Phosphorylation in Stem Cell Differentiation: Monitoring Techniques
SEP 23, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Phosphorylation Mechanisms in Stem Cell Development
Phosphorylation represents a critical post-translational modification that regulates numerous cellular processes, including stem cell differentiation and development. This biochemical process involves the addition of a phosphate group to proteins, altering their function, activity, localization, or interaction with other molecules. In stem cell biology, phosphorylation events serve as molecular switches that control the delicate balance between self-renewal and differentiation pathways.
The primary phosphorylation mechanisms in stem cell development involve several key kinase families. Mitogen-activated protein kinases (MAPKs) play essential roles in transducing extracellular signals to intracellular responses, influencing stem cell fate decisions. Similarly, cyclin-dependent kinases (CDKs) regulate cell cycle progression and are intimately linked to stem cell proliferation and differentiation timing.
Protein kinase A (PKA) and protein kinase C (PKC) represent another class of important kinases that respond to second messengers like cAMP and calcium, respectively. These kinases phosphorylate transcription factors and other regulatory proteins that control gene expression patterns specific to stem cell states. The PI3K/AKT pathway, activated by growth factors, contributes to stem cell survival and metabolic regulation through phosphorylation of downstream targets.
Tyrosine kinase receptors, including fibroblast growth factor receptors (FGFRs) and epidermal growth factor receptors (EGFRs), initiate phosphorylation cascades upon ligand binding that ultimately influence stem cell behavior. These receptors activate multiple downstream pathways, including STAT proteins, which when phosphorylated, translocate to the nucleus and regulate transcription of genes involved in differentiation.
Phosphorylation dynamics exhibit remarkable temporal and spatial specificity during stem cell development. Early differentiation events often feature rapid, transient phosphorylation patterns that trigger initial lineage commitment, while later stages involve more sustained phosphorylation states that stabilize cell identity. This temporal regulation ensures proper developmental progression and prevents aberrant differentiation.
Mechanistically, phosphorylation affects stem cell development through several modes of action. It can directly modulate the activity of transcription factors like SMAD proteins in the TGF-β pathway or β-catenin in the Wnt pathway. Phosphorylation also regulates chromatin remodeling enzymes and histone modifications, thereby influencing epigenetic landscapes that define cell identity during differentiation.
The interplay between kinases and phosphatases creates a dynamic equilibrium that fine-tunes phosphorylation levels. This balance is crucial for maintaining stem cell plasticity while allowing for directed differentiation when appropriate signals are received. Disruption of these phosphorylation mechanisms has been implicated in developmental disorders and various diseases, highlighting their fundamental importance in stem cell biology.
The primary phosphorylation mechanisms in stem cell development involve several key kinase families. Mitogen-activated protein kinases (MAPKs) play essential roles in transducing extracellular signals to intracellular responses, influencing stem cell fate decisions. Similarly, cyclin-dependent kinases (CDKs) regulate cell cycle progression and are intimately linked to stem cell proliferation and differentiation timing.
Protein kinase A (PKA) and protein kinase C (PKC) represent another class of important kinases that respond to second messengers like cAMP and calcium, respectively. These kinases phosphorylate transcription factors and other regulatory proteins that control gene expression patterns specific to stem cell states. The PI3K/AKT pathway, activated by growth factors, contributes to stem cell survival and metabolic regulation through phosphorylation of downstream targets.
Tyrosine kinase receptors, including fibroblast growth factor receptors (FGFRs) and epidermal growth factor receptors (EGFRs), initiate phosphorylation cascades upon ligand binding that ultimately influence stem cell behavior. These receptors activate multiple downstream pathways, including STAT proteins, which when phosphorylated, translocate to the nucleus and regulate transcription of genes involved in differentiation.
Phosphorylation dynamics exhibit remarkable temporal and spatial specificity during stem cell development. Early differentiation events often feature rapid, transient phosphorylation patterns that trigger initial lineage commitment, while later stages involve more sustained phosphorylation states that stabilize cell identity. This temporal regulation ensures proper developmental progression and prevents aberrant differentiation.
Mechanistically, phosphorylation affects stem cell development through several modes of action. It can directly modulate the activity of transcription factors like SMAD proteins in the TGF-β pathway or β-catenin in the Wnt pathway. Phosphorylation also regulates chromatin remodeling enzymes and histone modifications, thereby influencing epigenetic landscapes that define cell identity during differentiation.
The interplay between kinases and phosphatases creates a dynamic equilibrium that fine-tunes phosphorylation levels. This balance is crucial for maintaining stem cell plasticity while allowing for directed differentiation when appropriate signals are received. Disruption of these phosphorylation mechanisms has been implicated in developmental disorders and various diseases, highlighting their fundamental importance in stem cell biology.
Market Analysis of Stem Cell Differentiation Monitoring Tools
The global market for stem cell differentiation monitoring tools is experiencing robust growth, driven by increasing investments in regenerative medicine and cell therapy research. Currently valued at approximately 3.2 billion USD, this market segment is projected to expand at a compound annual growth rate of 12.7% through 2028, according to recent industry analyses. This growth trajectory is supported by rising demand from academic research institutions, pharmaceutical companies, and biotechnology firms seeking to advance stem cell-based therapies.
Phosphorylation monitoring technologies represent a significant portion of this market, accounting for roughly 18% of the total market share. These technologies are particularly valuable as phosphorylation events serve as critical molecular switches during stem cell differentiation processes. The ability to accurately track these events in real-time provides researchers with essential insights into cellular development pathways.
Geographically, North America dominates the market with approximately 42% share, followed by Europe (28%) and Asia-Pacific (22%). The Asia-Pacific region, particularly China and South Korea, is witnessing the fastest growth rate at 15.3% annually, fueled by increasing government funding and expanding research infrastructure. Japan's regenerative medicine initiatives have also contributed significantly to regional market expansion.
By application segment, the market can be divided into basic research (45%), drug discovery and development (32%), and clinical applications (23%). The drug discovery segment is expected to grow most rapidly as pharmaceutical companies increasingly incorporate stem cell models into their development pipelines to improve predictive efficacy and reduce attrition rates in clinical trials.
Key customer segments include academic and research institutions (38%), pharmaceutical and biotechnology companies (34%), contract research organizations (18%), and hospitals and diagnostic centers (10%). The pharmaceutical sector is showing the strongest growth in adoption as companies seek to leverage stem cell technologies for developing novel therapeutics.
Price sensitivity varies significantly across market segments, with academic institutions being most price-sensitive while pharmaceutical companies prioritize performance and reliability over cost. The average price point for comprehensive phosphorylation monitoring systems ranges from 75,000 to 250,000 USD, with consumables and reagents generating substantial recurring revenue streams for manufacturers.
Market barriers include high initial investment costs, technical complexity requiring specialized expertise, and regulatory challenges related to clinical applications. Additionally, the lack of standardized protocols for phosphorylation monitoring in stem cell differentiation represents a significant challenge that industry stakeholders are actively working to address through collaborative initiatives and consortium-based approaches.
Phosphorylation monitoring technologies represent a significant portion of this market, accounting for roughly 18% of the total market share. These technologies are particularly valuable as phosphorylation events serve as critical molecular switches during stem cell differentiation processes. The ability to accurately track these events in real-time provides researchers with essential insights into cellular development pathways.
Geographically, North America dominates the market with approximately 42% share, followed by Europe (28%) and Asia-Pacific (22%). The Asia-Pacific region, particularly China and South Korea, is witnessing the fastest growth rate at 15.3% annually, fueled by increasing government funding and expanding research infrastructure. Japan's regenerative medicine initiatives have also contributed significantly to regional market expansion.
By application segment, the market can be divided into basic research (45%), drug discovery and development (32%), and clinical applications (23%). The drug discovery segment is expected to grow most rapidly as pharmaceutical companies increasingly incorporate stem cell models into their development pipelines to improve predictive efficacy and reduce attrition rates in clinical trials.
Key customer segments include academic and research institutions (38%), pharmaceutical and biotechnology companies (34%), contract research organizations (18%), and hospitals and diagnostic centers (10%). The pharmaceutical sector is showing the strongest growth in adoption as companies seek to leverage stem cell technologies for developing novel therapeutics.
Price sensitivity varies significantly across market segments, with academic institutions being most price-sensitive while pharmaceutical companies prioritize performance and reliability over cost. The average price point for comprehensive phosphorylation monitoring systems ranges from 75,000 to 250,000 USD, with consumables and reagents generating substantial recurring revenue streams for manufacturers.
Market barriers include high initial investment costs, technical complexity requiring specialized expertise, and regulatory challenges related to clinical applications. Additionally, the lack of standardized protocols for phosphorylation monitoring in stem cell differentiation represents a significant challenge that industry stakeholders are actively working to address through collaborative initiatives and consortium-based approaches.
Current Challenges in Phosphorylation Detection Technologies
Despite significant advancements in phosphorylation detection technologies, several critical challenges persist that limit our ability to effectively monitor phosphorylation events during stem cell differentiation. The dynamic and transient nature of phosphorylation presents a fundamental obstacle, as these modifications often occur rapidly and may be reversed within minutes or seconds. This temporal complexity makes capturing the complete phosphorylation landscape at precise differentiation stages exceptionally difficult.
Sensitivity limitations represent another major hurdle in current detection methods. Many important phosphorylation events occur at extremely low abundance, particularly in the early stages of differentiation signaling cascades. Conventional techniques often fail to detect these low-abundance phosphorylation sites, potentially missing critical regulatory events that initiate differentiation pathways.
Spatial resolution remains inadequate in most current technologies. Phosphorylation events are highly localized within specific cellular compartments, and this spatial organization is crucial for proper signal transduction. However, many detection methods provide only whole-cell or tissue-level information, obscuring the subcellular context that is essential for understanding phosphorylation's role in differentiation.
The heterogeneity of stem cell populations compounds these challenges. Even seemingly homogeneous stem cell cultures contain cells at slightly different stages of differentiation or activation. This cellular heterogeneity creates significant noise in phosphorylation measurements, as signals from relevant subpopulations may be diluted by signals from other cells.
Technical limitations in sample preparation also impede progress. Phosphorylation states are notoriously labile and can be rapidly altered during cell lysis and protein extraction. Current preservation methods often fail to maintain the native phosphorylation landscape, introducing artifacts that complicate data interpretation.
Quantification accuracy presents ongoing difficulties, particularly for multiplexed analyses. While mass spectrometry has revolutionized phosphoproteomics, challenges in standardization, reproducibility, and absolute quantification persist. These issues are especially problematic when attempting to compare phosphorylation levels across different experimental conditions or time points during differentiation.
Finally, there exists a significant computational challenge in integrating phosphorylation data with other molecular information. The biological significance of detected phosphorylation events cannot be fully understood without contextualizing them within broader signaling networks and epigenetic landscapes. Current bioinformatic approaches struggle to effectively integrate these multi-omic datasets to provide meaningful insights into the regulatory mechanisms governing stem cell differentiation.
Sensitivity limitations represent another major hurdle in current detection methods. Many important phosphorylation events occur at extremely low abundance, particularly in the early stages of differentiation signaling cascades. Conventional techniques often fail to detect these low-abundance phosphorylation sites, potentially missing critical regulatory events that initiate differentiation pathways.
Spatial resolution remains inadequate in most current technologies. Phosphorylation events are highly localized within specific cellular compartments, and this spatial organization is crucial for proper signal transduction. However, many detection methods provide only whole-cell or tissue-level information, obscuring the subcellular context that is essential for understanding phosphorylation's role in differentiation.
The heterogeneity of stem cell populations compounds these challenges. Even seemingly homogeneous stem cell cultures contain cells at slightly different stages of differentiation or activation. This cellular heterogeneity creates significant noise in phosphorylation measurements, as signals from relevant subpopulations may be diluted by signals from other cells.
Technical limitations in sample preparation also impede progress. Phosphorylation states are notoriously labile and can be rapidly altered during cell lysis and protein extraction. Current preservation methods often fail to maintain the native phosphorylation landscape, introducing artifacts that complicate data interpretation.
Quantification accuracy presents ongoing difficulties, particularly for multiplexed analyses. While mass spectrometry has revolutionized phosphoproteomics, challenges in standardization, reproducibility, and absolute quantification persist. These issues are especially problematic when attempting to compare phosphorylation levels across different experimental conditions or time points during differentiation.
Finally, there exists a significant computational challenge in integrating phosphorylation data with other molecular information. The biological significance of detected phosphorylation events cannot be fully understood without contextualizing them within broader signaling networks and epigenetic landscapes. Current bioinformatic approaches struggle to effectively integrate these multi-omic datasets to provide meaningful insights into the regulatory mechanisms governing stem cell differentiation.
Established Phosphorylation Monitoring Methodologies
01 Methods for detecting protein phosphorylation
Various techniques have been developed to detect and monitor protein phosphorylation, which is a critical post-translational modification in cellular signaling pathways. These methods include the use of phospho-specific antibodies, mass spectrometry, and fluorescence-based assays that can specifically recognize phosphorylated proteins or peptides. These techniques allow researchers to identify phosphorylation sites and quantify phosphorylation levels in biological samples.- Methods for detecting protein phosphorylation: Various techniques have been developed to monitor protein phosphorylation, including antibody-based assays, mass spectrometry, and fluorescence-based detection methods. These approaches allow researchers to identify phosphorylated proteins and quantify phosphorylation levels in biological samples. Such methods are crucial for understanding cellular signaling pathways and protein regulation mechanisms.
- Phosphorylation monitoring in disease diagnosis and treatment: Monitoring protein phosphorylation patterns can serve as biomarkers for disease diagnosis, progression monitoring, and treatment response assessment. Abnormal phosphorylation is associated with various diseases including cancer, neurodegenerative disorders, and metabolic conditions. Technologies that detect specific phosphorylation signatures enable personalized medicine approaches and targeted therapeutic interventions.
- Real-time phosphorylation monitoring systems: Real-time monitoring systems for protein phosphorylation allow continuous assessment of kinase activity and signaling pathway dynamics in living cells. These systems often employ biosensors, reporter constructs, or label-free detection methods that provide temporal information about phosphorylation events. Such technologies are valuable for studying rapid cellular responses and dynamic regulation of protein function.
- High-throughput phosphorylation screening platforms: High-throughput platforms enable simultaneous monitoring of multiple phosphorylation events across numerous samples. These technologies incorporate microarray formats, multiplexed detection systems, and automated analysis tools to efficiently process large datasets. Such platforms are particularly useful for drug discovery, kinase inhibitor screening, and systems biology approaches to understanding complex signaling networks.
- Phosphorylation monitoring in cellular signaling pathways: Techniques specifically designed to monitor phosphorylation events within cellular signaling cascades help elucidate signal transduction mechanisms. These methods can track phosphorylation patterns following receptor activation, identify feedback loops, and characterize cross-talk between different pathways. Understanding these signaling networks is essential for developing targeted therapeutics and predicting cellular responses to various stimuli.
02 Kinase activity monitoring systems
Systems for monitoring kinase activity involve the use of substrates that change their properties upon phosphorylation. These systems often utilize fluorescent or luminescent reporters that respond to phosphorylation events, allowing real-time monitoring of kinase activity in vitro or in living cells. Such monitoring systems are valuable tools for studying signal transduction pathways and for screening potential kinase inhibitors in drug discovery.Expand Specific Solutions03 Phosphorylation biomarkers for disease diagnosis
Phosphorylation patterns of certain proteins can serve as biomarkers for various diseases, including cancer, neurodegenerative disorders, and metabolic diseases. Monitoring these phosphorylation biomarkers can aid in early disease detection, prognosis assessment, and treatment response evaluation. Technologies have been developed to detect specific phosphorylation signatures in patient samples for diagnostic purposes.Expand Specific Solutions04 High-throughput phosphoproteomics platforms
Advanced platforms for high-throughput analysis of the phosphoproteome enable comprehensive monitoring of phosphorylation events across thousands of proteins simultaneously. These platforms typically combine phosphopeptide enrichment techniques with sophisticated mass spectrometry and bioinformatics tools. Such approaches allow researchers to map complex phosphorylation networks and study dynamic changes in phosphorylation in response to various stimuli or disease states.Expand Specific Solutions05 Phosphorylation sensors for live cell imaging
Genetically encoded biosensors have been developed to visualize protein phosphorylation events in living cells with high spatial and temporal resolution. These sensors typically consist of fluorescent proteins combined with phosphopeptide binding domains that undergo conformational changes upon phosphorylation, resulting in measurable changes in fluorescence properties. Such tools enable researchers to monitor signaling dynamics in real-time within intact cellular environments.Expand Specific Solutions
Leading Research Institutions and Biotech Companies
Phosphorylation monitoring in stem cell differentiation is currently in a growth phase, with the market expanding rapidly due to increasing applications in regenerative medicine and drug discovery. The global market size is estimated to reach several billion dollars by 2025, driven by rising investments in stem cell research. Technologically, the field shows moderate maturity with established techniques like mass spectrometry and immunoassays, but significant innovation continues. Leading players include academic institutions like The Regents of the University of California and University of Michigan, alongside commercial entities such as Illumina, FUJIFILM Wako Pure Chemical, and Bayer Pharma AG. Research centers like the German Cancer Research Center and Dalian Institute of Chemical Physics are advancing novel monitoring approaches, while companies like Revvity Health Sciences and Cytiva Sweden provide essential analytical tools for phosphorylation detection.
Illumina, Inc.
Technical Solution: Illumina has developed advanced sequencing-based phosphoproteomics platforms specifically optimized for stem cell research. Their technology combines next-generation sequencing with phosphopeptide enrichment techniques to provide comprehensive phosphorylation profiling during stem cell differentiation. Their proprietary TruSeq Phospho-Seq technology enables researchers to identify thousands of phosphorylation sites simultaneously with high sensitivity and specificity. The platform integrates seamlessly with their bioinformatics tools that can track phosphorylation changes across different timepoints during stem cell differentiation, allowing for temporal mapping of signaling cascades. Illumina's systems incorporate machine learning algorithms to identify key phosphorylation events that serve as molecular switches during lineage commitment.
Strengths: Unparalleled throughput capacity allowing comprehensive phosphoproteome coverage; integrated bioinformatics solutions for complex data interpretation. Weaknesses: High initial equipment investment; requires specialized technical expertise for optimal utilization.
Olympus Corp.
Technical Solution: Olympus has developed specialized microscopy platforms for visualizing phosphorylation dynamics in living stem cells during differentiation processes. Their FV3000 confocal system incorporates proprietary TruSpectral detection technology that enables simultaneous imaging of multiple phosphorylation sites with minimal spectral overlap, crucial for dissecting complex signaling networks. Olympus' SpinSR10 super-resolution microscope achieves sub-diffraction imaging of phosphorylation events at specific subcellular locations, revealing spatial organization of signaling hubs during stem cell fate decisions. Their systems integrate with microincubation chambers specifically designed to maintain stem cell viability during long-term phosphorylation imaging experiments. Olympus has also pioneered FRET-based biosensors compatible with their microscopy platforms that enable real-time visualization of kinase activities driving phosphorylation events during differentiation.
Strengths: Exceptional spatial resolution for localizing phosphorylation events within cellular compartments; non-destructive monitoring allowing longitudinal studies of the same cells. Weaknesses: Limited throughput compared to biochemical assays; requires development of specific phospho-sensors for each target of interest.
Key Patents in Phosphoproteomic Analysis
A system and method for electrochemical detection and quantification of cell characteristics and functions
PatentPendingIN202211006300A
Innovation
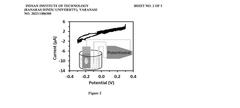
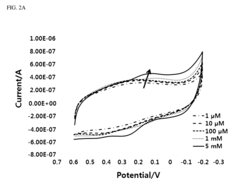
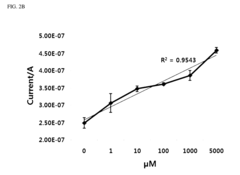
- A three-electrode system using a polydimethylsiloxane (PDMS) working electrode with cyclic voltammetry and differential pulse voltammetry for electrochemical detection and quantification of cell surface markers/antigens, allowing cells to adhere and remain viable, without the need for fluorophores, and enabling real-time characterization.
Sensor for detecting stem cell differentiation based on electrochemical methods
PatentInactiveUS9650663B2
Innovation
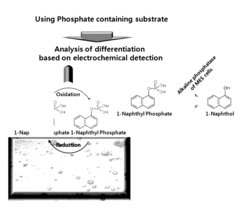
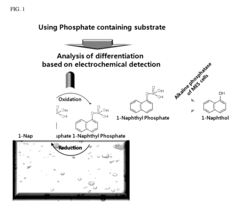
- A novel sensor system utilizing a three-electrode setup with a gold, copper, or glass carbon electrode and a substrate like 1-naphthyl phosphate for alkaline phosphatase, which measures the phosphorylation or dephosphorylation of the substrate using electrical signals to determine stem cell differentiation.
Regulatory Framework for Stem Cell Research Technologies
The regulatory landscape governing stem cell research technologies, particularly those involving phosphorylation monitoring in stem cell differentiation, presents a complex framework that varies significantly across global jurisdictions. In the United States, the Food and Drug Administration (FDA) has established specific guidelines for stem cell research technologies through the Center for Biologics Evaluation and Research (CBER), which oversees the approval process for monitoring techniques that track phosphorylation events during differentiation. These regulations emphasize validation protocols, reproducibility standards, and safety assessments for both research and clinical applications.
The European Medicines Agency (EMA) has implemented the Advanced Therapy Medicinal Products (ATMP) regulation framework, which includes specific provisions for technologies monitoring cellular signaling pathways. This framework requires comprehensive documentation of phosphorylation monitoring methodologies, with particular attention to standardization of detection limits and signal-to-noise ratios in measurement techniques. The Committee for Advanced Therapies (CAT) provides scientific recommendations on these technologies, ensuring alignment with Good Laboratory Practice (GLP) and Good Manufacturing Practice (GMP) standards.
In Asia, regulatory approaches vary substantially. Japan's Act on the Safety of Regenerative Medicine provides an accelerated pathway for stem cell technologies, including monitoring techniques, through conditional and time-limited approvals. China has recently strengthened its regulatory framework through the National Medical Products Administration (NMPA), implementing specific technical guidelines for phosphorylation detection methodologies in stem cell research.
International harmonization efforts are being coordinated through the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH), which is developing consensus guidelines for analytical techniques in stem cell research. These guidelines specifically address the validation of phosphorylation monitoring technologies, including mass spectrometry, immunoassays, and real-time imaging techniques.
Ethical considerations form a critical component of the regulatory framework, with institutional review boards (IRBs) and ethics committees evaluating research protocols involving phosphorylation monitoring in human stem cells. These evaluations focus on consent procedures, data privacy, and the ethical implications of manipulating cellular differentiation pathways through targeted interventions based on phosphorylation data.
Recent regulatory trends indicate movement toward adaptive licensing approaches that accommodate the rapid evolution of monitoring technologies. These frameworks incorporate continuous assessment protocols that evaluate the performance of phosphorylation detection methods throughout the research and development lifecycle, allowing for methodological refinements while maintaining regulatory oversight.
The European Medicines Agency (EMA) has implemented the Advanced Therapy Medicinal Products (ATMP) regulation framework, which includes specific provisions for technologies monitoring cellular signaling pathways. This framework requires comprehensive documentation of phosphorylation monitoring methodologies, with particular attention to standardization of detection limits and signal-to-noise ratios in measurement techniques. The Committee for Advanced Therapies (CAT) provides scientific recommendations on these technologies, ensuring alignment with Good Laboratory Practice (GLP) and Good Manufacturing Practice (GMP) standards.
In Asia, regulatory approaches vary substantially. Japan's Act on the Safety of Regenerative Medicine provides an accelerated pathway for stem cell technologies, including monitoring techniques, through conditional and time-limited approvals. China has recently strengthened its regulatory framework through the National Medical Products Administration (NMPA), implementing specific technical guidelines for phosphorylation detection methodologies in stem cell research.
International harmonization efforts are being coordinated through the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH), which is developing consensus guidelines for analytical techniques in stem cell research. These guidelines specifically address the validation of phosphorylation monitoring technologies, including mass spectrometry, immunoassays, and real-time imaging techniques.
Ethical considerations form a critical component of the regulatory framework, with institutional review boards (IRBs) and ethics committees evaluating research protocols involving phosphorylation monitoring in human stem cells. These evaluations focus on consent procedures, data privacy, and the ethical implications of manipulating cellular differentiation pathways through targeted interventions based on phosphorylation data.
Recent regulatory trends indicate movement toward adaptive licensing approaches that accommodate the rapid evolution of monitoring technologies. These frameworks incorporate continuous assessment protocols that evaluate the performance of phosphorylation detection methods throughout the research and development lifecycle, allowing for methodological refinements while maintaining regulatory oversight.
Ethical Implications of Stem Cell Differentiation Control
The ethical landscape surrounding stem cell differentiation control techniques, particularly those involving phosphorylation monitoring, presents complex challenges for researchers, clinicians, and policymakers. As these technologies advance, they raise fundamental questions about human identity, autonomy, and the boundaries of scientific intervention in natural processes.
The ability to precisely control stem cell differentiation through phosphorylation pathways introduces concerns regarding genetic manipulation and potential unintended consequences. While these techniques offer unprecedented therapeutic possibilities for regenerative medicine, they simultaneously create ethical tensions regarding the extent to which human development should be artificially directed. The monitoring technologies that track phosphorylation events during differentiation further complicate these considerations by enabling increasingly granular control over cellular fate.
Consent and autonomy emerge as critical ethical dimensions, particularly when considering applications involving embryonic stem cells or induced pluripotent stem cells derived from individuals. Questions arise regarding appropriate consent procedures for donors whose cells might be perpetually maintained in laboratories, potentially generating therapies or discoveries decades after donation. The right to withdraw consent becomes particularly problematic in this context.
Regulatory frameworks worldwide demonstrate significant variation in their approach to stem cell differentiation technologies. Some jurisdictions have established permissive environments with robust ethical oversight, while others maintain restrictive policies that limit research applications. This regulatory heterogeneity creates potential for "ethics arbitrage," where researchers might conduct work in regions with less stringent oversight, raising concerns about global governance of these powerful technologies.
The commercialization of phosphorylation-based differentiation control techniques introduces additional ethical considerations regarding equitable access. As these technologies transition from research tools to therapeutic applications, questions of intellectual property, pricing, and healthcare disparities become increasingly relevant. The potential for these advanced therapies to exacerbate existing healthcare inequalities requires careful consideration of justice principles in their development and deployment.
Religious and cultural perspectives on stem cell manipulation vary considerably, with some traditions expressing fundamental concerns about interfering with natural developmental processes. Respecting this diversity of viewpoints while advancing beneficial medical applications represents a significant ethical challenge. Inclusive stakeholder engagement that acknowledges these diverse perspectives is essential for developing socially responsible approaches to these technologies.
The ability to precisely control stem cell differentiation through phosphorylation pathways introduces concerns regarding genetic manipulation and potential unintended consequences. While these techniques offer unprecedented therapeutic possibilities for regenerative medicine, they simultaneously create ethical tensions regarding the extent to which human development should be artificially directed. The monitoring technologies that track phosphorylation events during differentiation further complicate these considerations by enabling increasingly granular control over cellular fate.
Consent and autonomy emerge as critical ethical dimensions, particularly when considering applications involving embryonic stem cells or induced pluripotent stem cells derived from individuals. Questions arise regarding appropriate consent procedures for donors whose cells might be perpetually maintained in laboratories, potentially generating therapies or discoveries decades after donation. The right to withdraw consent becomes particularly problematic in this context.
Regulatory frameworks worldwide demonstrate significant variation in their approach to stem cell differentiation technologies. Some jurisdictions have established permissive environments with robust ethical oversight, while others maintain restrictive policies that limit research applications. This regulatory heterogeneity creates potential for "ethics arbitrage," where researchers might conduct work in regions with less stringent oversight, raising concerns about global governance of these powerful technologies.
The commercialization of phosphorylation-based differentiation control techniques introduces additional ethical considerations regarding equitable access. As these technologies transition from research tools to therapeutic applications, questions of intellectual property, pricing, and healthcare disparities become increasingly relevant. The potential for these advanced therapies to exacerbate existing healthcare inequalities requires careful consideration of justice principles in their development and deployment.
Religious and cultural perspectives on stem cell manipulation vary considerably, with some traditions expressing fundamental concerns about interfering with natural developmental processes. Respecting this diversity of viewpoints while advancing beneficial medical applications represents a significant ethical challenge. Inclusive stakeholder engagement that acknowledges these diverse perspectives is essential for developing socially responsible approaches to these technologies.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!