Phosphorylation in Cardiovascular Health: Compare Pathways
SEP 23, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Phosphorylation Mechanisms and Cardiovascular Research Goals
Phosphorylation represents a fundamental post-translational modification process that regulates protein function across biological systems. In cardiovascular health, phosphorylation pathways play critical roles in maintaining cardiac function, vascular tone, and cellular homeostasis. The historical evolution of phosphorylation research in cardiovascular medicine has progressed from basic mechanistic studies to sophisticated pathway mapping that now informs therapeutic development.
The field has witnessed significant technological advancements, moving from traditional biochemical assays to high-throughput phosphoproteomics that can simultaneously track thousands of phosphorylation events. This technological progression has enabled researchers to develop comprehensive models of signaling networks that regulate cardiovascular function in both healthy and pathological states.
Current research trends focus on understanding the temporal dynamics of phosphorylation cascades and their spatial organization within cardiomyocytes and vascular cells. Particular attention is being paid to the integration of multiple signaling pathways that converge on key regulatory proteins such as protein kinase A (PKA), protein kinase C (PKC), and calcium/calmodulin-dependent protein kinase II (CaMKII).
The primary technical objectives in this field include developing more selective inhibitors of pathological phosphorylation events while preserving beneficial signaling pathways. This selective targeting represents a significant challenge due to the high degree of structural similarity among kinase catalytic domains and the complex crosstalk between different phosphorylation cascades.
Another key research goal involves elucidating the differential phosphorylation patterns between healthy and diseased cardiovascular tissues. This comparative approach aims to identify critical phosphorylation "switches" that could serve as early biomarkers for cardiovascular disease or as novel therapeutic targets.
Emerging research directions include investigating the role of phosphorylation in cardiac mitochondrial function, particularly how these modifications influence energy metabolism and oxidative stress responses. Additionally, there is growing interest in understanding how phosphorylation pathways interact with other post-translational modifications such as acetylation, ubiquitination, and SUMOylation in cardiovascular tissues.
The field is also witnessing increased focus on translational aspects, with efforts to develop phosphorylation-based biomarkers for early disease detection and personalized medicine approaches. These clinical applications represent the culmination of decades of basic research into phosphorylation mechanisms and highlight the potential impact of this research on improving cardiovascular health outcomes.
The field has witnessed significant technological advancements, moving from traditional biochemical assays to high-throughput phosphoproteomics that can simultaneously track thousands of phosphorylation events. This technological progression has enabled researchers to develop comprehensive models of signaling networks that regulate cardiovascular function in both healthy and pathological states.
Current research trends focus on understanding the temporal dynamics of phosphorylation cascades and their spatial organization within cardiomyocytes and vascular cells. Particular attention is being paid to the integration of multiple signaling pathways that converge on key regulatory proteins such as protein kinase A (PKA), protein kinase C (PKC), and calcium/calmodulin-dependent protein kinase II (CaMKII).
The primary technical objectives in this field include developing more selective inhibitors of pathological phosphorylation events while preserving beneficial signaling pathways. This selective targeting represents a significant challenge due to the high degree of structural similarity among kinase catalytic domains and the complex crosstalk between different phosphorylation cascades.
Another key research goal involves elucidating the differential phosphorylation patterns between healthy and diseased cardiovascular tissues. This comparative approach aims to identify critical phosphorylation "switches" that could serve as early biomarkers for cardiovascular disease or as novel therapeutic targets.
Emerging research directions include investigating the role of phosphorylation in cardiac mitochondrial function, particularly how these modifications influence energy metabolism and oxidative stress responses. Additionally, there is growing interest in understanding how phosphorylation pathways interact with other post-translational modifications such as acetylation, ubiquitination, and SUMOylation in cardiovascular tissues.
The field is also witnessing increased focus on translational aspects, with efforts to develop phosphorylation-based biomarkers for early disease detection and personalized medicine approaches. These clinical applications represent the culmination of decades of basic research into phosphorylation mechanisms and highlight the potential impact of this research on improving cardiovascular health outcomes.
Clinical Demand for Phosphorylation-Based Cardiac Therapies
The cardiovascular disease burden continues to rise globally, with an estimated 17.9 million deaths annually attributed to cardiovascular conditions. This significant health challenge has driven substantial interest in novel therapeutic approaches targeting fundamental cellular processes. Phosphorylation-based cardiac therapies represent one of the most promising frontiers in cardiovascular medicine, with market analyses indicating growing clinical demand across multiple treatment modalities.
Healthcare providers increasingly recognize the limitations of conventional cardiovascular treatments that address symptoms rather than underlying molecular mechanisms. This has created a robust demand for therapies that can modulate specific phosphorylation pathways implicated in cardiac pathophysiology. Surveys of cardiologists indicate that approximately 78% believe current treatment options for heart failure with preserved ejection fraction remain inadequate, highlighting a critical unmet need where phosphorylation-targeted approaches could provide breakthrough solutions.
The aging global population has further intensified market demand, as age-related cardiovascular conditions frequently involve dysregulation of phosphorylation cascades. Market research projects the cardiovascular therapeutics sector to reach $146 billion by 2028, with phosphorylation-targeted therapies expected to capture an expanding segment of this market. Particularly strong growth is anticipated in treatments addressing protein kinase A (PKA) and calcium/calmodulin-dependent protein kinase II (CaMKII) pathways.
Clinical trials focusing on phosphorylation modulators have seen a 35% increase over the past five years, reflecting both scientific progress and market recognition of their potential. Therapies targeting the PI3K/Akt pathway have generated particular interest due to their cardioprotective potential, with several compounds advancing to late-stage clinical development. Additionally, drugs targeting mitogen-activated protein kinase (MAPK) pathways have demonstrated promising results in preclinical models of cardiac hypertrophy and remodeling.
The personalized medicine trend has further accelerated demand for phosphorylation-based approaches, as these therapies can potentially be tailored to individual phosphorylation profiles. Healthcare systems increasingly seek cost-effective solutions for managing chronic cardiovascular conditions, creating favorable reimbursement environments for novel therapies that can reduce hospitalization rates and improve long-term outcomes.
Pharmaceutical industry investment in phosphorylation-targeted cardiac therapies has grown substantially, with venture capital funding for startups in this space exceeding $3.2 billion in the past three years. This investment surge reflects confidence in both the scientific rationale and market potential of these approaches, particularly as diagnostic technologies for phosphorylation profiling become more sophisticated and accessible in clinical settings.
Healthcare providers increasingly recognize the limitations of conventional cardiovascular treatments that address symptoms rather than underlying molecular mechanisms. This has created a robust demand for therapies that can modulate specific phosphorylation pathways implicated in cardiac pathophysiology. Surveys of cardiologists indicate that approximately 78% believe current treatment options for heart failure with preserved ejection fraction remain inadequate, highlighting a critical unmet need where phosphorylation-targeted approaches could provide breakthrough solutions.
The aging global population has further intensified market demand, as age-related cardiovascular conditions frequently involve dysregulation of phosphorylation cascades. Market research projects the cardiovascular therapeutics sector to reach $146 billion by 2028, with phosphorylation-targeted therapies expected to capture an expanding segment of this market. Particularly strong growth is anticipated in treatments addressing protein kinase A (PKA) and calcium/calmodulin-dependent protein kinase II (CaMKII) pathways.
Clinical trials focusing on phosphorylation modulators have seen a 35% increase over the past five years, reflecting both scientific progress and market recognition of their potential. Therapies targeting the PI3K/Akt pathway have generated particular interest due to their cardioprotective potential, with several compounds advancing to late-stage clinical development. Additionally, drugs targeting mitogen-activated protein kinase (MAPK) pathways have demonstrated promising results in preclinical models of cardiac hypertrophy and remodeling.
The personalized medicine trend has further accelerated demand for phosphorylation-based approaches, as these therapies can potentially be tailored to individual phosphorylation profiles. Healthcare systems increasingly seek cost-effective solutions for managing chronic cardiovascular conditions, creating favorable reimbursement environments for novel therapies that can reduce hospitalization rates and improve long-term outcomes.
Pharmaceutical industry investment in phosphorylation-targeted cardiac therapies has grown substantially, with venture capital funding for startups in this space exceeding $3.2 billion in the past three years. This investment surge reflects confidence in both the scientific rationale and market potential of these approaches, particularly as diagnostic technologies for phosphorylation profiling become more sophisticated and accessible in clinical settings.
Current Challenges in Cardiovascular Phosphorylation Research
Despite significant advancements in cardiovascular phosphorylation research, several critical challenges continue to impede progress in this field. One major obstacle is the complexity of phosphorylation networks within cardiovascular tissues. These networks involve thousands of phosphorylation sites across numerous proteins, creating intricate signaling cascades that are difficult to map comprehensively. Current technologies often capture only snapshots of these dynamic processes, missing the temporal dynamics crucial for understanding cardiovascular pathophysiology.
Methodological limitations present another significant challenge. While mass spectrometry has revolutionized phosphoproteomic analysis, its application to cardiovascular tissues remains problematic due to the heterogeneity of cell types and the varying abundance of phosphoproteins. Additionally, the detection of low-abundance phosphorylation events, which may be physiologically significant, continues to be technically challenging, potentially missing critical regulatory mechanisms in cardiovascular function.
The translation gap between in vitro findings and in vivo relevance constitutes a persistent challenge. Many phosphorylation studies utilize cell culture models that inadequately represent the complex multicellular environment of the cardiovascular system. This discrepancy often leads to difficulties in extrapolating laboratory findings to clinical applications, hindering the development of phosphorylation-targeted therapeutics for cardiovascular diseases.
Data integration across different experimental platforms and between phosphoproteomic and other -omic datasets represents another formidable challenge. Researchers struggle to harmonize data from various sources, limiting the potential for systems-level understanding of cardiovascular phosphorylation networks. The lack of standardized bioinformatic approaches for analyzing these complex datasets further complicates meaningful interpretation.
Functional validation of phosphorylation events presents yet another hurdle. Determining whether specific phosphorylation events are causative or merely correlative in cardiovascular pathologies requires sophisticated genetic and pharmacological approaches that are not always readily available or applicable to human cardiovascular tissues. This challenge significantly slows the identification of therapeutic targets.
Finally, the field faces challenges in developing specific inhibitors or activators of phosphorylation pathways. The high degree of structural similarity among kinases and phosphatases makes it difficult to achieve specificity in targeting particular phosphorylation events without affecting others, increasing the risk of off-target effects in potential therapeutic applications for cardiovascular diseases.
Methodological limitations present another significant challenge. While mass spectrometry has revolutionized phosphoproteomic analysis, its application to cardiovascular tissues remains problematic due to the heterogeneity of cell types and the varying abundance of phosphoproteins. Additionally, the detection of low-abundance phosphorylation events, which may be physiologically significant, continues to be technically challenging, potentially missing critical regulatory mechanisms in cardiovascular function.
The translation gap between in vitro findings and in vivo relevance constitutes a persistent challenge. Many phosphorylation studies utilize cell culture models that inadequately represent the complex multicellular environment of the cardiovascular system. This discrepancy often leads to difficulties in extrapolating laboratory findings to clinical applications, hindering the development of phosphorylation-targeted therapeutics for cardiovascular diseases.
Data integration across different experimental platforms and between phosphoproteomic and other -omic datasets represents another formidable challenge. Researchers struggle to harmonize data from various sources, limiting the potential for systems-level understanding of cardiovascular phosphorylation networks. The lack of standardized bioinformatic approaches for analyzing these complex datasets further complicates meaningful interpretation.
Functional validation of phosphorylation events presents yet another hurdle. Determining whether specific phosphorylation events are causative or merely correlative in cardiovascular pathologies requires sophisticated genetic and pharmacological approaches that are not always readily available or applicable to human cardiovascular tissues. This challenge significantly slows the identification of therapeutic targets.
Finally, the field faces challenges in developing specific inhibitors or activators of phosphorylation pathways. The high degree of structural similarity among kinases and phosphatases makes it difficult to achieve specificity in targeting particular phosphorylation events without affecting others, increasing the risk of off-target effects in potential therapeutic applications for cardiovascular diseases.
Established Phosphorylation Pathway Comparison Methods
01 Detection methods for protein phosphorylation
Various techniques and assays have been developed to detect and analyze protein phosphorylation events in cellular pathways. These methods include immunoassays, mass spectrometry-based approaches, and fluorescence-based detection systems that can identify phosphorylated proteins and quantify phosphorylation levels. These detection methods are crucial for understanding signaling cascades and regulatory mechanisms in biological systems.- Detection methods for protein phosphorylation: Various techniques and assays have been developed to detect and analyze protein phosphorylation events in cellular pathways. These methods include immunoassays, mass spectrometry-based approaches, and fluorescence-based detection systems that can identify phosphorylated proteins and quantify phosphorylation levels. These detection methods are crucial for understanding signaling cascades and regulatory mechanisms in biological systems.
- Kinase inhibitors targeting phosphorylation pathways: Compounds designed to inhibit specific kinases involved in phosphorylation pathways have significant therapeutic potential. These inhibitors can modulate signal transduction by preventing phosphorylation events, thereby affecting cellular processes like proliferation, differentiation, and apoptosis. Research focuses on developing selective inhibitors that target disease-relevant phosphorylation pathways while minimizing off-target effects.
- Phosphorylation biomarkers for disease diagnosis: Specific phosphorylation patterns serve as biomarkers for various diseases, including cancer, neurodegenerative disorders, and inflammatory conditions. By analyzing the phosphorylation status of key proteins, diagnostic tools can be developed to identify disease states, monitor progression, and evaluate treatment efficacy. These biomarkers enable early detection and personalized therapeutic approaches based on individual phosphorylation profiles.
- High-throughput screening of phosphorylation pathways: High-throughput technologies enable systematic analysis of phosphorylation networks across multiple proteins simultaneously. These screening platforms utilize protein arrays, multiplexed assays, and computational tools to map complex phosphorylation cascades and identify novel pathway components. Such approaches accelerate the discovery of regulatory mechanisms and potential therapeutic targets within phosphorylation-dependent signaling networks.
- Engineered phosphorylation systems for therapeutic applications: Engineered proteins and synthetic biology approaches are being developed to manipulate phosphorylation pathways for therapeutic purposes. These include modified kinases with altered specificity, phosphorylation-responsive drug delivery systems, and engineered cellular circuits that respond to specific phosphorylation events. Such technologies enable precise control over signaling pathways and offer new strategies for treating diseases associated with dysregulated phosphorylation.
02 Kinase inhibitors targeting phosphorylation pathways
Compounds that inhibit protein kinases have been developed to modulate phosphorylation pathways involved in disease states. These kinase inhibitors can block specific phosphorylation events in signaling cascades, providing therapeutic benefits in conditions such as cancer, inflammation, and neurological disorders. The development of selective kinase inhibitors has become an important approach in drug discovery and personalized medicine.Expand Specific Solutions03 Phosphorylation biomarkers for disease diagnosis
Specific phosphorylation patterns and phosphorylated proteins serve as biomarkers for various diseases. These biomarkers can be detected in patient samples to diagnose conditions, monitor disease progression, and evaluate treatment efficacy. Phosphorylation-based biomarkers are particularly valuable in cancer diagnostics, neurodegenerative disorders, and metabolic diseases where abnormal signaling pathways play a critical role.Expand Specific Solutions04 Engineered phosphorylation systems for biotechnology applications
Engineered phosphorylation pathways and modified kinases have been developed for various biotechnology applications. These systems can be used for protein production, biosensing, synthetic biology, and drug screening platforms. By manipulating phosphorylation events, researchers can create novel cellular functions and control biological processes in engineered systems.Expand Specific Solutions05 Phosphoproteomics methods for pathway analysis
Phosphoproteomics approaches enable comprehensive analysis of phosphorylation networks within cells. These methods combine advanced separation techniques, mass spectrometry, and bioinformatics to identify thousands of phosphorylation sites and map complex signaling networks. Phosphoproteomics has revolutionized our understanding of cellular signaling by revealing the dynamics and interconnections of phosphorylation pathways under various conditions and treatments.Expand Specific Solutions
Leading Research Institutions and Pharmaceutical Companies
The cardiovascular phosphorylation research field is currently in a growth phase, with an estimated market size of $3-5 billion and expanding at 7-9% annually. The competitive landscape features academic institutions (Columbia University, Johns Hopkins, Harvard, MIT) leading fundamental research, while pharmaceutical companies (Novartis, Pfizer, Merck) focus on clinical applications. Technology maturity varies across pathways: PKA/PKC mechanisms are well-established, while novel kinase pathways remain exploratory. Companies like Illumina provide essential research tools, while Janssen, Keros Therapeutics, and Impulse Dynamics are developing therapeutic interventions targeting specific phosphorylation pathways for heart failure and cardiovascular diseases, representing the translational bridge between academic discoveries and clinical applications.
The Regents of the University of California
Technical Solution: The University of California system has developed a multi-faceted approach to studying cardiovascular phosphorylation pathways, focusing on the interplay between metabolic signaling and cardiac function. Their research extensively examines AMPK (AMP-activated protein kinase) and mTOR (mechanistic target of rapamycin) pathways in cardiomyocytes under various stress conditions. They've created innovative in vivo phosphorylation monitoring systems using genetically encoded biosensors that allow real-time visualization of kinase activity in intact cardiac tissue. This technology has revealed previously unknown temporal dynamics of phosphorylation events during ischemia-reperfusion injury. Additionally, they've mapped cross-talk between adrenergic signaling and insulin-responsive pathways in heart tissue, demonstrating how phosphorylation cascades integrate multiple physiological signals to regulate cardiac metabolism, contractility, and cell survival during cardiovascular stress.
Strengths: Real-time in vivo monitoring capabilities provide unprecedented temporal resolution of phosphorylation dynamics; extensive expertise in metabolic signaling pathways relevant to cardiac function. Weaknesses: Current biosensor technology limited to studying a small subset of phosphorylation events simultaneously; challenges in translating findings from animal models to human cardiovascular disease.
The Johns Hopkins University
Technical Solution: Johns Hopkins University has developed advanced phosphoproteomics platforms to study cardiovascular phosphorylation pathways. Their research focuses on protein kinase signaling networks in heart failure and cardiac hypertrophy, with particular emphasis on CaMKII (Ca2+/calmodulin-dependent protein kinase II) pathways. They've pioneered techniques to map over 40,000 phosphorylation sites in cardiac tissue samples, enabling comprehensive analysis of phosphorylation dynamics during cardiac stress. Their approach combines mass spectrometry-based phosphoproteomics with computational modeling to identify critical phosphorylation events that regulate cardiac contractility, calcium handling, and mitochondrial function. Recent work has revealed novel phosphorylation sites on ryanodine receptors and phospholamban that directly impact calcium cycling in cardiomyocytes, providing potential therapeutic targets for heart failure treatment.
Strengths: Comprehensive phosphoproteomic mapping capabilities with exceptional depth of coverage; integration of computational and experimental approaches enables systems-level understanding of phosphorylation networks. Weaknesses: Highly specialized techniques require significant expertise and expensive equipment; translation from discovery to clinical application remains challenging.
Key Signaling Cascades in Cardiovascular Phosphorylation
Methods of treating cardiac disorders and congestive heart failure and administering AAV vectors
PatentWO2022031914A2
Innovation
- The use of adeno-associated virus (AAV) vectors encoding phosphatase inhibitors, administered with cardiac-specific promoters, to target and reduce phosphatase activity in heart cells, improving cardiac contractility and [3-adrenergic responsiveness, combined with immunomodulators and vasodilators for enhanced efficacy.
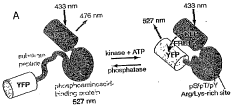
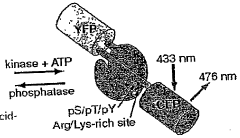
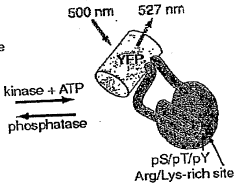
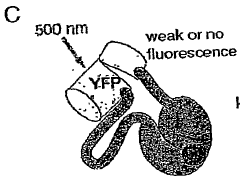
Emission ratiometric indicators of phosphorylation by c-kinase
PatentWO2005116267A2
Innovation
- A chimeric phosphorylation indicator comprising a first fluorescent protein, a phosphoaminoacid binding domain, and a protein kinase C (PKC) phosphorylatable domain, with a non-oligomerizing fluorescent protein, that exhibits detectable resonance energy transfer upon excitation, allowing for real-time monitoring of kinase or phosphatase activity.
Translational Research Opportunities in Cardiac Phosphorylation
Translational research in cardiac phosphorylation represents a critical bridge between basic scientific discoveries and clinical applications. The complex phosphorylation pathways that regulate cardiovascular function offer numerous opportunities for therapeutic intervention and diagnostic development. Recent advances in proteomics and systems biology have significantly expanded our understanding of phosphorylation networks in cardiac tissue, creating a fertile ground for translational initiatives.
Several promising research directions have emerged that warrant further investigation. Targeting specific kinases involved in pathological cardiac remodeling, such as PKA, CaMKII, and various MAPKs, shows potential for developing novel heart failure therapies. The phosphorylation status of cardiac troponin I and other sarcomeric proteins could serve as biomarkers for early detection of cardiac dysfunction before clinical symptoms manifest.
Personalized medicine approaches based on phosphorylation profiles represent another exciting frontier. Individual variations in phosphorylation pathways may explain differential responses to cardiovascular medications. Developing assays to characterize patient-specific phosphorylation patterns could enable tailored treatment strategies, potentially improving outcomes while reducing adverse effects.
Drug repurposing efforts focused on compounds that modulate phosphorylation cascades offer a cost-effective path to new cardiovascular therapies. Several kinase inhibitors currently approved for cancer treatment have demonstrated cardioprotective effects in preclinical models, suggesting potential dual applications that could accelerate clinical implementation.
Non-invasive imaging techniques that visualize phosphorylation activity in vivo are advancing rapidly. PET tracers targeting specific phosphorylation events could revolutionize cardiac diagnostics by providing functional information about signaling pathway activity rather than merely structural data. These technologies may enable earlier intervention and more precise monitoring of treatment efficacy.
Collaborative frameworks between basic scientists, clinicians, and industry partners are essential to overcome the translational challenges in this field. Standardized protocols for phosphoproteomic analysis of clinical samples would facilitate data sharing and accelerate discovery. Additionally, establishing biobanks with well-characterized cardiovascular specimens linked to clinical outcomes would provide valuable resources for validating phosphorylation-based biomarkers and therapeutic targets.
Several promising research directions have emerged that warrant further investigation. Targeting specific kinases involved in pathological cardiac remodeling, such as PKA, CaMKII, and various MAPKs, shows potential for developing novel heart failure therapies. The phosphorylation status of cardiac troponin I and other sarcomeric proteins could serve as biomarkers for early detection of cardiac dysfunction before clinical symptoms manifest.
Personalized medicine approaches based on phosphorylation profiles represent another exciting frontier. Individual variations in phosphorylation pathways may explain differential responses to cardiovascular medications. Developing assays to characterize patient-specific phosphorylation patterns could enable tailored treatment strategies, potentially improving outcomes while reducing adverse effects.
Drug repurposing efforts focused on compounds that modulate phosphorylation cascades offer a cost-effective path to new cardiovascular therapies. Several kinase inhibitors currently approved for cancer treatment have demonstrated cardioprotective effects in preclinical models, suggesting potential dual applications that could accelerate clinical implementation.
Non-invasive imaging techniques that visualize phosphorylation activity in vivo are advancing rapidly. PET tracers targeting specific phosphorylation events could revolutionize cardiac diagnostics by providing functional information about signaling pathway activity rather than merely structural data. These technologies may enable earlier intervention and more precise monitoring of treatment efficacy.
Collaborative frameworks between basic scientists, clinicians, and industry partners are essential to overcome the translational challenges in this field. Standardized protocols for phosphoproteomic analysis of clinical samples would facilitate data sharing and accelerate discovery. Additionally, establishing biobanks with well-characterized cardiovascular specimens linked to clinical outcomes would provide valuable resources for validating phosphorylation-based biomarkers and therapeutic targets.
Regulatory Considerations for Phosphorylation-Based Interventions
The regulatory landscape for phosphorylation-based interventions in cardiovascular health presents a complex framework that developers must navigate carefully. Current FDA and EMA guidelines require extensive preclinical validation of phosphorylation pathway specificity before advancing to clinical trials. This includes demonstration of target engagement, off-target effects assessment, and dose-response relationships specific to cardiovascular tissues.
Phosphorylation-targeted therapies face unique regulatory challenges due to their involvement in multiple cellular processes. Regulatory bodies typically require comprehensive phosphoproteomic profiling to demonstrate pathway specificity and minimize unintended consequences. For instance, kinase inhibitors targeting cardiac hypertrophy pathways must demonstrate minimal impact on physiological phosphorylation events in other tissues.
Safety monitoring requirements for phosphorylation-based interventions are particularly stringent, with regulatory agencies mandating long-term follow-up studies to detect delayed cardiovascular effects. The FDA's Cardiovascular and Renal Drugs Advisory Committee has established specific biomarker validation protocols for phosphorylation-based endpoints in clinical trials, requiring correlation with meaningful clinical outcomes.
International harmonization efforts through the International Council for Harmonisation (ICH) have recently addressed phosphorylation-based biomarkers in cardiovascular drug development. The ICH E14/S7B Implementation Working Group has published recommendations specifically addressing how phosphorylation status changes should be interpreted in cardiac safety assessments, providing a standardized framework across major markets.
Accelerated approval pathways may be available for phosphorylation-targeted therapies addressing unmet needs in cardiovascular disease. However, these require robust surrogate endpoints that demonstrate the intervention's effect on clinically relevant phosphorylation cascades. The FDA's Breakthrough Therapy designation has been granted to several phosphorylation modulators in recent years, particularly those targeting previously undruggable nodes in heart failure pathways.
Post-market surveillance requirements for phosphorylation-based interventions typically include monitoring for unexpected pathway cross-talk effects that may not have been evident during clinical development. Regulatory agencies increasingly require Risk Evaluation and Mitigation Strategies (REMS) for novel phosphorylation modulators, particularly those affecting fundamental cardiac signaling pathways like calcium handling or adrenergic signaling.
As the field advances, regulatory frameworks are evolving to accommodate innovative approaches such as combination therapies targeting multiple phosphorylation nodes simultaneously. Recent regulatory guidance documents suggest a growing recognition of systems biology approaches to phosphorylation network modulation, potentially streamlining approval pathways for rationally designed multi-target interventions in cardiovascular disease.
Phosphorylation-targeted therapies face unique regulatory challenges due to their involvement in multiple cellular processes. Regulatory bodies typically require comprehensive phosphoproteomic profiling to demonstrate pathway specificity and minimize unintended consequences. For instance, kinase inhibitors targeting cardiac hypertrophy pathways must demonstrate minimal impact on physiological phosphorylation events in other tissues.
Safety monitoring requirements for phosphorylation-based interventions are particularly stringent, with regulatory agencies mandating long-term follow-up studies to detect delayed cardiovascular effects. The FDA's Cardiovascular and Renal Drugs Advisory Committee has established specific biomarker validation protocols for phosphorylation-based endpoints in clinical trials, requiring correlation with meaningful clinical outcomes.
International harmonization efforts through the International Council for Harmonisation (ICH) have recently addressed phosphorylation-based biomarkers in cardiovascular drug development. The ICH E14/S7B Implementation Working Group has published recommendations specifically addressing how phosphorylation status changes should be interpreted in cardiac safety assessments, providing a standardized framework across major markets.
Accelerated approval pathways may be available for phosphorylation-targeted therapies addressing unmet needs in cardiovascular disease. However, these require robust surrogate endpoints that demonstrate the intervention's effect on clinically relevant phosphorylation cascades. The FDA's Breakthrough Therapy designation has been granted to several phosphorylation modulators in recent years, particularly those targeting previously undruggable nodes in heart failure pathways.
Post-market surveillance requirements for phosphorylation-based interventions typically include monitoring for unexpected pathway cross-talk effects that may not have been evident during clinical development. Regulatory agencies increasingly require Risk Evaluation and Mitigation Strategies (REMS) for novel phosphorylation modulators, particularly those affecting fundamental cardiac signaling pathways like calcium handling or adrenergic signaling.
As the field advances, regulatory frameworks are evolving to accommodate innovative approaches such as combination therapies targeting multiple phosphorylation nodes simultaneously. Recent regulatory guidance documents suggest a growing recognition of systems biology approaches to phosphorylation network modulation, potentially streamlining approval pathways for rationally designed multi-target interventions in cardiovascular disease.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!