Phosphorylation in Signal Cascades: Compare Subunit Roles
SEP 23, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Phosphorylation Signaling Background and Research Objectives
Phosphorylation represents one of the most fundamental post-translational modifications in cellular signaling pathways, with a rich history dating back to the 1950s when Edwin Krebs and Edmond Fischer first discovered this process. Over the decades, our understanding of phosphorylation has evolved from simple enzymatic reactions to complex regulatory networks that control virtually every aspect of cellular function. This biochemical process involves the addition of phosphate groups to proteins by kinases and their removal by phosphatases, creating a dynamic and reversible mechanism for signal transduction.
The evolution of phosphorylation research has progressed through several distinct phases. Initially, scientists focused on identifying individual kinases and their substrates. This was followed by the mapping of linear signaling pathways, and more recently, the field has advanced to understanding complex signaling networks and their cross-talk mechanisms. Modern technological breakthroughs in phosphoproteomics, structural biology, and computational modeling have dramatically accelerated our comprehension of these intricate systems.
Signal cascades represent hierarchical arrangements of phosphorylation events that amplify and integrate cellular signals. These cascades typically involve multiple subunits with distinct roles in signal propagation, modulation, and termination. The mitogen-activated protein kinase (MAPK) cascades exemplify this complexity, comprising three-tiered kinase modules (MAPKKK → MAPKK → MAPK) that translate extracellular stimuli into specific cellular responses.
The comparative analysis of subunit roles within phosphorylation cascades has emerged as a critical area of investigation. Different subunits can serve as signal amplifiers, integrators, or attenuators, depending on their position within the cascade and their specific biochemical properties. Understanding these differential roles is essential for deciphering how cells achieve specificity and fidelity in signal transduction despite using a limited set of signaling molecules.
Our technical objectives in this research domain are multifaceted. First, we aim to systematically characterize the structural and functional differences between subunits in major signaling cascades. Second, we seek to elucidate the molecular mechanisms that determine subunit-specific functions, including scaffold interactions, subcellular localization, and feedback regulation. Third, we intend to develop predictive models that can anticipate how alterations in specific subunits might affect overall cascade dynamics and downstream responses.
The ultimate goal of this technical exploration is to establish a comprehensive framework for understanding subunit-specific roles in phosphorylation cascades. This knowledge will not only advance our fundamental understanding of cellular signaling but also provide crucial insights for therapeutic interventions targeting specific components of these pathways in various disease contexts, particularly cancer, inflammatory disorders, and neurodegenerative conditions.
The evolution of phosphorylation research has progressed through several distinct phases. Initially, scientists focused on identifying individual kinases and their substrates. This was followed by the mapping of linear signaling pathways, and more recently, the field has advanced to understanding complex signaling networks and their cross-talk mechanisms. Modern technological breakthroughs in phosphoproteomics, structural biology, and computational modeling have dramatically accelerated our comprehension of these intricate systems.
Signal cascades represent hierarchical arrangements of phosphorylation events that amplify and integrate cellular signals. These cascades typically involve multiple subunits with distinct roles in signal propagation, modulation, and termination. The mitogen-activated protein kinase (MAPK) cascades exemplify this complexity, comprising three-tiered kinase modules (MAPKKK → MAPKK → MAPK) that translate extracellular stimuli into specific cellular responses.
The comparative analysis of subunit roles within phosphorylation cascades has emerged as a critical area of investigation. Different subunits can serve as signal amplifiers, integrators, or attenuators, depending on their position within the cascade and their specific biochemical properties. Understanding these differential roles is essential for deciphering how cells achieve specificity and fidelity in signal transduction despite using a limited set of signaling molecules.
Our technical objectives in this research domain are multifaceted. First, we aim to systematically characterize the structural and functional differences between subunits in major signaling cascades. Second, we seek to elucidate the molecular mechanisms that determine subunit-specific functions, including scaffold interactions, subcellular localization, and feedback regulation. Third, we intend to develop predictive models that can anticipate how alterations in specific subunits might affect overall cascade dynamics and downstream responses.
The ultimate goal of this technical exploration is to establish a comprehensive framework for understanding subunit-specific roles in phosphorylation cascades. This knowledge will not only advance our fundamental understanding of cellular signaling but also provide crucial insights for therapeutic interventions targeting specific components of these pathways in various disease contexts, particularly cancer, inflammatory disorders, and neurodegenerative conditions.
Market Applications of Phosphorylation Cascade Research
The phosphorylation cascade research market has expanded significantly across multiple industries, with pharmaceutical and biotechnology sectors leading adoption. These sectors leverage phosphorylation insights for drug discovery and development, particularly for kinase inhibitors that target specific phosphorylation events in disease pathways. The global kinase inhibitor market reached approximately $46 billion in 2022, with projections suggesting continued growth at a CAGR of 7.5% through 2030, highlighting the commercial significance of phosphorylation research.
Diagnostic applications represent another substantial market segment, where phosphorylation-based biomarkers enable early disease detection and personalized medicine approaches. Companies have developed diagnostic kits that measure phosphorylation states of specific proteins as indicators of disease progression or treatment response, particularly in oncology where aberrant phosphorylation drives many cancer types.
Agricultural biotechnology has emerged as a growing application area, with researchers exploring phosphorylation cascades in plants to develop crops with enhanced stress resistance, improved yield, and optimized resource utilization. Understanding phosphorylation's role in plant signaling pathways has led to innovative agricultural products that help address food security challenges while reducing environmental impact.
The research tools and reagents market supporting phosphorylation studies has also flourished, with specialized antibodies, protein arrays, and phospho-specific detection systems becoming essential components of modern biological research. This segment generates substantial revenue through high-value consumables and equipment used in both academic and industrial research settings.
Emerging applications include cosmetic and dermatological products that target cellular signaling pathways to address aging and skin conditions. Several skincare companies have developed formulations claiming to modulate phosphorylation events in skin cells to promote rejuvenation and repair, though regulatory oversight varies significantly across regions.
Digital health and AI-driven platforms represent the newest frontier, with companies developing computational tools that predict phosphorylation patterns and their biological consequences. These platforms help researchers identify novel therapeutic targets and optimize treatment strategies by modeling complex signaling networks and their responses to interventions.
Cross-industry collaborations are increasingly common, with pharmaceutical companies partnering with technology firms to develop integrated solutions that combine phosphorylation insights with advanced delivery systems or diagnostic capabilities, creating higher-value offerings that address multiple aspects of disease management.
Diagnostic applications represent another substantial market segment, where phosphorylation-based biomarkers enable early disease detection and personalized medicine approaches. Companies have developed diagnostic kits that measure phosphorylation states of specific proteins as indicators of disease progression or treatment response, particularly in oncology where aberrant phosphorylation drives many cancer types.
Agricultural biotechnology has emerged as a growing application area, with researchers exploring phosphorylation cascades in plants to develop crops with enhanced stress resistance, improved yield, and optimized resource utilization. Understanding phosphorylation's role in plant signaling pathways has led to innovative agricultural products that help address food security challenges while reducing environmental impact.
The research tools and reagents market supporting phosphorylation studies has also flourished, with specialized antibodies, protein arrays, and phospho-specific detection systems becoming essential components of modern biological research. This segment generates substantial revenue through high-value consumables and equipment used in both academic and industrial research settings.
Emerging applications include cosmetic and dermatological products that target cellular signaling pathways to address aging and skin conditions. Several skincare companies have developed formulations claiming to modulate phosphorylation events in skin cells to promote rejuvenation and repair, though regulatory oversight varies significantly across regions.
Digital health and AI-driven platforms represent the newest frontier, with companies developing computational tools that predict phosphorylation patterns and their biological consequences. These platforms help researchers identify novel therapeutic targets and optimize treatment strategies by modeling complex signaling networks and their responses to interventions.
Cross-industry collaborations are increasingly common, with pharmaceutical companies partnering with technology firms to develop integrated solutions that combine phosphorylation insights with advanced delivery systems or diagnostic capabilities, creating higher-value offerings that address multiple aspects of disease management.
Current Challenges in Subunit Phosphorylation Analysis
Despite significant advancements in phosphorylation research, several critical challenges persist in the analysis of subunit phosphorylation within signal cascades. The complexity of phosphorylation networks presents a fundamental obstacle, as these networks involve multiple kinases, phosphatases, and substrates interacting in intricate temporal and spatial patterns. This complexity makes it difficult to isolate and study the specific roles of individual subunits within larger protein complexes.
Technical limitations in detection methods continue to hamper comprehensive phosphorylation analysis. While mass spectrometry has revolutionized phosphoproteomic studies, it still struggles with low-abundance phosphorylation events and hydrophobic membrane proteins. Additionally, the transient nature of many phosphorylation events means they may be missed during standard analytical timeframes, particularly those serving as rapid intermediate steps in signaling cascades.
Contextual dependencies represent another significant challenge. Phosphorylation events often depend on cellular context, including cell type, developmental stage, and environmental conditions. This contextual variability makes it difficult to establish universal models of subunit phosphorylation roles across different biological systems and experimental conditions.
The combinatorial complexity of multi-site phosphorylation further complicates analysis. Many signaling proteins contain numerous phosphorylation sites, creating a vast array of possible phosphorylation patterns. Understanding how these combinations influence protein function and downstream signaling remains exceptionally challenging, particularly when comparing homologous subunits across different protein complexes.
Quantification issues persist despite technological advances. Accurately measuring the stoichiometry of phosphorylation at specific sites and determining the proportion of a protein population that is phosphorylated under various conditions remains difficult. This challenge is particularly pronounced when comparing phosphorylation levels between different subunits within the same complex.
Cross-talk between different post-translational modifications (PTMs) adds another layer of complexity. Phosphorylation events may influence or be influenced by other PTMs such as ubiquitination, acetylation, or methylation. Disentangling these interdependencies to understand the specific contribution of phosphorylation to subunit function requires sophisticated multi-modal analytical approaches that are still being developed.
Finally, computational challenges in data integration and modeling remain significant. The vast amounts of phosphoproteomic data generated require advanced computational tools to integrate, visualize, and interpret. Current algorithms still struggle to accurately predict phosphorylation sites, kinase-substrate relationships, and the functional consequences of phosphorylation events, particularly when comparing homologous subunits that may have evolved divergent regulatory mechanisms.
Technical limitations in detection methods continue to hamper comprehensive phosphorylation analysis. While mass spectrometry has revolutionized phosphoproteomic studies, it still struggles with low-abundance phosphorylation events and hydrophobic membrane proteins. Additionally, the transient nature of many phosphorylation events means they may be missed during standard analytical timeframes, particularly those serving as rapid intermediate steps in signaling cascades.
Contextual dependencies represent another significant challenge. Phosphorylation events often depend on cellular context, including cell type, developmental stage, and environmental conditions. This contextual variability makes it difficult to establish universal models of subunit phosphorylation roles across different biological systems and experimental conditions.
The combinatorial complexity of multi-site phosphorylation further complicates analysis. Many signaling proteins contain numerous phosphorylation sites, creating a vast array of possible phosphorylation patterns. Understanding how these combinations influence protein function and downstream signaling remains exceptionally challenging, particularly when comparing homologous subunits across different protein complexes.
Quantification issues persist despite technological advances. Accurately measuring the stoichiometry of phosphorylation at specific sites and determining the proportion of a protein population that is phosphorylated under various conditions remains difficult. This challenge is particularly pronounced when comparing phosphorylation levels between different subunits within the same complex.
Cross-talk between different post-translational modifications (PTMs) adds another layer of complexity. Phosphorylation events may influence or be influenced by other PTMs such as ubiquitination, acetylation, or methylation. Disentangling these interdependencies to understand the specific contribution of phosphorylation to subunit function requires sophisticated multi-modal analytical approaches that are still being developed.
Finally, computational challenges in data integration and modeling remain significant. The vast amounts of phosphoproteomic data generated require advanced computational tools to integrate, visualize, and interpret. Current algorithms still struggle to accurately predict phosphorylation sites, kinase-substrate relationships, and the functional consequences of phosphorylation events, particularly when comparing homologous subunits that may have evolved divergent regulatory mechanisms.
Established Methodologies for Subunit Comparison
01 Regulatory mechanisms of protein phosphorylation in signal transduction
Protein phosphorylation serves as a key regulatory mechanism in signal transduction pathways. Various kinases phosphorylate specific amino acid residues on target proteins, altering their conformation, activity, or binding properties. This post-translational modification plays a crucial role in transmitting signals from cell surface receptors to intracellular effectors, ultimately influencing cellular responses such as gene expression, metabolism, and cell division.- Regulatory mechanisms of protein phosphorylation in signal transduction: Protein phosphorylation plays a crucial role in regulating signal transduction pathways. This process involves the addition of phosphate groups to specific amino acid residues, primarily serine, threonine, and tyrosine, which can alter protein conformation, activity, and interactions. Regulatory mechanisms include feedback loops, crosstalk between pathways, and spatiotemporal control of kinase and phosphatase activities. These mechanisms ensure precise signal transmission and appropriate cellular responses to various stimuli.
- Subunit-specific phosphorylation in multi-protein complexes: In multi-protein signaling complexes, phosphorylation of specific subunits can dictate the assembly, stability, and function of the entire complex. Different subunits may undergo phosphorylation at distinct sites and with varying kinetics, allowing for fine-tuned regulation of complex activity. This subunit-specific phosphorylation can create binding sites for downstream effectors, alter subcellular localization, or induce conformational changes that propagate throughout the complex, thereby controlling signal cascade progression.
- Role of scaffold proteins in organizing phosphorylation cascades: Scaffold proteins serve as organizational hubs that bring together kinases, substrates, and regulatory molecules in close proximity to facilitate efficient signal transduction. These proteins can coordinate sequential phosphorylation events by positioning cascade components in the correct orientation and order. By compartmentalizing signaling molecules, scaffold proteins enhance specificity and efficiency while preventing inappropriate cross-talk between pathways. The phosphorylation state of scaffold proteins themselves can also regulate their assembly properties and interactions with cascade components.
- Phosphorylation-dependent conformational changes in signaling subunits: Phosphorylation events can trigger significant conformational changes in signaling proteins, exposing or concealing functional domains that mediate protein-protein interactions or enzymatic activities. These structural alterations can propagate through subunits of a signaling complex, creating allosteric effects that regulate the entire cascade. The precise positioning of phosphorylation sites within protein domains is critical for determining how conformational changes affect subunit interactions and signal propagation through the cascade.
- Methods for detecting and analyzing subunit phosphorylation patterns: Advanced techniques have been developed to identify and characterize phosphorylation patterns within signaling cascade subunits. These include phospho-specific antibodies, mass spectrometry, protein microarrays, and fluorescence-based assays that can detect phosphorylation events with high sensitivity and specificity. Computational approaches are also employed to predict phosphorylation sites and model how these modifications affect protein structure and function. These methods enable researchers to map complex phosphorylation networks and understand how they coordinate cellular responses.
02 Subunit-specific phosphorylation in multi-protein complexes
In multi-protein signaling complexes, phosphorylation of specific subunits can regulate the assembly, stability, and function of the entire complex. Different subunits may undergo phosphorylation at distinct sites and with varying kinetics, allowing for fine-tuned control of signal propagation. This subunit-specific phosphorylation creates a combinatorial code that determines downstream signaling outcomes and enables crosstalk between different signaling pathways.Expand Specific Solutions03 Methods for detecting and analyzing phosphorylation events in signaling cascades
Various analytical techniques have been developed to detect and quantify phosphorylation events within signaling cascades. These include phospho-specific antibodies, mass spectrometry, protein microarrays, and fluorescence-based assays. These methods enable researchers to identify phosphorylation sites, determine the temporal dynamics of phosphorylation, and elucidate the functional consequences of these modifications on subunit interactions and signal transduction.Expand Specific Solutions04 Therapeutic targeting of phosphorylation in disease-related signaling pathways
Dysregulated phosphorylation in signaling cascades is implicated in various diseases, including cancer, inflammatory disorders, and neurological conditions. Therapeutic approaches targeting specific phosphorylation events include kinase inhibitors, phosphatase activators, and molecules that disrupt protein-protein interactions dependent on phosphorylation. Understanding the roles of different subunits in phosphorylation-dependent signaling is crucial for developing targeted therapies with improved efficacy and reduced side effects.Expand Specific Solutions05 Scaffold proteins and adaptor molecules in phosphorylation-dependent signaling
Scaffold proteins and adaptor molecules play critical roles in organizing phosphorylation-dependent signaling complexes. These proteins facilitate the assembly of multi-component signaling modules by bringing kinases and their substrates into proximity. They can also regulate the specificity, efficiency, and spatial organization of phosphorylation events. Different subunits within these complexes may serve as scaffolds, catalytic components, or regulatory elements, collectively determining the outcome of signal transduction.Expand Specific Solutions
Leading Research Institutions and Biotech Companies
Phosphorylation in signal cascades represents a mature yet evolving field, currently in the growth phase with an estimated global market size of $5-7 billion. The competitive landscape features established pharmaceutical giants like Merck & Co. and Genentech (Roche) investing heavily in signal transduction research, alongside specialized biotechnology firms such as Sarepta Therapeutics and Cell Signaling Technology focusing on targeted applications. Academic institutions including Johns Hopkins University and New York University contribute significant foundational research. The technology demonstrates high maturity in cancer and immunology applications, with companies like Merck Patent GmbH and Agilent Technologies developing sophisticated analytical tools for phosphorylation detection. Emerging players from Asia, including Samsung and Huawei, are increasingly entering this space through bioelectronics and AI-driven drug discovery platforms.
Merck & Co., Inc.
Technical Solution: Merck has developed comprehensive phosphoproteomics platforms specifically designed to elucidate the roles of different subunits in signaling cascades. Their KinobeadsTM technology combines immobilized kinase inhibitors with mass spectrometry to profile the activity of hundreds of kinases simultaneously, providing insights into how different kinase subunits coordinate responses within signaling networks[9]. Merck's researchers have also developed phosphorylation-specific antibody arrays that can detect over 1,000 distinct phosphorylation sites across multiple signaling pathways, enabling researchers to compare the activation states of different subunits under various conditions. Their chemical proteomics approach uses covalent kinase inhibitors coupled with quantitative mass spectrometry to map kinase-substrate relationships with high precision, revealing how phosphorylation propagates through different subunits in a signaling cascade[10]. Additionally, Merck has pioneered the development of allosteric kinase modulators that can selectively alter the phosphorylation of specific substrates without affecting others, providing tools to dissect the roles of individual phosphorylation events within complex signaling networks.
Strengths: Exceptional chemical biology tools for probing kinase-substrate relationships; strong integration of phosphoproteomics with drug discovery; ability to profile hundreds of phosphorylation events simultaneously. Weaknesses: Technologies often optimized for drug development rather than basic research; proprietary nature of some tools limits accessibility; focus sometimes narrowed to therapeutically relevant pathways rather than comprehensive signaling networks.
Genentech, Inc.
Technical Solution: Genentech has developed sophisticated technologies for analyzing phosphorylation in signal cascades, particularly in the context of cancer and immunological pathways. Their phosphoproteomic platform combines high-resolution mass spectrometry with proprietary computational algorithms to quantify thousands of phosphorylation sites simultaneously across different experimental conditions[7]. This approach has been instrumental in mapping phosphorylation networks in receptor tyrosine kinase (RTK) signaling and identifying novel feedback mechanisms between different cascade subunits. Genentech has also pioneered the development of highly selective kinase inhibitors that target specific phosphorylation events within signaling cascades, allowing researchers to probe the functional consequences of inhibiting specific subunits. Their PhosphoFlow cytometry platform enables single-cell analysis of multiple phosphorylation events simultaneously, providing insights into cell-to-cell heterogeneity in signaling responses[8]. Additionally, Genentech has developed phosphorylation-specific degraders (PROTACs) that can selectively target phosphorylated proteins for degradation, offering a novel approach to study the roles of phosphorylated subunits in signaling cascades.
Strengths: Industry-leading integration of drug discovery with basic phosphorylation research; exceptional ability to translate findings into therapeutic applications; robust high-throughput screening capabilities. Weaknesses: Proprietary nature limits broader scientific community access; focus sometimes narrowed to disease-relevant pathways rather than fundamental mechanisms; technologies optimized for pharmaceutical applications rather than basic research.
Key Phosphorylation Mechanisms and Regulatory Patterns
Methods and Compositions for Detecting and Isolating Phosphorylated Molecules Using Hydrated Metal Oxides
PatentActiveUS20080261321A1
Innovation
- The use of phosphoaffinity materials containing hydrated metal oxides, such as yttrium oxide, yttrium iron garnet, and titanium dioxide, to bind and isolate phosphomolecules from samples, allowing for their separation and detection.
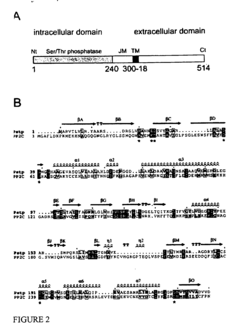
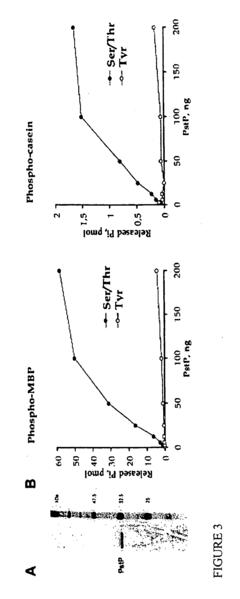
PknB kinase and pstP phosphatase and methods of identifying inhibitory substances
PatentInactiveUS20060019324A1
Innovation
- The pknB kinase and pstP phosphatase, which are part of a conserved operon with genes involved in peptidoglycan synthesis, are identified and characterized for their role in signal transduction pathways, with PstP dephosphorylating PknB, thereby regulating its kinase activity, suggesting a potential target for antibacterial agents.
Computational Modeling of Subunit Interactions
Computational modeling has emerged as a powerful approach for understanding the complex dynamics of phosphorylation in signal cascades. These models integrate experimental data with mathematical frameworks to simulate how different subunits interact during signaling events. Agent-based models have proven particularly effective for representing individual protein subunits and their phosphorylation states, allowing researchers to track signal propagation through cascades with remarkable precision.
Recent advances in molecular dynamics simulations have enabled more accurate representation of conformational changes that occur during phosphorylation events. These simulations can now capture the subtle structural alterations that facilitate or inhibit interactions between regulatory and catalytic subunits, providing insights into how phosphorylation modulates protein function at the atomic level.
Stochastic modeling approaches have revealed the inherent variability in phosphorylation-dependent interactions, demonstrating how noise and randomness in molecular encounters can significantly impact signaling outcomes. These models have been instrumental in explaining why identical cells can exhibit different responses to the same stimulus, highlighting the probabilistic nature of subunit interactions during signal transduction.
Differential equation-based models have successfully captured the temporal dynamics of phosphorylation cascades, revealing how the timing of subunit activation creates distinct signaling signatures. These models demonstrate that the sequence and duration of phosphorylation events often matter as much as their magnitude, particularly in systems where feedback loops regulate signal amplification or attenuation.
Network-based computational approaches have mapped the complex interplay between multiple subunits across different signaling pathways. These models illustrate how phosphorylation events can create nodes of cross-talk between pathways, allowing signals to branch, converge, or form feedback loops that fine-tune cellular responses.
Machine learning algorithms are increasingly being applied to predict novel subunit interactions based on structural and sequence features. These computational tools can identify potential phosphorylation sites and predict how modifications might alter binding affinities between subunits, accelerating the discovery of previously unknown regulatory mechanisms in signal cascades.
The integration of multi-scale modeling approaches has emerged as a frontier in the field, connecting molecular-level subunit interactions to cellular and tissue-level outcomes. These comprehensive models bridge the gap between microscopic phosphorylation events and macroscopic physiological responses, providing a more holistic understanding of how signal cascades coordinate complex biological processes.
Recent advances in molecular dynamics simulations have enabled more accurate representation of conformational changes that occur during phosphorylation events. These simulations can now capture the subtle structural alterations that facilitate or inhibit interactions between regulatory and catalytic subunits, providing insights into how phosphorylation modulates protein function at the atomic level.
Stochastic modeling approaches have revealed the inherent variability in phosphorylation-dependent interactions, demonstrating how noise and randomness in molecular encounters can significantly impact signaling outcomes. These models have been instrumental in explaining why identical cells can exhibit different responses to the same stimulus, highlighting the probabilistic nature of subunit interactions during signal transduction.
Differential equation-based models have successfully captured the temporal dynamics of phosphorylation cascades, revealing how the timing of subunit activation creates distinct signaling signatures. These models demonstrate that the sequence and duration of phosphorylation events often matter as much as their magnitude, particularly in systems where feedback loops regulate signal amplification or attenuation.
Network-based computational approaches have mapped the complex interplay between multiple subunits across different signaling pathways. These models illustrate how phosphorylation events can create nodes of cross-talk between pathways, allowing signals to branch, converge, or form feedback loops that fine-tune cellular responses.
Machine learning algorithms are increasingly being applied to predict novel subunit interactions based on structural and sequence features. These computational tools can identify potential phosphorylation sites and predict how modifications might alter binding affinities between subunits, accelerating the discovery of previously unknown regulatory mechanisms in signal cascades.
The integration of multi-scale modeling approaches has emerged as a frontier in the field, connecting molecular-level subunit interactions to cellular and tissue-level outcomes. These comprehensive models bridge the gap between microscopic phosphorylation events and macroscopic physiological responses, providing a more holistic understanding of how signal cascades coordinate complex biological processes.
Therapeutic Implications of Subunit-Specific Targeting
The selective targeting of specific phosphorylation subunits presents a revolutionary approach in modern therapeutic development. By understanding the differential roles of subunits within phosphorylation cascades, pharmaceutical interventions can achieve unprecedented specificity, potentially reducing off-target effects that plague many current treatments. This precision medicine approach leverages the unique structural and functional characteristics of individual subunits to create targeted therapies with improved efficacy and safety profiles.
Recent clinical trials have demonstrated promising results for subunit-specific inhibitors in oncology, where aberrant phosphorylation pathways drive malignant transformation. For instance, selective inhibition of p110α catalytic subunit of PI3K has shown remarkable efficacy against tumors harboring PIK3CA mutations while sparing normal tissues from toxicity associated with pan-PI3K inhibition. Similarly, targeting specific JAK family members rather than employing pan-JAK inhibition has yielded improved outcomes in inflammatory conditions.
The therapeutic potential extends beyond cancer and inflammation to neurodegenerative disorders, where dysregulated phosphorylation contributes to pathogenesis. Selective inhibitors targeting specific GSK-3β phosphorylation sites have demonstrated neuroprotective effects in preclinical models of Alzheimer's disease without disrupting essential GSK-3β functions in other tissues. This approach may circumvent the failures of previous broad-spectrum kinase inhibitors in neurological disease trials.
Emerging technologies in drug delivery systems further enhance the therapeutic potential of subunit-specific targeting. Nanoparticle-based delivery platforms can now transport inhibitors to specific tissue microenvironments, while antibody-drug conjugates enable precise cellular targeting. These advances address historical challenges in achieving sufficient bioavailability and tissue penetration for phosphorylation modulators.
Combination therapies targeting multiple subunits within a signaling cascade represent another frontier in treatment optimization. By simultaneously modulating different nodes in the phosphorylation network, these approaches can overcome compensatory mechanisms that often lead to treatment resistance. Clinical data suggests that dual inhibition of complementary phosphorylation pathways can produce synergistic effects while allowing dose reduction of individual agents.
Pharmacogenomic approaches are increasingly vital in identifying patient populations most likely to benefit from subunit-specific interventions. Genetic profiling can reveal individuals with particular subunit mutations or expression patterns that predict therapeutic response, enabling truly personalized treatment regimens. This strategy has already improved outcomes in several kinase inhibitor therapies by matching patients with optimal molecular targets.
The economic implications of subunit-specific targeting are substantial, with potential for reduced healthcare costs through decreased adverse events and improved treatment efficacy. While development costs for these precision therapeutics may be higher initially, their improved therapeutic index and potential for biomarker-guided patient selection offer compelling value propositions for healthcare systems worldwide.
Recent clinical trials have demonstrated promising results for subunit-specific inhibitors in oncology, where aberrant phosphorylation pathways drive malignant transformation. For instance, selective inhibition of p110α catalytic subunit of PI3K has shown remarkable efficacy against tumors harboring PIK3CA mutations while sparing normal tissues from toxicity associated with pan-PI3K inhibition. Similarly, targeting specific JAK family members rather than employing pan-JAK inhibition has yielded improved outcomes in inflammatory conditions.
The therapeutic potential extends beyond cancer and inflammation to neurodegenerative disorders, where dysregulated phosphorylation contributes to pathogenesis. Selective inhibitors targeting specific GSK-3β phosphorylation sites have demonstrated neuroprotective effects in preclinical models of Alzheimer's disease without disrupting essential GSK-3β functions in other tissues. This approach may circumvent the failures of previous broad-spectrum kinase inhibitors in neurological disease trials.
Emerging technologies in drug delivery systems further enhance the therapeutic potential of subunit-specific targeting. Nanoparticle-based delivery platforms can now transport inhibitors to specific tissue microenvironments, while antibody-drug conjugates enable precise cellular targeting. These advances address historical challenges in achieving sufficient bioavailability and tissue penetration for phosphorylation modulators.
Combination therapies targeting multiple subunits within a signaling cascade represent another frontier in treatment optimization. By simultaneously modulating different nodes in the phosphorylation network, these approaches can overcome compensatory mechanisms that often lead to treatment resistance. Clinical data suggests that dual inhibition of complementary phosphorylation pathways can produce synergistic effects while allowing dose reduction of individual agents.
Pharmacogenomic approaches are increasingly vital in identifying patient populations most likely to benefit from subunit-specific interventions. Genetic profiling can reveal individuals with particular subunit mutations or expression patterns that predict therapeutic response, enabling truly personalized treatment regimens. This strategy has already improved outcomes in several kinase inhibitor therapies by matching patients with optimal molecular targets.
The economic implications of subunit-specific targeting are substantial, with potential for reduced healthcare costs through decreased adverse events and improved treatment efficacy. While development costs for these precision therapeutics may be higher initially, their improved therapeutic index and potential for biomarker-guided patient selection offer compelling value propositions for healthcare systems worldwide.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!