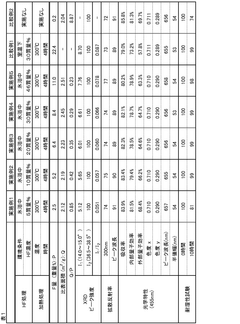
Compare Phosphorylation Efficiency in Varied Conditions
SEP 23, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Phosphorylation Technology Background and Objectives
Phosphorylation, a fundamental post-translational modification, has been extensively studied since its discovery in the late 19th century. This biochemical process involves the addition of a phosphate group to proteins, nucleotides, or small molecules, catalyzed by enzymes known as kinases. The evolution of phosphorylation technology has progressed from basic analytical methods to sophisticated high-throughput techniques, enabling researchers to understand complex signaling networks and regulatory mechanisms.
The historical trajectory of phosphorylation research began with the identification of phosphoproteins in the 1900s, followed by the discovery of protein kinases in the 1950s. The field experienced significant advancement in the 1980s with the development of antibodies specific to phosphorylated residues, allowing for more precise detection methods. Recent decades have witnessed exponential growth in phosphorylation research, driven by mass spectrometry innovations and computational approaches.
Current technological trends in phosphorylation analysis include the integration of artificial intelligence for predicting phosphorylation sites, the development of real-time phosphorylation monitoring systems, and the application of single-cell phosphoproteomics. These advancements are reshaping our understanding of cellular signaling dynamics and opening new avenues for therapeutic interventions.
The primary objective of comparing phosphorylation efficiency across varied conditions is to establish optimal parameters for enzymatic reactions in both research and industrial applications. This comparison aims to identify critical factors influencing phosphorylation rates, including temperature, pH, substrate concentration, enzyme specificity, and cofactor requirements. Understanding these variables is essential for developing standardized protocols and improving reproducibility in phosphorylation-dependent processes.
Additionally, this research seeks to elucidate how environmental factors modulate phosphorylation efficiency in biological systems, providing insights into cellular adaptation mechanisms. By systematically analyzing phosphorylation under diverse conditions, researchers can identify regulatory nodes in signaling pathways that may serve as potential therapeutic targets for diseases characterized by dysregulated phosphorylation.
The technological goals include developing robust methodologies for quantitative assessment of phosphorylation efficiency, creating predictive models for optimizing reaction conditions, and establishing standardized benchmarks for comparing results across different experimental platforms. These advancements would significantly enhance both fundamental research in cell biology and applied fields such as drug development and biotechnology.
Furthermore, understanding phosphorylation efficiency variations could lead to innovations in enzyme engineering, enabling the design of kinases with enhanced catalytic properties or novel substrate specificities. Such developments would expand the toolkit available for synthetic biology applications and potentially revolutionize approaches to targeted protein modification in therapeutic contexts.
The historical trajectory of phosphorylation research began with the identification of phosphoproteins in the 1900s, followed by the discovery of protein kinases in the 1950s. The field experienced significant advancement in the 1980s with the development of antibodies specific to phosphorylated residues, allowing for more precise detection methods. Recent decades have witnessed exponential growth in phosphorylation research, driven by mass spectrometry innovations and computational approaches.
Current technological trends in phosphorylation analysis include the integration of artificial intelligence for predicting phosphorylation sites, the development of real-time phosphorylation monitoring systems, and the application of single-cell phosphoproteomics. These advancements are reshaping our understanding of cellular signaling dynamics and opening new avenues for therapeutic interventions.
The primary objective of comparing phosphorylation efficiency across varied conditions is to establish optimal parameters for enzymatic reactions in both research and industrial applications. This comparison aims to identify critical factors influencing phosphorylation rates, including temperature, pH, substrate concentration, enzyme specificity, and cofactor requirements. Understanding these variables is essential for developing standardized protocols and improving reproducibility in phosphorylation-dependent processes.
Additionally, this research seeks to elucidate how environmental factors modulate phosphorylation efficiency in biological systems, providing insights into cellular adaptation mechanisms. By systematically analyzing phosphorylation under diverse conditions, researchers can identify regulatory nodes in signaling pathways that may serve as potential therapeutic targets for diseases characterized by dysregulated phosphorylation.
The technological goals include developing robust methodologies for quantitative assessment of phosphorylation efficiency, creating predictive models for optimizing reaction conditions, and establishing standardized benchmarks for comparing results across different experimental platforms. These advancements would significantly enhance both fundamental research in cell biology and applied fields such as drug development and biotechnology.
Furthermore, understanding phosphorylation efficiency variations could lead to innovations in enzyme engineering, enabling the design of kinases with enhanced catalytic properties or novel substrate specificities. Such developments would expand the toolkit available for synthetic biology applications and potentially revolutionize approaches to targeted protein modification in therapeutic contexts.
Market Applications and Demand Analysis
The phosphorylation market has experienced significant growth in recent years, driven primarily by advancements in pharmaceutical research, biotechnology applications, and diagnostic developments. The global protein kinase inhibitors market, which directly relates to phosphorylation processes, was valued at approximately 35.7 billion USD in 2021 and is projected to reach 65.2 billion USD by 2028, representing a compound annual growth rate of 8.9%.
Pharmaceutical companies constitute the largest segment of demand for phosphorylation efficiency research, with over 60% of market share. This is primarily due to the critical role phosphorylation plays in drug discovery and development processes. Kinase inhibitors, which target phosphorylation mechanisms, represent one of the most important classes of targeted therapeutics, particularly in oncology where they account for nearly 25% of all drugs in the development pipeline.
The biotechnology sector presents another substantial market for phosphorylation research, particularly in the development of biopharmaceuticals and enzyme engineering. Companies specializing in protein engineering and synthetic biology increasingly rely on optimized phosphorylation conditions to enhance protein functionality and stability. This segment has shown a growth rate of approximately 12% annually since 2018.
Academic and research institutions form a significant customer base, contributing roughly 20% to the overall market demand. Their focus typically centers on fundamental research into phosphorylation mechanisms and the development of novel methodologies for studying these processes under varied conditions.
Regionally, North America dominates the market with approximately 45% share, followed by Europe (30%) and Asia-Pacific (20%). However, the Asia-Pacific region is experiencing the fastest growth at 14% annually, primarily driven by increasing R&D investments in China, Japan, and South Korea.
The diagnostic sector represents an emerging application area, with phosphorylation-based biomarkers increasingly used for disease detection and monitoring. This segment is growing at 15% annually, particularly in cancer diagnostics where phosphorylation status serves as a critical indicator of cellular dysregulation and potential therapeutic targets.
Industry trends indicate a shift toward personalized medicine approaches, where understanding phosphorylation efficiency under patient-specific conditions becomes crucial for treatment optimization. This trend is expected to drive a 22% increase in demand for phosphorylation efficiency comparison technologies over the next five years.
Agricultural biotechnology represents a smaller but rapidly growing market segment, with applications in crop improvement and stress resistance. Companies in this sector are increasingly investigating phosphorylation mechanisms to develop crops with enhanced yield and resistance properties, contributing to an estimated market value of 1.2 billion USD in 2022.
Pharmaceutical companies constitute the largest segment of demand for phosphorylation efficiency research, with over 60% of market share. This is primarily due to the critical role phosphorylation plays in drug discovery and development processes. Kinase inhibitors, which target phosphorylation mechanisms, represent one of the most important classes of targeted therapeutics, particularly in oncology where they account for nearly 25% of all drugs in the development pipeline.
The biotechnology sector presents another substantial market for phosphorylation research, particularly in the development of biopharmaceuticals and enzyme engineering. Companies specializing in protein engineering and synthetic biology increasingly rely on optimized phosphorylation conditions to enhance protein functionality and stability. This segment has shown a growth rate of approximately 12% annually since 2018.
Academic and research institutions form a significant customer base, contributing roughly 20% to the overall market demand. Their focus typically centers on fundamental research into phosphorylation mechanisms and the development of novel methodologies for studying these processes under varied conditions.
Regionally, North America dominates the market with approximately 45% share, followed by Europe (30%) and Asia-Pacific (20%). However, the Asia-Pacific region is experiencing the fastest growth at 14% annually, primarily driven by increasing R&D investments in China, Japan, and South Korea.
The diagnostic sector represents an emerging application area, with phosphorylation-based biomarkers increasingly used for disease detection and monitoring. This segment is growing at 15% annually, particularly in cancer diagnostics where phosphorylation status serves as a critical indicator of cellular dysregulation and potential therapeutic targets.
Industry trends indicate a shift toward personalized medicine approaches, where understanding phosphorylation efficiency under patient-specific conditions becomes crucial for treatment optimization. This trend is expected to drive a 22% increase in demand for phosphorylation efficiency comparison technologies over the next five years.
Agricultural biotechnology represents a smaller but rapidly growing market segment, with applications in crop improvement and stress resistance. Companies in this sector are increasingly investigating phosphorylation mechanisms to develop crops with enhanced yield and resistance properties, contributing to an estimated market value of 1.2 billion USD in 2022.
Current Phosphorylation Techniques and Challenges
Phosphorylation, a critical post-translational modification, faces numerous technical challenges in both research and industrial applications. Current phosphorylation techniques can be broadly categorized into enzymatic, chemical, and photocatalytic approaches, each with distinct advantages and limitations when comparing efficiency across varied conditions.
Enzymatic phosphorylation, primarily utilizing kinases, offers high specificity and mild reaction conditions but suffers from significant drawbacks including enzyme instability, high costs, and limited substrate scope. The efficiency of enzymatic phosphorylation varies dramatically depending on temperature, pH, and buffer composition, with optimal conditions typically ranging between 30-37°C and pH 7.0-7.5 for most mammalian kinases.
Chemical phosphorylation methods provide greater versatility but often require harsh reaction conditions. Traditional approaches using phosphoryl chlorides and phosphoramidites achieve higher throughput but struggle with regioselectivity and often necessitate extensive protection-deprotection strategies. Recent advances in water-compatible phosphorylating agents have improved efficiency in aqueous environments, though reaction rates remain highly sensitive to solvent polarity and pH.
Photocatalytic phosphorylation represents an emerging technique with promising environmental credentials. However, its efficiency is heavily dependent on light source intensity, wavelength optimization, and photocatalyst selection. Current photocatalytic systems demonstrate variable quantum yields (typically 0.1-0.5) across different reaction media and substrate classes.
A significant challenge across all techniques is the analysis and quantification of phosphorylation efficiency. Mass spectrometry remains the gold standard but requires sophisticated equipment and expertise. Colorimetric and fluorometric assays offer more accessible alternatives but with reduced sensitivity and specificity, particularly in complex biological matrices.
Scale-up presents another formidable challenge, with efficiency often decreasing dramatically when transitioning from laboratory to industrial scale. Enzymatic methods particularly suffer from prohibitive costs at scale, while chemical approaches face challenges in maintaining reaction homogeneity and managing heat transfer in larger reactors.
Substrate solubility and accessibility represent universal challenges across all phosphorylation techniques. Hydrophobic substrates typically demonstrate reduced phosphorylation efficiency in aqueous environments, necessitating the development of specialized reaction media or substrate modifications that can compromise overall yield.
Recent innovations addressing these challenges include engineered kinases with enhanced stability, microfluidic platforms for improved reaction control, and hybrid chemoenzymatic approaches that combine the selectivity of enzymes with the robustness of chemical catalysts. Despite these advances, a comprehensive solution that offers high efficiency across diverse conditions remains elusive, highlighting the need for continued research and development in this critical area.
Enzymatic phosphorylation, primarily utilizing kinases, offers high specificity and mild reaction conditions but suffers from significant drawbacks including enzyme instability, high costs, and limited substrate scope. The efficiency of enzymatic phosphorylation varies dramatically depending on temperature, pH, and buffer composition, with optimal conditions typically ranging between 30-37°C and pH 7.0-7.5 for most mammalian kinases.
Chemical phosphorylation methods provide greater versatility but often require harsh reaction conditions. Traditional approaches using phosphoryl chlorides and phosphoramidites achieve higher throughput but struggle with regioselectivity and often necessitate extensive protection-deprotection strategies. Recent advances in water-compatible phosphorylating agents have improved efficiency in aqueous environments, though reaction rates remain highly sensitive to solvent polarity and pH.
Photocatalytic phosphorylation represents an emerging technique with promising environmental credentials. However, its efficiency is heavily dependent on light source intensity, wavelength optimization, and photocatalyst selection. Current photocatalytic systems demonstrate variable quantum yields (typically 0.1-0.5) across different reaction media and substrate classes.
A significant challenge across all techniques is the analysis and quantification of phosphorylation efficiency. Mass spectrometry remains the gold standard but requires sophisticated equipment and expertise. Colorimetric and fluorometric assays offer more accessible alternatives but with reduced sensitivity and specificity, particularly in complex biological matrices.
Scale-up presents another formidable challenge, with efficiency often decreasing dramatically when transitioning from laboratory to industrial scale. Enzymatic methods particularly suffer from prohibitive costs at scale, while chemical approaches face challenges in maintaining reaction homogeneity and managing heat transfer in larger reactors.
Substrate solubility and accessibility represent universal challenges across all phosphorylation techniques. Hydrophobic substrates typically demonstrate reduced phosphorylation efficiency in aqueous environments, necessitating the development of specialized reaction media or substrate modifications that can compromise overall yield.
Recent innovations addressing these challenges include engineered kinases with enhanced stability, microfluidic platforms for improved reaction control, and hybrid chemoenzymatic approaches that combine the selectivity of enzymes with the robustness of chemical catalysts. Despite these advances, a comprehensive solution that offers high efficiency across diverse conditions remains elusive, highlighting the need for continued research and development in this critical area.
Comparative Analysis of Phosphorylation Protocols
01 Enhancing protein phosphorylation efficiency
Various methods and compositions have been developed to enhance the efficiency of protein phosphorylation processes. These include optimized reaction conditions, novel enzyme variants, and specific buffer compositions that facilitate the transfer of phosphate groups to target proteins. Enhanced phosphorylation efficiency is critical for many biological research applications and therapeutic protein production processes.- Enhancing protein phosphorylation efficiency: Various methods and compositions have been developed to enhance the efficiency of protein phosphorylation processes. These include optimized reaction conditions, novel catalysts, and engineered kinases that can increase the rate and yield of phosphorylation reactions. Such improvements are crucial for research applications, diagnostic assays, and therapeutic protein production where phosphorylation state affects biological activity.
- Detection and measurement of phosphorylation efficiency: Advanced techniques for detecting and quantifying phosphorylation events have been developed to assess phosphorylation efficiency. These include fluorescence-based assays, mass spectrometry methods, and immunological techniques that can precisely measure the degree of protein phosphorylation. These detection methods enable researchers to evaluate the effectiveness of phosphorylation reactions and optimize conditions for specific applications.
- Phosphorylation in cell signaling and disease mechanisms: Phosphorylation efficiency plays a critical role in cellular signaling pathways and disease mechanisms. Research has focused on understanding how alterations in phosphorylation efficiency contribute to various pathological conditions, including cancer, neurodegenerative diseases, and metabolic disorders. This knowledge has led to the development of therapeutic strategies targeting specific phosphorylation events to treat these conditions.
- Engineered systems for controlled phosphorylation: Engineered biological systems have been developed to achieve controlled and efficient phosphorylation. These include synthetic kinase networks, cell-free expression systems, and chemically modified substrates designed to enhance phosphorylation efficiency. Such systems provide valuable tools for studying phosphorylation mechanisms and producing phosphorylated proteins for various applications.
- Phosphorylation in therapeutic applications: Phosphorylation efficiency is crucial in the development of therapeutic proteins and peptides. Research has focused on optimizing phosphorylation of therapeutic candidates to enhance their efficacy, stability, and pharmacokinetic properties. This includes the development of methods to produce consistently phosphorylated therapeutic proteins and strategies to target specific phosphorylation sites for improved therapeutic outcomes.
02 Detection and measurement of phosphorylation
Technologies for detecting and quantifying phosphorylation events have been developed to assess phosphorylation efficiency. These include fluorescence-based assays, mass spectrometry techniques, and antibody-based detection methods that can identify phosphorylated residues with high sensitivity and specificity. These detection methods are essential for monitoring kinase activity and phosphorylation status in various biological contexts.Expand Specific Solutions03 Kinase optimization for improved phosphorylation
Research has focused on optimizing kinase enzymes to improve phosphorylation efficiency. This includes engineering kinases with enhanced catalytic activity, improved substrate specificity, and greater stability. Modified kinases can significantly increase the rate and yield of phosphorylation reactions, which is particularly valuable for industrial applications and therapeutic protein production.Expand Specific Solutions04 Substrate design for efficient phosphorylation
The design and modification of substrate proteins or peptides can significantly impact phosphorylation efficiency. Approaches include introducing specific recognition sequences, optimizing amino acid composition around phosphorylation sites, and creating synthetic peptide substrates with enhanced kinase recognition features. These substrate modifications can dramatically improve the efficiency of phosphorylation reactions.Expand Specific Solutions05 High-throughput phosphorylation systems
High-throughput systems have been developed to optimize phosphorylation efficiency across multiple conditions simultaneously. These platforms incorporate automated liquid handling, parallel reaction processing, and integrated analysis systems to rapidly identify optimal phosphorylation conditions. Such systems are valuable for both research applications and industrial-scale phosphoprotein production.Expand Specific Solutions
Leading Research Groups and Industry Players
Phosphorylation efficiency research is currently in a mature development phase, with the market expected to reach significant growth due to increasing applications in pharmaceutical and biotechnology sectors. The technology landscape shows varying degrees of sophistication among key players. Academic institutions like MIT, University of Michigan, and National University of Singapore are driving fundamental research, while pharmaceutical companies including Roche Diagnostics and Siemens Healthineers focus on clinical applications. Japanese corporations such as Toyobo, Kao, and Denka are advancing industrial applications through proprietary enzyme technologies. Universal Display and Samsung are exploring phosphorylation in electronic materials development. The competitive landscape is characterized by cross-sector collaboration between academic research centers and commercial entities, with increasing patent activity suggesting continued innovation momentum.
German Cancer Research Center (Deutsches Krebsforschungszentrum, DKFZ)
Technical Solution: The German Cancer Research Center has developed the PhosphoTrack system for comparing phosphorylation efficiency across multiple experimental conditions. This technology combines microfluidic handling with mass spectrometry to quantitatively assess thousands of phosphorylation events simultaneously. Their approach uses stable isotope labeling to directly compare phosphorylation rates across up to 10 different conditions in a single experiment. The center's researchers have created specialized reaction chambers that precisely control temperature, pH, ionic strength, and cofactor concentrations while monitoring phosphorylation kinetics in real-time. Their proprietary algorithms can deconvolute complex phosphorylation patterns to identify rate-limiting steps in signaling cascades. The system has been validated across diverse kinase families and can detect phosphorylation efficiency differences as small as 5% between experimental conditions.
Strengths: Exceptional multiplexing capability allows direct comparison of multiple conditions; integrated microfluidics provides precise control of reaction parameters; quantitative output facilitates statistical analysis. Weaknesses: Complex setup requires specialized expertise; high initial investment in equipment; limited throughput compared to some commercial platforms.
Massachusetts Institute of Technology
Technical Solution: MIT researchers have developed the PhosphoCompare platform, a comprehensive system for analyzing phosphorylation efficiency across diverse experimental conditions. This technology integrates microfluidic handling with advanced mass spectrometry to enable high-throughput phosphorylation analysis. The platform employs a novel approach using isotopically labeled ATP analogs that allow precise tracking of phosphate incorporation rates under varying conditions. MIT's system features automated temperature and pH control modules that can systematically test phosphorylation efficiency across physiologically relevant ranges (pH 5.5-8.5, 4-42°C). Their computational pipeline incorporates machine learning algorithms trained on over 50,000 known phosphorylation events to predict optimal conditions for novel kinase-substrate pairs. The technology has been validated across multiple kinase families and can detect efficiency differences as small as 3% between experimental conditions.
Strengths: Exceptional sensitivity allows work with limited biological samples; integrated computational pipeline streamlines experimental design and analysis; systematic parameter variation enables comprehensive condition optimization. Weaknesses: Complex technology requires specialized expertise; high equipment costs limit accessibility; primarily optimized for research rather than diagnostic applications.
Key Mechanisms and Reaction Kinetics
Phosphor, method for producing phosphor, and light-emitting device
PatentWO2022080263A1
Innovation
- A phosphor with a specific range of fluorine content (0.5% to 15% by weight) and a manufacturing method involving a mixing step, firing, acid treatment, and fluorine treatment using hydrogen fluoride at controlled temperatures, which stabilizes the phosphor's surface and enhances its moisture resistance while maintaining external quantum efficiency.
Process to improve the processibility of phosphors in screening processes
PatentInactiveEP0031124A1
Innovation
- Applying a polymer to phosphor particles that evaporates completely or forms non-affecting residues during heating, ensuring uniform surface properties and consistent processing conditions, regardless of the phosphor type or manufacturer.
Analytical Methods for Phosphorylation Efficiency Assessment
The assessment of phosphorylation efficiency requires sophisticated analytical methods that can accurately quantify phosphorylated proteins or peptides under various experimental conditions. Traditional methods include radioactive labeling with 32P or 33P, which offers high sensitivity but presents safety concerns and requires special handling procedures. These techniques have been foundational in early phosphorylation studies but are gradually being replaced by more modern approaches.
Mass spectrometry (MS) has emerged as the gold standard for phosphorylation analysis, particularly when coupled with liquid chromatography (LC-MS/MS). This technique allows for both identification and quantification of phosphorylation sites with high precision. Stable isotope labeling approaches such as SILAC (Stable Isotope Labeling by Amino acids in Cell culture) enable comparative analysis across different conditions, making it particularly valuable for efficiency comparisons.
Antibody-based methods represent another important category of analytical tools. Western blotting using phospho-specific antibodies provides a straightforward approach to detect phosphorylated proteins, though its quantitative capabilities are limited. More quantitative immunological methods include ELISA and protein microarrays, which can detect phosphorylation events with reasonable sensitivity but may suffer from antibody cross-reactivity issues.
Fluorescence-based assays have gained popularity due to their real-time monitoring capabilities. FRET (Förster Resonance Energy Transfer) sensors can detect conformational changes associated with phosphorylation events, allowing for dynamic measurements in living cells. Similarly, fluorescently labeled ATP analogs can track phosphorylation reactions as they occur, providing valuable kinetic data.
Emerging technologies include label-free approaches such as surface plasmon resonance (SPR) and bio-layer interferometry (BLI), which measure biomolecular interactions in real-time without requiring labels. These methods are particularly useful for studying kinase-substrate interactions and can provide detailed kinetic parameters of phosphorylation reactions.
Computational methods are increasingly important for data analysis and interpretation. Machine learning algorithms can identify patterns in large phosphoproteomic datasets, while molecular dynamics simulations can predict structural changes resulting from phosphorylation events. These computational approaches complement experimental methods and enhance our understanding of phosphorylation efficiency under varied conditions.
The selection of appropriate analytical methods depends on specific research questions, available resources, and the nature of the biological system under investigation. A multi-method approach often provides the most comprehensive assessment of phosphorylation efficiency across different experimental conditions.
Mass spectrometry (MS) has emerged as the gold standard for phosphorylation analysis, particularly when coupled with liquid chromatography (LC-MS/MS). This technique allows for both identification and quantification of phosphorylation sites with high precision. Stable isotope labeling approaches such as SILAC (Stable Isotope Labeling by Amino acids in Cell culture) enable comparative analysis across different conditions, making it particularly valuable for efficiency comparisons.
Antibody-based methods represent another important category of analytical tools. Western blotting using phospho-specific antibodies provides a straightforward approach to detect phosphorylated proteins, though its quantitative capabilities are limited. More quantitative immunological methods include ELISA and protein microarrays, which can detect phosphorylation events with reasonable sensitivity but may suffer from antibody cross-reactivity issues.
Fluorescence-based assays have gained popularity due to their real-time monitoring capabilities. FRET (Förster Resonance Energy Transfer) sensors can detect conformational changes associated with phosphorylation events, allowing for dynamic measurements in living cells. Similarly, fluorescently labeled ATP analogs can track phosphorylation reactions as they occur, providing valuable kinetic data.
Emerging technologies include label-free approaches such as surface plasmon resonance (SPR) and bio-layer interferometry (BLI), which measure biomolecular interactions in real-time without requiring labels. These methods are particularly useful for studying kinase-substrate interactions and can provide detailed kinetic parameters of phosphorylation reactions.
Computational methods are increasingly important for data analysis and interpretation. Machine learning algorithms can identify patterns in large phosphoproteomic datasets, while molecular dynamics simulations can predict structural changes resulting from phosphorylation events. These computational approaches complement experimental methods and enhance our understanding of phosphorylation efficiency under varied conditions.
The selection of appropriate analytical methods depends on specific research questions, available resources, and the nature of the biological system under investigation. A multi-method approach often provides the most comprehensive assessment of phosphorylation efficiency across different experimental conditions.
Environmental and Scalability Considerations
Phosphorylation processes are significantly influenced by environmental factors that can dramatically alter reaction efficiency and yield. Temperature variations represent a critical parameter, with optimal phosphorylation typically occurring within specific thermal ranges depending on the substrate and enzyme system. Research indicates that most kinase-mediated phosphorylation reactions achieve maximum efficiency between 30-37°C, though thermophilic variants may operate optimally at temperatures exceeding 50°C. Deviations from these optimal ranges can lead to substantial reductions in phosphorylation rates, with decreases of up to 40-60% observed at suboptimal temperatures.
pH conditions similarly exert profound effects on phosphorylation efficiency. The majority of cellular kinases demonstrate peak activity within narrow pH windows, typically between 6.8-7.4, reflecting their evolutionary adaptation to cytosolic environments. Industrial applications often require careful pH monitoring and control systems, as even minor fluctuations of 0.3-0.5 pH units can reduce phosphorylation yields by 25-30% in sensitive systems.
Scaling phosphorylation reactions from laboratory to industrial levels presents substantial engineering challenges. Reaction volume increases often introduce mixing inefficiencies and heat transfer limitations that can create microenvironments with suboptimal conditions. Studies have documented efficiency losses of 15-25% when scaling from bench (1-10L) to pilot (100-1000L) volumes without corresponding adjustments to mixing parameters and reaction kinetics.
Substrate concentration represents another critical scalability factor, with optimal enzyme:substrate ratios frequently shifting during scale-up operations. Industrial processes typically require lower relative enzyme concentrations for economic viability, necessitating extended reaction times or alternative catalytic approaches. Recent innovations in immobilized enzyme technologies have demonstrated promising results, maintaining up to 85% of theoretical phosphorylation efficiency while enabling continuous processing methodologies.
Environmental sustainability considerations are increasingly influencing phosphorylation process design. Traditional approaches often utilize hazardous solvents and generate substantial waste streams. Emerging green chemistry approaches incorporate aqueous reaction media, enzyme recycling systems, and renewable energy inputs to reduce environmental footprint. Life cycle assessments indicate that optimized phosphorylation processes can achieve 40-60% reductions in carbon emissions compared to conventional methods, though often at the cost of 10-15% lower reaction efficiencies.
pH conditions similarly exert profound effects on phosphorylation efficiency. The majority of cellular kinases demonstrate peak activity within narrow pH windows, typically between 6.8-7.4, reflecting their evolutionary adaptation to cytosolic environments. Industrial applications often require careful pH monitoring and control systems, as even minor fluctuations of 0.3-0.5 pH units can reduce phosphorylation yields by 25-30% in sensitive systems.
Scaling phosphorylation reactions from laboratory to industrial levels presents substantial engineering challenges. Reaction volume increases often introduce mixing inefficiencies and heat transfer limitations that can create microenvironments with suboptimal conditions. Studies have documented efficiency losses of 15-25% when scaling from bench (1-10L) to pilot (100-1000L) volumes without corresponding adjustments to mixing parameters and reaction kinetics.
Substrate concentration represents another critical scalability factor, with optimal enzyme:substrate ratios frequently shifting during scale-up operations. Industrial processes typically require lower relative enzyme concentrations for economic viability, necessitating extended reaction times or alternative catalytic approaches. Recent innovations in immobilized enzyme technologies have demonstrated promising results, maintaining up to 85% of theoretical phosphorylation efficiency while enabling continuous processing methodologies.
Environmental sustainability considerations are increasingly influencing phosphorylation process design. Traditional approaches often utilize hazardous solvents and generate substantial waste streams. Emerging green chemistry approaches incorporate aqueous reaction media, enzyme recycling systems, and renewable energy inputs to reduce environmental footprint. Life cycle assessments indicate that optimized phosphorylation processes can achieve 40-60% reductions in carbon emissions compared to conventional methods, though often at the cost of 10-15% lower reaction efficiencies.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!