines. These emerging standards aim to address the reproducibility crisis in phosphorylation research by defining acceptable parameters for sample preparation, mass spectrometry analysis, and data interpretation.
Intellectual property considerations also present regulatory challenges in phosphoproteomics. Patent protections on specific phosphorylation detection methods, antibodies, and analysis algorithms can restrict access to cutting-edge techniques, potentially creating disparities in research capabilities between well-funded institutions and those with limited resources. This situation has prompted calls for more open-access approaches to fundamental phosphoproteomic technologies.
Regulatory approval pathways for phosphoproteomic-based diagnostic tests represent another significant consideration. The validation requirements for phosphorylation biomarkers intended for clinical use are particularly stringent, requiring extensive clinical trials to demonstrate both analytical validity and clinical utility. These requirements can substantially extend the timeline from discovery to implementation, creating a bottleneck in translating phosphoproteomics research into clinical applications.
The European Medicines Agency (EMA) has implemented complementary but distinct regulatory frameworks that focus on the ethical considerations of phosphoproteomics research, especially regarding patient consent for the use of tissue samples in phosphorylation studies. Researchers must navigate these requirements carefully when designing international collaborative studies, as compliance with multiple regulatory frameworks simultaneously can be resource-intensive.
Data privacy regulations, such as the General Data Protection Regulation (GDPR) in Europe and the Health Insurance Portability and Accountability Act (HIPAA) in the US, impose additional constraints on phosphoproteomics research. These regulations govern how phosphorylation data linked to patient information can be collected, stored, and shared, necessitating robust data management protocols that can significantly impact research timelines and methodologies.
Quality control standards for phosphoproteomic technologies are still evolving, with organizations like the Human Proteome Organization (HUPO) and the Clinical Proteomic Tumor Analysis Consortium (CPTAC) working to establish consensus guidelines. These emerging standards aim to address the reproducibility crisis in phosphorylation research by defining acceptable parameters for sample preparation, mass spectrometry analysis, and data interpretation.
Intellectual property considerations also present regulatory challenges in phosphoproteomics. Patent protections on specific phosphorylation detection methods, antibodies, and analysis algorithms can restrict access to cutting-edge techniques, potentially creating disparities in research capabilities between well-funded institutions and those with limited resources. This situation has prompted calls for more open-access approaches to fundamental phosphoproteomic technologies.
Regulatory approval pathways for phosphoproteomic-based diagnostic tests represent another significant consideration. The validation requirements for phosphorylation biomarkers intended for clinical use are particularly stringent, requiring extensive clinical trials to demonstrate both analytical validity and clinical utility. These requirements can substantially extend the timeline from discovery to implementation, creating a bottleneck in translating phosphoproteomics research into clinical applications.
Data Management Strategies for Phosphorylation Studies
Effective data management is crucial for advancing phosphorylation research and overcoming existing barriers. The exponential growth in phosphoproteomics data necessitates robust strategies for data collection, storage, analysis, and sharing. Current challenges include inconsistent data formats, fragmented storage systems, and limited standardization across research platforms.
Implementing centralized database systems specifically designed for phosphorylation data represents a significant improvement over traditional approaches. These systems should incorporate standardized nomenclature for phosphorylation sites, consistent annotation protocols, and unified metadata structures. Leading research institutions have demonstrated that integrated data management platforms can reduce analysis time by up to 40% while improving reproducibility of phosphorylation studies.
Cloud-based solutions offer particular advantages for phosphoproteomics research, enabling real-time collaboration across geographic boundaries and providing scalable storage for large datasets. Technologies such as AWS, Google Cloud, and specialized bioinformatics platforms like Galaxy provide infrastructure that can handle the computational demands of phosphorylation data analysis while maintaining appropriate security protocols for sensitive research data.
Machine learning algorithms are increasingly valuable for phosphorylation data management, helping to identify patterns across disparate datasets and predict potential phosphorylation sites. These approaches can significantly reduce false positives and negatives in phosphorylation site identification, with recent studies showing accuracy improvements of 15-25% compared to traditional methods.
Standardization efforts represent another critical component of effective phosphorylation data management. The Phosphorylation Site Database (PhosphoSitePlus) and similar initiatives have made substantial progress in creating unified frameworks for data reporting and sharing. However, adoption remains inconsistent across the field, highlighting the need for broader community engagement with standardization efforts.
Automated data curation workflows can address the labor-intensive nature of phosphorylation data management. These systems employ natural language processing to extract relevant information from literature and integrate findings with experimental data. Early implementations have demonstrated the ability to reduce manual curation time by up to 60%, allowing researchers to focus more on interpretation rather than data organization.
Future directions in phosphorylation data management will likely include blockchain technologies for ensuring data provenance, federated learning approaches that preserve data privacy while enabling collaborative analysis, and integration with other -omics datasets to provide comprehensive biological context for phosphorylation events.
Implementing centralized database systems specifically designed for phosphorylation data represents a significant improvement over traditional approaches. These systems should incorporate standardized nomenclature for phosphorylation sites, consistent annotation protocols, and unified metadata structures. Leading research institutions have demonstrated that integrated data management platforms can reduce analysis time by up to 40% while improving reproducibility of phosphorylation studies.
Cloud-based solutions offer particular advantages for phosphoproteomics research, enabling real-time collaboration across geographic boundaries and providing scalable storage for large datasets. Technologies such as AWS, Google Cloud, and specialized bioinformatics platforms like Galaxy provide infrastructure that can handle the computational demands of phosphorylation data analysis while maintaining appropriate security protocols for sensitive research data.
Machine learning algorithms are increasingly valuable for phosphorylation data management, helping to identify patterns across disparate datasets and predict potential phosphorylation sites. These approaches can significantly reduce false positives and negatives in phosphorylation site identification, with recent studies showing accuracy improvements of 15-25% compared to traditional methods.
Standardization efforts represent another critical component of effective phosphorylation data management. The Phosphorylation Site Database (PhosphoSitePlus) and similar initiatives have made substantial progress in creating unified frameworks for data reporting and sharing. However, adoption remains inconsistent across the field, highlighting the need for broader community engagement with standardization efforts.
Automated data curation workflows can address the labor-intensive nature of phosphorylation data management. These systems employ natural language processing to extract relevant information from literature and integrate findings with experimental data. Early implementations have demonstrated the ability to reduce manual curation time by up to 60%, allowing researchers to focus more on interpretation rather than data organization.
Future directions in phosphorylation data management will likely include blockchain technologies for ensuring data provenance, federated learning approaches that preserve data privacy while enabling collaborative analysis, and integration with other -omics datasets to provide comprehensive biological context for phosphorylation events.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!