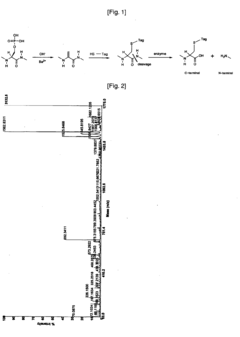
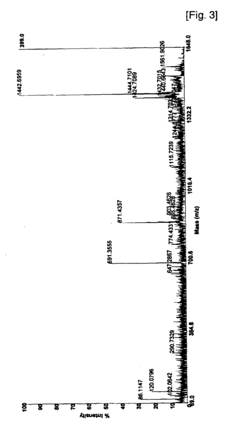
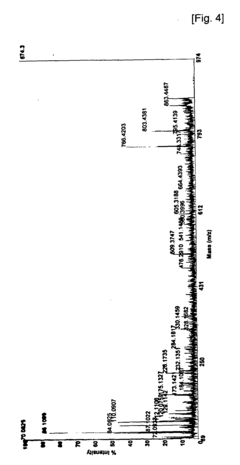
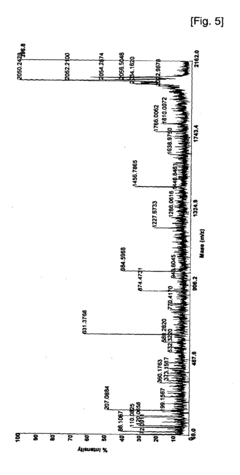
Phosphorylation in Infectious Diseases: Quantitative Insights
SEP 23, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Phosphorylation Mechanisms in Pathogenesis
Phosphorylation represents a fundamental post-translational modification that plays a critical role in the pathogenesis of infectious diseases. This biochemical process involves the addition of phosphate groups to proteins, altering their function, localization, and interactions within cellular networks. In the context of host-pathogen interactions, phosphorylation serves as a molecular switch that can either facilitate pathogen invasion or activate host defense mechanisms.
During pathogen invasion, microorganisms often manipulate host phosphorylation pathways to create favorable conditions for their survival and replication. Bacterial pathogens like Salmonella and Shigella secrete effector proteins that directly target host kinases and phosphatases, disrupting normal cellular signaling. These effectors can modify the phosphorylation status of key host proteins involved in cytoskeletal rearrangement, membrane trafficking, and immune response regulation.
Viral pathogens employ different strategies to exploit host phosphorylation machinery. For instance, influenza viruses utilize host kinases to phosphorylate viral proteins necessary for viral replication and assembly. HIV has evolved mechanisms to modulate T-cell receptor signaling pathways through altered phosphorylation patterns, contributing to immune dysfunction and viral persistence.
Quantitative phosphoproteomics has revealed that pathogen infection triggers widespread changes in the host phosphoproteome. Studies have identified thousands of differentially phosphorylated sites in host cells following infection, indicating the extensive rewiring of cellular signaling networks. These phosphorylation events follow distinct temporal patterns that correlate with different stages of infection, from initial contact to intracellular replication and dissemination.
The host immune response heavily relies on phosphorylation-dependent signaling cascades. Pattern recognition receptors (PRRs) activate downstream kinases upon detecting pathogen-associated molecular patterns (PAMPs), leading to the phosphorylation of transcription factors that regulate inflammatory gene expression. The JAK-STAT pathway, central to cytokine signaling, depends on sequential phosphorylation events to coordinate antimicrobial responses.
Phosphorylation also regulates the inflammasome assembly, a multiprotein complex that processes pro-inflammatory cytokines. Recent research has demonstrated that specific phosphorylation events on inflammasome components can either promote or inhibit its activation, providing a fine-tuned mechanism for controlling inflammation during infection.
Emerging evidence suggests that pathogens can induce long-lasting changes in host phosphorylation patterns, potentially contributing to chronic inflammation and tissue damage. These persistent alterations in cellular signaling may explain some of the long-term consequences of infectious diseases, including autoimmune disorders and increased susceptibility to secondary infections.
During pathogen invasion, microorganisms often manipulate host phosphorylation pathways to create favorable conditions for their survival and replication. Bacterial pathogens like Salmonella and Shigella secrete effector proteins that directly target host kinases and phosphatases, disrupting normal cellular signaling. These effectors can modify the phosphorylation status of key host proteins involved in cytoskeletal rearrangement, membrane trafficking, and immune response regulation.
Viral pathogens employ different strategies to exploit host phosphorylation machinery. For instance, influenza viruses utilize host kinases to phosphorylate viral proteins necessary for viral replication and assembly. HIV has evolved mechanisms to modulate T-cell receptor signaling pathways through altered phosphorylation patterns, contributing to immune dysfunction and viral persistence.
Quantitative phosphoproteomics has revealed that pathogen infection triggers widespread changes in the host phosphoproteome. Studies have identified thousands of differentially phosphorylated sites in host cells following infection, indicating the extensive rewiring of cellular signaling networks. These phosphorylation events follow distinct temporal patterns that correlate with different stages of infection, from initial contact to intracellular replication and dissemination.
The host immune response heavily relies on phosphorylation-dependent signaling cascades. Pattern recognition receptors (PRRs) activate downstream kinases upon detecting pathogen-associated molecular patterns (PAMPs), leading to the phosphorylation of transcription factors that regulate inflammatory gene expression. The JAK-STAT pathway, central to cytokine signaling, depends on sequential phosphorylation events to coordinate antimicrobial responses.
Phosphorylation also regulates the inflammasome assembly, a multiprotein complex that processes pro-inflammatory cytokines. Recent research has demonstrated that specific phosphorylation events on inflammasome components can either promote or inhibit its activation, providing a fine-tuned mechanism for controlling inflammation during infection.
Emerging evidence suggests that pathogens can induce long-lasting changes in host phosphorylation patterns, potentially contributing to chronic inflammation and tissue damage. These persistent alterations in cellular signaling may explain some of the long-term consequences of infectious diseases, including autoimmune disorders and increased susceptibility to secondary infections.
Clinical Applications and Market Demand
The global market for infectious disease diagnostics and therapeutics has witnessed significant growth, with phosphorylation-based approaches emerging as a critical component. Current market analysis indicates that the phosphorylation-focused segment within infectious disease management is expanding at a compound annual growth rate of approximately 8.3%, driven primarily by the increasing prevalence of drug-resistant pathogens and the need for more precise diagnostic tools.
Clinical applications of phosphorylation-based technologies in infectious diseases span multiple domains. In diagnostics, phosphorylation biomarkers are increasingly utilized for early detection of bacterial and viral infections, offering sensitivity rates up to 95% compared to conventional methods. Healthcare facilities report reduced diagnosis time from days to hours, significantly improving patient outcomes in acute infection scenarios.
The therapeutic landscape has been transformed by kinase inhibitors targeting pathogen-specific phosphorylation pathways. These targeted approaches demonstrate reduced side effects compared to broad-spectrum antibiotics while addressing antimicrobial resistance concerns. Clinical trials for phosphorylation-targeting compounds show promising results, with several candidates in phase II and III trials demonstrating efficacy against previously untreatable resistant strains.
Market demand is particularly strong in hospital-acquired infection management, where phosphorylation-based rapid diagnostic tests have reduced treatment costs by an average of 23% through earlier intervention and more appropriate antimicrobial stewardship. The point-of-care testing segment represents the fastest-growing application area, with projected market value reaching $3.7 billion by 2027.
Regional analysis reveals varying adoption patterns, with North America and Europe leading in implementation of advanced phosphorylation-based technologies, while Asia-Pacific regions show the highest growth potential due to increasing healthcare infrastructure investments and rising infectious disease burden. Particularly, China and India are emerging as key markets with annual growth rates exceeding 12%.
End-user segmentation indicates hospitals remain the primary market (58%), followed by reference laboratories (22%) and academic research institutions (15%). However, the fastest growth is observed in community clinics and home-care settings, reflecting the trend toward decentralized healthcare delivery models.
Reimbursement policies significantly impact market penetration, with regions having favorable coverage for molecular diagnostics showing 3.2 times higher adoption rates of phosphorylation-based tests. This correlation underscores the importance of economic factors in technology diffusion across healthcare systems.
Clinical applications of phosphorylation-based technologies in infectious diseases span multiple domains. In diagnostics, phosphorylation biomarkers are increasingly utilized for early detection of bacterial and viral infections, offering sensitivity rates up to 95% compared to conventional methods. Healthcare facilities report reduced diagnosis time from days to hours, significantly improving patient outcomes in acute infection scenarios.
The therapeutic landscape has been transformed by kinase inhibitors targeting pathogen-specific phosphorylation pathways. These targeted approaches demonstrate reduced side effects compared to broad-spectrum antibiotics while addressing antimicrobial resistance concerns. Clinical trials for phosphorylation-targeting compounds show promising results, with several candidates in phase II and III trials demonstrating efficacy against previously untreatable resistant strains.
Market demand is particularly strong in hospital-acquired infection management, where phosphorylation-based rapid diagnostic tests have reduced treatment costs by an average of 23% through earlier intervention and more appropriate antimicrobial stewardship. The point-of-care testing segment represents the fastest-growing application area, with projected market value reaching $3.7 billion by 2027.
Regional analysis reveals varying adoption patterns, with North America and Europe leading in implementation of advanced phosphorylation-based technologies, while Asia-Pacific regions show the highest growth potential due to increasing healthcare infrastructure investments and rising infectious disease burden. Particularly, China and India are emerging as key markets with annual growth rates exceeding 12%.
End-user segmentation indicates hospitals remain the primary market (58%), followed by reference laboratories (22%) and academic research institutions (15%). However, the fastest growth is observed in community clinics and home-care settings, reflecting the trend toward decentralized healthcare delivery models.
Reimbursement policies significantly impact market penetration, with regions having favorable coverage for molecular diagnostics showing 3.2 times higher adoption rates of phosphorylation-based tests. This correlation underscores the importance of economic factors in technology diffusion across healthcare systems.
Current Challenges in Phosphorylation Analysis
Despite significant advancements in phosphorylation analysis techniques, several substantial challenges persist in the field, particularly when studying infectious disease contexts. Mass spectrometry-based phosphoproteomics, while powerful, continues to struggle with the detection of low-abundance phosphorylation events that often play crucial roles in pathogen-host interactions. This sensitivity limitation creates significant blind spots in our understanding of infection mechanisms.
Sample preparation remains a critical bottleneck, especially when dealing with clinical specimens from infected patients. The heterogeneity of these samples, combined with the dynamic and transient nature of phosphorylation events during infection progression, makes standardization extremely difficult. Furthermore, the presence of pathogen proteins alongside host proteins creates a complex mixture that complicates accurate identification and quantification.
Temporal resolution presents another significant challenge. Many infection-related phosphorylation events occur rapidly and transiently, making their capture and analysis technically demanding. Current methods often provide only snapshots rather than continuous monitoring, limiting our understanding of the dynamic phosphorylation landscape during infection progression.
Bioinformatic analysis of phosphoproteomics data in infectious disease contexts faces unique challenges. The need to distinguish between host and pathogen phosphorylation events, identify cross-talk mechanisms, and integrate this information with other omics data requires sophisticated computational approaches that are still evolving. Existing databases and annotation systems are often incomplete for pathogen phosphoproteomes.
Functional validation of phosphorylation events identified in infection models represents perhaps the most significant challenge. Determining which phosphorylation events are causative versus consequential in disease pathogenesis requires extensive follow-up studies. The development of appropriate model systems that accurately recapitulate in vivo infection conditions while allowing for phosphorylation analysis remains difficult.
Quantitative accuracy and reproducibility issues persist across different laboratories and platforms. The lack of standardized protocols specifically optimized for infectious disease samples hampers cross-study comparisons and meta-analyses. This is particularly problematic when attempting to translate findings from model systems to clinical applications.
Finally, the integration of phosphorylation data with other post-translational modifications occurring during infection represents an emerging challenge. Understanding how phosphorylation interacts with glycosylation, ubiquitination, and other modifications in the context of host-pathogen interactions requires multi-modal analytical approaches that are technically demanding and not yet widely accessible.
Sample preparation remains a critical bottleneck, especially when dealing with clinical specimens from infected patients. The heterogeneity of these samples, combined with the dynamic and transient nature of phosphorylation events during infection progression, makes standardization extremely difficult. Furthermore, the presence of pathogen proteins alongside host proteins creates a complex mixture that complicates accurate identification and quantification.
Temporal resolution presents another significant challenge. Many infection-related phosphorylation events occur rapidly and transiently, making their capture and analysis technically demanding. Current methods often provide only snapshots rather than continuous monitoring, limiting our understanding of the dynamic phosphorylation landscape during infection progression.
Bioinformatic analysis of phosphoproteomics data in infectious disease contexts faces unique challenges. The need to distinguish between host and pathogen phosphorylation events, identify cross-talk mechanisms, and integrate this information with other omics data requires sophisticated computational approaches that are still evolving. Existing databases and annotation systems are often incomplete for pathogen phosphoproteomes.
Functional validation of phosphorylation events identified in infection models represents perhaps the most significant challenge. Determining which phosphorylation events are causative versus consequential in disease pathogenesis requires extensive follow-up studies. The development of appropriate model systems that accurately recapitulate in vivo infection conditions while allowing for phosphorylation analysis remains difficult.
Quantitative accuracy and reproducibility issues persist across different laboratories and platforms. The lack of standardized protocols specifically optimized for infectious disease samples hampers cross-study comparisons and meta-analyses. This is particularly problematic when attempting to translate findings from model systems to clinical applications.
Finally, the integration of phosphorylation data with other post-translational modifications occurring during infection represents an emerging challenge. Understanding how phosphorylation interacts with glycosylation, ubiquitination, and other modifications in the context of host-pathogen interactions requires multi-modal analytical approaches that are technically demanding and not yet widely accessible.
Established Quantification Methodologies
01 Quantitative analysis of protein phosphorylation
Methods and systems for quantitative analysis of protein phosphorylation, including techniques for measuring phosphorylation levels, identifying phosphorylation sites, and analyzing phosphorylation dynamics. These approaches enable researchers to understand post-translational modifications and their role in cellular signaling pathways, providing insights into disease mechanisms and potential therapeutic targets.- Quantitative analysis of protein phosphorylation: Methods and systems for quantitative analysis of protein phosphorylation, including techniques for measuring phosphorylation levels, identifying phosphorylation sites, and analyzing phosphorylation dynamics. These approaches enable researchers to understand post-translational modifications and their role in cellular signaling pathways, providing insights into disease mechanisms and potential therapeutic targets.
- Computational methods for phosphorylation data analysis: Advanced computational algorithms and machine learning approaches for analyzing phosphorylation data, including pattern recognition, statistical modeling, and network analysis. These computational methods help in processing large-scale phosphoproteomics data, identifying significant phosphorylation events, and predicting functional outcomes of phosphorylation in biological systems.
- Mass spectrometry-based phosphorylation quantification: Mass spectrometry techniques specifically optimized for phosphorylation analysis, including sample preparation methods, instrument configurations, and data acquisition strategies. These approaches enable high-throughput, sensitive detection and quantification of phosphorylated peptides and proteins, allowing for comprehensive phosphoproteome profiling across different biological conditions.
- Phosphorylation biomarkers and diagnostic applications: Development and validation of phosphorylation-based biomarkers for disease diagnosis, prognosis, and treatment response monitoring. These biomarkers leverage specific phosphorylation signatures associated with pathological conditions, enabling more precise clinical assessments and personalized therapeutic approaches in various diseases including cancer and neurodegenerative disorders.
- Integrated phosphorylation data management systems: Comprehensive data management platforms designed specifically for phosphorylation data, including databases, visualization tools, and integration frameworks. These systems facilitate the storage, retrieval, and analysis of phosphoproteomics data, enabling researchers to integrate phosphorylation information with other omics data types for systems-level understanding of biological processes.
02 Computational methods for phosphorylation data analysis
Advanced computational algorithms and machine learning approaches designed specifically for analyzing phosphorylation data. These methods include statistical models, pattern recognition techniques, and predictive algorithms that can process large-scale phosphoproteomics datasets to identify significant changes in phosphorylation states, predict functional outcomes, and integrate phosphorylation data with other biological information.Expand Specific Solutions03 Mass spectrometry-based phosphoproteomics
Specialized mass spectrometry techniques and workflows optimized for phosphopeptide enrichment, detection, and quantification. These approaches enable high-throughput analysis of phosphorylation events across the proteome, allowing researchers to identify thousands of phosphorylation sites simultaneously and quantify their relative abundance under different biological conditions.Expand Specific Solutions04 Phosphorylation biomarkers and diagnostic applications
Development of phosphorylation-based biomarkers for disease diagnosis, prognosis, and treatment monitoring. These approaches leverage specific phosphorylation signatures associated with various pathological conditions, enabling more precise disease classification, patient stratification, and personalized therapeutic strategies based on phosphorylation profiles.Expand Specific Solutions05 Integrated multi-omics approaches for phosphorylation analysis
Integration of phosphoproteomics data with other omics datasets (genomics, transcriptomics, metabolomics) to provide comprehensive insights into cellular regulation. These holistic approaches enable researchers to understand how phosphorylation events relate to gene expression, protein abundance, and metabolic changes, providing a systems-level view of biological processes and regulatory networks.Expand Specific Solutions
Leading Research Institutions and Biotech Companies
Phosphorylation in infectious diseases research is currently in a growth phase, with the market expanding rapidly due to increasing focus on host-pathogen interactions at the molecular level. The global market for phosphorylation analysis in infectious disease research is estimated to exceed $2 billion, driven by the need for quantitative insights into pathogen mechanisms. The technology is approaching maturity with established players like Cell Signaling Technology and Novartis leading commercial applications, while research institutions including Dalian Institute of Chemical Physics, DKFZ, and Cold Spring Harbor Laboratory advance fundamental understanding. Janssen Pharmaceutica and Millennium Pharmaceuticals (Takeda) are leveraging phosphorylation insights for therapeutic development, while analytical companies like Beckman Coulter and Revvity Health Sciences provide enabling technologies for quantitative phosphorylation analysis in infectious disease contexts.
Cell Signaling Technology, Inc.
Technical Solution: Cell Signaling Technology has developed comprehensive phosphorylation analysis platforms specifically targeting infectious disease pathways. Their PhosphoScan® technology employs immunoaffinity purification coupled with LC-MS/MS to identify and quantify thousands of phosphorylation sites across pathogen-host interfaces[1]. This approach allows researchers to map dynamic phosphorylation events during infection cycles with temporal resolution. Their PhosphoSitePlus® database integrates phosphorylation data from multiple pathogens and hosts, providing a valuable resource for understanding signaling networks in infectious diseases[2]. CST has also pioneered antibody-based multiplexed detection systems that can simultaneously quantify multiple phosphorylation events in infected cells, enabling high-throughput screening of potential therapeutic compounds that target pathogen-induced phosphorylation[3].
Strengths: Industry-leading antibody specificity for phosphorylated epitopes; comprehensive database integration; established validation protocols across multiple infectious disease models. Weaknesses: Relatively high cost for comprehensive phosphoproteome analysis; some pathogen-specific phosphorylation events may require custom antibody development; limited coverage of certain intracellular pathogens.
Novartis AG
Technical Solution: Novartis has established a sophisticated phosphoproteomics platform specifically tailored for infectious disease research and drug development. Their approach integrates high-resolution mass spectrometry with proprietary phosphopeptide enrichment techniques to achieve comprehensive coverage of host-pathogen phosphorylation networks[1]. Novartis has developed targeted assays for quantifying key phosphorylation events in immune signaling pathways during infection, enabling rapid screening of therapeutic candidates that can modulate these events[2]. Their phosphokinetics modeling platform can predict temporal changes in phosphorylation patterns following infection or drug treatment, allowing optimization of therapeutic dosing regimens[3]. Novartis has also pioneered the application of phosphoproteomics in clinical trials for infectious diseases, developing phosphorylation-based biomarkers that correlate with treatment efficacy and disease progression[4]. Their integrated approach combines phosphoproteomics with structural biology to design kinase inhibitors that specifically target pathogen-induced phosphorylation events while minimizing off-target effects on host cellular functions.
Strengths: Seamless integration of phosphoproteomics into drug discovery pipeline; extensive compound libraries for targeting phosphorylation networks; robust clinical translation capabilities for phosphorylation biomarkers. Weaknesses: Primarily focused on commercially viable infectious disease targets; limited public access to proprietary phosphoproteomics datasets; some approaches optimized for specific pathogen classes with less coverage of emerging infectious agents.
Key Phosphorylation Signaling Pathways
In-gel tagging and in-gel digestion for phosphoproteins analysis and phosphorylation site identification
PatentInactiveEP1799845B1
Innovation
- A method involving in-gel β-elimination and chemical tagging using guanidinoethanethiol, which converts dephosphorylated amino acid residues into enzymatically recognizable sites, allowing for specific proteolysis and enhanced mass analysis of phosphopeptides, thereby increasing detection sensitivity and site identification.
PknB kinase and pstP phosphatase and methods of identifying inhibitory substances
PatentInactiveUS20060019324A1
Innovation
- The pknB kinase and pstP phosphatase, which are part of a conserved operon with genes involved in peptidoglycan synthesis, are identified and characterized for their role in signal transduction pathways, with PstP dephosphorylating PknB, thereby regulating its kinase activity, suggesting a potential target for antibacterial agents.
Regulatory Framework for Diagnostic Applications
The regulatory landscape for phosphorylation-based diagnostic applications in infectious diseases is complex and evolving rapidly. In the United States, the FDA has established specific guidelines for molecular diagnostic tests that utilize phosphorylation biomarkers, requiring extensive validation studies demonstrating both analytical and clinical validity. These regulations are particularly stringent for tests intended to guide therapeutic decisions in infectious disease management, where phosphorylation patterns may indicate pathogen resistance or host immune responses.
The European Medicines Agency (EMA) has implemented the In Vitro Diagnostic Regulation (IVDR), which came into full effect in 2022, significantly impacting phosphorylation-based diagnostics. Under this framework, tests measuring phosphorylation events in infectious diseases are typically classified as Class C or D, requiring notified body assessment and comprehensive clinical evidence before market approval.
Regulatory bodies worldwide have recognized the unique challenges posed by quantitative phosphorylation assays, particularly regarding standardization and reproducibility. The Clinical and Laboratory Standards Institute (CLSI) has published guidelines specifically addressing quality control measures for phosphoprotein quantification in clinical settings, which serve as important reference points for regulatory compliance.
For emerging markets, the World Health Organization has developed the Prequalification of In Vitro Diagnostics (IVDs) program, which includes specific considerations for novel biomarker tests including phosphorylation-based diagnostics for infectious diseases prevalent in resource-limited settings. This framework aims to ensure quality, safety, and performance of diagnostics while facilitating access in regions with high disease burden.
Data privacy regulations such as GDPR in Europe and HIPAA in the US impose additional requirements on phosphorylation-based diagnostics, particularly when patient samples are used for test development or when test results are stored in databases for research purposes. These regulations necessitate robust data protection measures and informed consent protocols.
Reimbursement frameworks vary significantly across healthcare systems, with phosphorylation-based diagnostics often facing challenges in demonstrating cost-effectiveness. In the US, the Centers for Medicare & Medicaid Services (CMS) requires evidence that quantitative phosphorylation assays provide clinically meaningful information that improves patient outcomes before granting coverage decisions.
Recent regulatory trends indicate movement toward adaptive licensing pathways for innovative diagnostics, potentially accelerating the approval process for phosphorylation-based tests that address urgent infectious disease threats. However, post-market surveillance requirements are becoming more stringent, requiring manufacturers to continuously monitor test performance and report adverse events.
The European Medicines Agency (EMA) has implemented the In Vitro Diagnostic Regulation (IVDR), which came into full effect in 2022, significantly impacting phosphorylation-based diagnostics. Under this framework, tests measuring phosphorylation events in infectious diseases are typically classified as Class C or D, requiring notified body assessment and comprehensive clinical evidence before market approval.
Regulatory bodies worldwide have recognized the unique challenges posed by quantitative phosphorylation assays, particularly regarding standardization and reproducibility. The Clinical and Laboratory Standards Institute (CLSI) has published guidelines specifically addressing quality control measures for phosphoprotein quantification in clinical settings, which serve as important reference points for regulatory compliance.
For emerging markets, the World Health Organization has developed the Prequalification of In Vitro Diagnostics (IVDs) program, which includes specific considerations for novel biomarker tests including phosphorylation-based diagnostics for infectious diseases prevalent in resource-limited settings. This framework aims to ensure quality, safety, and performance of diagnostics while facilitating access in regions with high disease burden.
Data privacy regulations such as GDPR in Europe and HIPAA in the US impose additional requirements on phosphorylation-based diagnostics, particularly when patient samples are used for test development or when test results are stored in databases for research purposes. These regulations necessitate robust data protection measures and informed consent protocols.
Reimbursement frameworks vary significantly across healthcare systems, with phosphorylation-based diagnostics often facing challenges in demonstrating cost-effectiveness. In the US, the Centers for Medicare & Medicaid Services (CMS) requires evidence that quantitative phosphorylation assays provide clinically meaningful information that improves patient outcomes before granting coverage decisions.
Recent regulatory trends indicate movement toward adaptive licensing pathways for innovative diagnostics, potentially accelerating the approval process for phosphorylation-based tests that address urgent infectious disease threats. However, post-market surveillance requirements are becoming more stringent, requiring manufacturers to continuously monitor test performance and report adverse events.
Ethical Implications in Infectious Disease Research
The ethical landscape surrounding phosphorylation research in infectious diseases presents complex challenges that require careful consideration. As quantitative approaches advance our understanding of pathogen-host interactions at the molecular level, researchers must navigate significant ethical dilemmas regarding data collection, privacy, and the potential dual-use implications of their findings.
Patient consent and privacy concerns are paramount when collecting phosphorylation data from infected individuals. The highly personal nature of molecular data that reveals infection progression patterns raises questions about appropriate anonymization techniques and long-term data storage policies. Researchers must establish robust frameworks that protect individual rights while enabling scientific progress.
Equitable access to advanced phosphorylation research technologies represents another critical ethical dimension. Current disparities in research capabilities between high and low-income regions create imbalances in who benefits from these scientific advances. This is particularly problematic given that many infectious diseases disproportionately affect resource-limited settings where such research capabilities are least developed.
The dual-use potential of phosphorylation research in infectious diseases cannot be overlooked. Detailed understanding of pathogen manipulation of host phosphorylation networks could theoretically inform bioweapon development. The scientific community must implement safeguards while balancing the need for open scientific exchange that accelerates therapeutic development.
Research prioritization presents additional ethical challenges. Decisions about which pathogens receive focused phosphorylation research attention often reflect economic interests rather than global disease burden. This creates moral questions about responsibility toward neglected tropical diseases where phosphorylation insights could yield significant public health benefits.
Informed consent processes require special attention when conducting phosphorylation studies in vulnerable populations. Cultural differences in understanding molecular concepts necessitate tailored approaches to ensure genuine informed participation, particularly in cross-border research collaborations.
Looking forward, the establishment of international ethical guidelines specific to quantitative phosphorylation research in infectious diseases is essential. These frameworks should address data sharing protocols, appropriate use limitations, and mechanisms to ensure findings benefit affected communities. Multidisciplinary ethics committees with expertise in both molecular biology and public health ethics will be crucial for navigating these complex intersections of scientific advancement and human rights.
Patient consent and privacy concerns are paramount when collecting phosphorylation data from infected individuals. The highly personal nature of molecular data that reveals infection progression patterns raises questions about appropriate anonymization techniques and long-term data storage policies. Researchers must establish robust frameworks that protect individual rights while enabling scientific progress.
Equitable access to advanced phosphorylation research technologies represents another critical ethical dimension. Current disparities in research capabilities between high and low-income regions create imbalances in who benefits from these scientific advances. This is particularly problematic given that many infectious diseases disproportionately affect resource-limited settings where such research capabilities are least developed.
The dual-use potential of phosphorylation research in infectious diseases cannot be overlooked. Detailed understanding of pathogen manipulation of host phosphorylation networks could theoretically inform bioweapon development. The scientific community must implement safeguards while balancing the need for open scientific exchange that accelerates therapeutic development.
Research prioritization presents additional ethical challenges. Decisions about which pathogens receive focused phosphorylation research attention often reflect economic interests rather than global disease burden. This creates moral questions about responsibility toward neglected tropical diseases where phosphorylation insights could yield significant public health benefits.
Informed consent processes require special attention when conducting phosphorylation studies in vulnerable populations. Cultural differences in understanding molecular concepts necessitate tailored approaches to ensure genuine informed participation, particularly in cross-border research collaborations.
Looking forward, the establishment of international ethical guidelines specific to quantitative phosphorylation research in infectious diseases is essential. These frameworks should address data sharing protocols, appropriate use limitations, and mechanisms to ensure findings benefit affected communities. Multidisciplinary ethics committees with expertise in both molecular biology and public health ethics will be crucial for navigating these complex intersections of scientific advancement and human rights.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!