Measure Phosphorylation-Induced Protein Synthesis Boosts
SEP 23, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Phosphorylation-Induced Protein Synthesis Background & Objectives
Protein phosphorylation represents one of the most critical post-translational modifications in cellular biology, serving as a fundamental mechanism for signal transduction and regulation of protein function. The study of phosphorylation-induced protein synthesis boosts has emerged as a pivotal area of research over the past three decades, evolving from basic biochemical observations to sophisticated molecular understanding with significant implications for therapeutic development.
The historical trajectory of this field began in the 1980s with the discovery of key protein kinases and their roles in cellular signaling cascades. By the 1990s, researchers had established clear connections between phosphorylation events and the activation of translation machinery, particularly through the mTOR (mechanistic target of rapamycin) pathway. The early 2000s witnessed significant breakthroughs in understanding how phosphorylation of translation initiation factors, particularly eIF2α and 4E-BP1, directly modulates protein synthesis rates.
Recent technological advances have dramatically enhanced our ability to measure these phosphorylation-induced changes in protein synthesis. Mass spectrometry-based phosphoproteomics, combined with ribosome profiling and advanced imaging techniques, now allow for unprecedented temporal and spatial resolution in monitoring translation dynamics following phosphorylation events.
The current technological landscape presents both opportunities and challenges. While we can detect thousands of phosphorylation sites simultaneously, accurately quantifying their specific impacts on protein synthesis rates remains technically demanding. Traditional methods like radioactive amino acid incorporation provide overall synthesis rates but lack specificity for individual proteins and temporal resolution.
The primary objectives of research in this field now center on developing more precise, high-throughput methodologies to measure protein synthesis boosts following specific phosphorylation events. These objectives include creating real-time monitoring systems for translation initiation following kinase activation, establishing quantitative relationships between phosphorylation stoichiometry and resulting protein synthesis rates, and developing computational models that can predict translation outcomes based on phosphorylation patterns.
Additionally, there is growing interest in understanding how phosphorylation-induced protein synthesis contributes to disease states, particularly in cancer, neurodegeneration, and metabolic disorders. This has led to the parallel objective of identifying potential therapeutic targets within these phosphorylation-dependent translation pathways.
The field is now moving toward integrating multi-omics approaches, combining phosphoproteomics, translatome analysis, and functional genomics to create comprehensive models of how phosphorylation cascades regulate the cellular proteome. This integration represents the next frontier in understanding this fundamental biological process and harnessing it for medical applications.
The historical trajectory of this field began in the 1980s with the discovery of key protein kinases and their roles in cellular signaling cascades. By the 1990s, researchers had established clear connections between phosphorylation events and the activation of translation machinery, particularly through the mTOR (mechanistic target of rapamycin) pathway. The early 2000s witnessed significant breakthroughs in understanding how phosphorylation of translation initiation factors, particularly eIF2α and 4E-BP1, directly modulates protein synthesis rates.
Recent technological advances have dramatically enhanced our ability to measure these phosphorylation-induced changes in protein synthesis. Mass spectrometry-based phosphoproteomics, combined with ribosome profiling and advanced imaging techniques, now allow for unprecedented temporal and spatial resolution in monitoring translation dynamics following phosphorylation events.
The current technological landscape presents both opportunities and challenges. While we can detect thousands of phosphorylation sites simultaneously, accurately quantifying their specific impacts on protein synthesis rates remains technically demanding. Traditional methods like radioactive amino acid incorporation provide overall synthesis rates but lack specificity for individual proteins and temporal resolution.
The primary objectives of research in this field now center on developing more precise, high-throughput methodologies to measure protein synthesis boosts following specific phosphorylation events. These objectives include creating real-time monitoring systems for translation initiation following kinase activation, establishing quantitative relationships between phosphorylation stoichiometry and resulting protein synthesis rates, and developing computational models that can predict translation outcomes based on phosphorylation patterns.
Additionally, there is growing interest in understanding how phosphorylation-induced protein synthesis contributes to disease states, particularly in cancer, neurodegeneration, and metabolic disorders. This has led to the parallel objective of identifying potential therapeutic targets within these phosphorylation-dependent translation pathways.
The field is now moving toward integrating multi-omics approaches, combining phosphoproteomics, translatome analysis, and functional genomics to create comprehensive models of how phosphorylation cascades regulate the cellular proteome. This integration represents the next frontier in understanding this fundamental biological process and harnessing it for medical applications.
Market Analysis for Protein Synthesis Detection Technologies
The protein synthesis detection technology market is experiencing robust growth, driven by increasing research in cellular signaling pathways and post-translational modifications. The global market for protein analysis technologies reached approximately $32.5 billion in 2022 and is projected to grow at a CAGR of 9.8% through 2028, with phosphorylation detection technologies representing a significant segment.
Phosphorylation-induced protein synthesis measurement technologies serve diverse end-user segments, with academic and research institutions accounting for 45% of the market share, followed by pharmaceutical and biotechnology companies at 35%, and clinical diagnostic laboratories at 20%. This distribution reflects the critical role these technologies play in both fundamental research and drug development processes.
Regionally, North America dominates the market with 40% share due to substantial research funding and presence of major life science companies. Europe follows at 30%, while Asia-Pacific represents the fastest-growing region with a 12.5% annual growth rate, primarily driven by expanding research infrastructure in China, Japan, and South Korea.
Key market drivers include the rising prevalence of chronic diseases necessitating better understanding of protein regulation mechanisms, growing investments in proteomics research, and increasing demand for personalized medicine approaches that rely on protein biomarker identification. The expanding application of phosphorylation analysis in cancer research particularly fuels market growth, as phosphorylation events are critical in oncogenic signaling pathways.
Technological advancements in detection sensitivity and throughput capabilities are reshaping market dynamics. Mass spectrometry-based approaches currently hold 38% market share, while antibody-based detection methods account for 32%. Emerging technologies like biosensors and fluorescence-based real-time detection systems are gaining traction, growing at 15% annually.
Customer demand increasingly focuses on integrated solutions that offer higher sensitivity, improved specificity for detecting phosphorylation events, and capabilities for real-time monitoring of protein synthesis. There is particular interest in technologies that can measure phosphorylation-induced protein synthesis in living cells with minimal disruption to cellular processes.
Market challenges include high instrumentation costs, technical complexity requiring specialized expertise, and reproducibility issues across different experimental conditions. These factors create significant barriers to entry for smaller laboratories and institutions with limited resources, influencing purchasing decisions and technology adoption rates.
Phosphorylation-induced protein synthesis measurement technologies serve diverse end-user segments, with academic and research institutions accounting for 45% of the market share, followed by pharmaceutical and biotechnology companies at 35%, and clinical diagnostic laboratories at 20%. This distribution reflects the critical role these technologies play in both fundamental research and drug development processes.
Regionally, North America dominates the market with 40% share due to substantial research funding and presence of major life science companies. Europe follows at 30%, while Asia-Pacific represents the fastest-growing region with a 12.5% annual growth rate, primarily driven by expanding research infrastructure in China, Japan, and South Korea.
Key market drivers include the rising prevalence of chronic diseases necessitating better understanding of protein regulation mechanisms, growing investments in proteomics research, and increasing demand for personalized medicine approaches that rely on protein biomarker identification. The expanding application of phosphorylation analysis in cancer research particularly fuels market growth, as phosphorylation events are critical in oncogenic signaling pathways.
Technological advancements in detection sensitivity and throughput capabilities are reshaping market dynamics. Mass spectrometry-based approaches currently hold 38% market share, while antibody-based detection methods account for 32%. Emerging technologies like biosensors and fluorescence-based real-time detection systems are gaining traction, growing at 15% annually.
Customer demand increasingly focuses on integrated solutions that offer higher sensitivity, improved specificity for detecting phosphorylation events, and capabilities for real-time monitoring of protein synthesis. There is particular interest in technologies that can measure phosphorylation-induced protein synthesis in living cells with minimal disruption to cellular processes.
Market challenges include high instrumentation costs, technical complexity requiring specialized expertise, and reproducibility issues across different experimental conditions. These factors create significant barriers to entry for smaller laboratories and institutions with limited resources, influencing purchasing decisions and technology adoption rates.
Current Challenges in Measuring Phosphorylation-Induced Protein Synthesis
Despite significant advancements in molecular biology techniques, measuring phosphorylation-induced protein synthesis presents numerous technical challenges that impede accurate quantification and interpretation. Current methodologies suffer from temporal resolution limitations, making it difficult to capture the dynamic nature of phosphorylation events that often occur within seconds to minutes. This rapid signaling creates a substantial barrier to establishing clear cause-effect relationships between specific phosphorylation events and subsequent protein synthesis boosts.
Sample heterogeneity represents another major obstacle, as cells within the same population may exhibit varying phosphorylation states and protein synthesis rates. This biological variability necessitates single-cell analysis approaches, yet many existing techniques require bulk measurements that mask individual cellular responses and potentially obscure important biological insights.
The signal-to-noise ratio remains problematic in most detection systems, particularly when measuring low-abundance proteins or subtle changes in synthesis rates. Background signals from pre-existing proteins often overwhelm the detection of newly synthesized proteins, creating significant analytical challenges that require sophisticated computational approaches to overcome.
Technical limitations extend to spatial resolution capabilities as well. Phosphorylation events and subsequent protein synthesis often occur in specific subcellular compartments, but many current techniques lack the spatial resolution to distinguish these localized events. This spatial information gap prevents comprehensive understanding of how phosphorylation cascades influence protein synthesis in different cellular regions.
Multiplexing capabilities represent another significant challenge. Phosphorylation networks typically involve multiple proteins and pathways simultaneously, yet most techniques can only measure a limited number of targets concurrently. This restriction hampers system-level analysis and comprehensive pathway mapping.
Quantitative accuracy presents persistent difficulties, as absolute quantification of newly synthesized proteins remains technically challenging. Most methods provide relative measurements that complicate cross-experimental comparisons and standardization efforts across different laboratories and platforms.
Physiological relevance constitutes a final major challenge, as many measurement techniques require non-physiological conditions or introduce artifacts that may alter normal cellular processes. Cell manipulation procedures like transfection or the introduction of labeled amino acids can potentially disrupt the very phosphorylation-dependent synthesis mechanisms being studied, raising questions about data interpretation and biological significance.
Sample heterogeneity represents another major obstacle, as cells within the same population may exhibit varying phosphorylation states and protein synthesis rates. This biological variability necessitates single-cell analysis approaches, yet many existing techniques require bulk measurements that mask individual cellular responses and potentially obscure important biological insights.
The signal-to-noise ratio remains problematic in most detection systems, particularly when measuring low-abundance proteins or subtle changes in synthesis rates. Background signals from pre-existing proteins often overwhelm the detection of newly synthesized proteins, creating significant analytical challenges that require sophisticated computational approaches to overcome.
Technical limitations extend to spatial resolution capabilities as well. Phosphorylation events and subsequent protein synthesis often occur in specific subcellular compartments, but many current techniques lack the spatial resolution to distinguish these localized events. This spatial information gap prevents comprehensive understanding of how phosphorylation cascades influence protein synthesis in different cellular regions.
Multiplexing capabilities represent another significant challenge. Phosphorylation networks typically involve multiple proteins and pathways simultaneously, yet most techniques can only measure a limited number of targets concurrently. This restriction hampers system-level analysis and comprehensive pathway mapping.
Quantitative accuracy presents persistent difficulties, as absolute quantification of newly synthesized proteins remains technically challenging. Most methods provide relative measurements that complicate cross-experimental comparisons and standardization efforts across different laboratories and platforms.
Physiological relevance constitutes a final major challenge, as many measurement techniques require non-physiological conditions or introduce artifacts that may alter normal cellular processes. Cell manipulation procedures like transfection or the introduction of labeled amino acids can potentially disrupt the very phosphorylation-dependent synthesis mechanisms being studied, raising questions about data interpretation and biological significance.
Current Methodologies for Measuring Phosphorylation-Induced Protein Boosts
01 Phosphorylation signaling pathways in protein synthesis regulation
Phosphorylation cascades play a critical role in regulating protein synthesis by activating or inhibiting key translation factors. These signaling pathways, including mTOR and MAPK, respond to various stimuli such as growth factors, nutrients, and stress conditions to modulate the rate of protein synthesis. Phosphorylation of translation initiation factors like eIF2α and eIF4E can either enhance or suppress protein synthesis depending on the cellular context and specific phosphorylation sites.- Phosphorylation pathways in protein synthesis regulation: Phosphorylation cascades play a crucial role in regulating protein synthesis by activating or inhibiting key signaling molecules. These pathways, including mTOR and MAPK, respond to various stimuli such as growth factors and nutrients to modulate translation initiation and elongation factors. The phosphorylation state of these factors directly impacts the rate of protein synthesis, allowing cells to rapidly adjust protein production in response to changing environmental conditions.
- Detection methods for phosphorylation-induced protein synthesis: Various analytical techniques have been developed to detect and quantify phosphorylation events that lead to enhanced protein synthesis. These methods include phospho-specific antibodies, mass spectrometry, fluorescence-based assays, and biosensors that can monitor phosphorylation states in real-time. Such detection systems enable researchers to track the relationship between specific phosphorylation events and subsequent increases in protein synthesis, providing valuable tools for both basic research and drug development.
- Therapeutic modulation of phosphorylation-dependent protein synthesis: Compounds that target phosphorylation-dependent protein synthesis pathways have significant therapeutic potential. By selectively inhibiting or enhancing specific phosphorylation events, these agents can modulate protein synthesis rates in diseased cells. Applications include cancer treatment, where inhibiting aberrant protein synthesis can slow tumor growth, and neurodegenerative disorders, where boosting specific protein production may be beneficial. The development of such targeted therapies represents a promising approach for conditions characterized by dysregulated protein synthesis.
- Kinase-mediated enhancement of protein synthesis: Specific kinases serve as critical mediators for enhancing protein synthesis through phosphorylation events. These enzymes, including PI3K, Akt, and S6K, phosphorylate translation factors and ribosomal proteins to promote translation initiation and elongation. The coordinated action of these kinases creates a signaling network that can rapidly boost protein synthesis in response to cellular needs. Understanding the precise mechanisms by which these kinases regulate translation provides insights into fundamental cellular processes and potential therapeutic targets.
- Phosphorylation-responsive elements in translation machinery: The translation machinery contains numerous components that respond to phosphorylation signals to enhance protein synthesis. These include eukaryotic initiation factors (eIFs), elongation factors, and ribosomal proteins whose activity is modulated by their phosphorylation state. Additionally, regulatory elements in mRNA, such as specific sequences in the 5' untranslated region, can mediate phosphorylation-dependent translation enhancement. These responsive elements collectively form a sophisticated system that allows for precise control of protein synthesis rates in response to cellular signaling events.
02 Detection methods for phosphorylation-dependent protein synthesis
Various analytical techniques have been developed to detect and quantify phosphorylation events that influence protein synthesis rates. These methods include phospho-specific antibodies, mass spectrometry-based approaches, and fluorescent biosensors that can monitor phosphorylation status in real-time. Such detection systems enable researchers to understand the relationship between specific phosphorylation events and subsequent changes in protein synthesis, providing valuable tools for both basic research and drug development.Expand Specific Solutions03 Therapeutic modulation of phosphorylation-induced protein synthesis
Pharmaceutical compounds that target phosphorylation-dependent protein synthesis pathways show promise for treating various diseases. By selectively inhibiting or enhancing specific phosphorylation events, these therapeutics can modulate protein synthesis rates in target tissues. Applications include cancer treatment, where inhibiting aberrant protein synthesis can slow tumor growth, and neurodegenerative disorders, where boosting specific protein production may be beneficial. Small molecule inhibitors, peptide-based drugs, and biologics have been developed to target these pathways.Expand Specific Solutions04 Engineered systems for enhanced phosphorylation-dependent protein synthesis
Biotechnological approaches have been developed to create engineered cellular systems with enhanced phosphorylation-dependent protein synthesis capabilities. These systems include modified translation machinery components that respond more efficiently to phosphorylation signals, synthetic phosphorylation circuits that can boost protein production in response to specific stimuli, and cell-free protein synthesis platforms incorporating phosphorylation-responsive elements. Such technologies have applications in biomanufacturing, synthetic biology, and the production of therapeutic proteins.Expand Specific Solutions05 Phosphorylation-induced protein synthesis in cellular stress responses
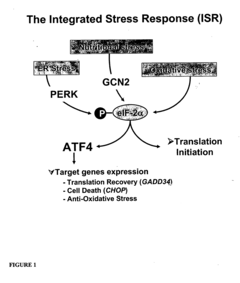
Cellular stress conditions trigger specific phosphorylation events that modulate protein synthesis to promote cell survival or adaptation. These phosphorylation cascades can either globally suppress protein synthesis to conserve energy or selectively enhance the translation of stress-response proteins. Key pathways include the integrated stress response, which involves phosphorylation of eIF2α, and stress-activated protein kinases that phosphorylate various translation factors. Understanding these mechanisms provides insights into cellular adaptation to environmental challenges and potential therapeutic targets for stress-related disorders.Expand Specific Solutions
Leading Research Groups and Companies in Protein Synthesis Detection
The phosphorylation-induced protein synthesis boost technology market is currently in an early growth phase, characterized by intensive research and emerging commercial applications. The market size remains relatively modest but is expanding rapidly due to increasing applications in drug discovery and personalized medicine. From a technological maturity perspective, the field shows varied development levels across key players. Established pharmaceutical companies like Bayer Pharma AG and Merck Sharp & Dohme are leveraging their R&D infrastructure to advance clinical applications, while specialized biotechnology firms such as Life Technologies Corp. and Beckman Coulter are developing innovative measurement platforms. Academic institutions including Dana-Farber Cancer Institute and The Broad Institute are driving fundamental research breakthroughs. Research organizations like the Dalian Institute of Chemical Physics are contributing novel analytical approaches, creating a competitive landscape balanced between commercial development and basic research advancement.
Life Technologies Corp.
Technical Solution: Life Technologies has developed the Click-iT™ Phosphorylation & Synthesis Correlation (PSC) platform specifically designed to measure phosphorylation-induced protein synthesis boosts. This technology combines their established phosphoprotein detection methods with metabolic labeling approaches to simultaneously track phosphorylation events and subsequent protein synthesis in the same biological sample[2]. The system utilizes their proprietary Click chemistry to incorporate modified amino acids into newly synthesized proteins, allowing precise temporal correlation between specific phosphorylation events and enhanced translation rates. Their workflow includes automated phosphoprotein enrichment using their IMAC-Select™ technology followed by quantitative fluorescence detection of both phosphorylated proteins and newly synthesized proteins[5]. The platform is complemented by their Attune™ NxT flow cytometer configuration that enables single-cell analysis of phosphorylation-induced synthesis boosts, providing insights into cell-to-cell variability in translational responses. Their Molecular Probes™ division has also developed specialized fluorescent probes that can simultaneously report on kinase activity and ribosomal engagement in live cells.
Strengths: Excellent compatibility with diverse biological systems including primary cells and tissues; ability to perform single-cell analysis of phosphorylation-synthesis relationships; good sensitivity for detecting moderate changes in synthesis rates; comprehensive reagent kits requiring minimal optimization. Weaknesses: More limited phosphoproteome coverage compared to mass spectrometry approaches; potential artifacts from metabolic labeling affecting normal cellular physiology; challenges in multiplexing beyond a few phosphorylation targets simultaneously.
The Regents of the University of Michigan
Technical Solution: The University of Michigan has developed the Phospho-Translational Nexus (PTN) platform for measuring phosphorylation-induced protein synthesis boosts. This academic technology integrates custom microfluidic devices with advanced mass spectrometry to achieve temporal resolution of phosphorylation events and subsequent translational enhancement[1]. Their approach employs a novel "pulse-chase-pulse" labeling strategy where cells are first labeled to establish baseline protein synthesis rates, then exposed to stimuli that induce phosphorylation, followed by a second distinct label to quantify changes in synthesis rates[4]. The platform incorporates their proprietary computational framework that applies Bayesian statistical models to identify causal relationships between specific phosphorylation events and enhanced protein synthesis. Their microfluidic sample processing system enables analysis from limited biological material, making it particularly valuable for clinical specimens. The technology has been validated across multiple signaling pathways, including mTOR, MAPK, and PKA networks, demonstrating its ability to identify previously unknown phosphorylation sites that significantly boost protein synthesis rates in disease states.
Strengths: Exceptional sensitivity for limited sample inputs; sophisticated statistical modeling for establishing causality; excellent temporal resolution of phosphorylation-synthesis relationships; validated across multiple biological systems including patient samples. Weaknesses: Currently limited throughput compared to commercial platforms; requires specialized expertise in both microfluidics and mass spectrometry; more complex data analysis pipeline; technology still primarily in academic setting with limited standardization.
Key Technical Innovations in Phosphoproteomics and Translation Analysis
Methods of screening test compounds using GADD34L, an eIF2alpha-specific phosphatase subunit
PatentInactiveUS20040142345A1
Innovation
- Inhibiting the activity of GADD34L, a regulatory subunit of PP1c, to promote the accumulation of phosphorylated eIF2α and activate the Integrated Stress Response (ISR) pathway without causing stress, thereby identifying substances that can prevent or treat oxidative stress-related diseases.
Method for quantifying phosphokinase activity on proteins
PatentInactiveUS20120135426A1
Innovation
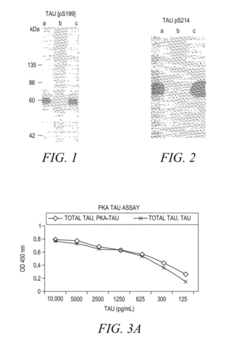
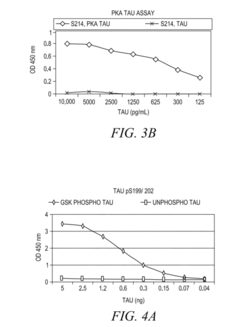
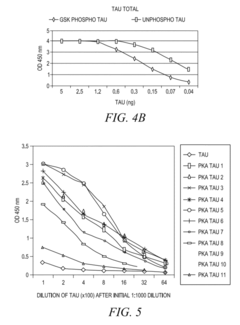
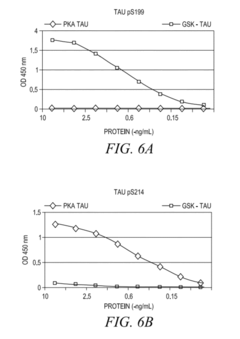
- The development of phosphorylation site-specific antibodies (PSSAs) that bind to specific phosphorylated amino acid residues, allowing for the detection and quantification of phosphorylated proteins at individual sites, enabling the measurement of protein kinase activity on whole proteins or their fragments, including Tau, Retinoblastoma protein, and Epidermal Growth Factor Receptor, with high specificity and sensitivity.
Bioethical Considerations in Protein Modification Research
The ethical implications of protein modification research, particularly in the field of phosphorylation-induced protein synthesis enhancement, demand careful consideration as this technology advances. The ability to measure and manipulate phosphorylation events that boost protein synthesis raises significant bioethical questions regarding the boundaries of human intervention in fundamental biological processes.
Informed consent represents a primary ethical concern in this domain. Research participants must fully understand the potential long-term consequences of phosphorylation modifications, which may persist beyond the study period. The current limitations in predicting all possible outcomes of such modifications necessitates transparent communication about known and unknown risks.
The principle of non-maleficence becomes particularly relevant when considering the potential for unintended consequences. Phosphorylation pathways interact with numerous cellular processes, and alterations may trigger cascading effects throughout biological systems. Scientists must establish robust safety protocols and monitoring mechanisms to prevent harm to research subjects and eventual therapeutic recipients.
Questions of equitable access emerge as phosphorylation-based technologies demonstrate promising applications in treating protein deficiency disorders. If successful, these technologies could create new therapeutic options, but their complexity may result in prohibitively expensive treatments accessible only to privileged populations, potentially exacerbating existing healthcare disparities.
The dual-use potential of phosphorylation enhancement technologies presents another ethical dimension. While developed for legitimate medical applications, these techniques could be repurposed for non-therapeutic enhancements, raising concerns about the creation of biological advantages for select individuals. This necessitates careful regulatory frameworks that distinguish between therapeutic applications and enhancement purposes.
Environmental considerations must also be addressed, as modified organisms containing enhanced phosphorylation pathways could potentially interact with natural ecosystems if containment measures fail. The ecological impact of such interactions remains largely unknown and requires thorough risk assessment protocols.
The governance of phosphorylation research demands international coordination to establish consistent ethical standards. Different cultural and regulatory approaches to biotechnology could lead to regulatory arbitrage, where research migrates to jurisdictions with less stringent oversight. Harmonized global frameworks would help ensure responsible advancement of this promising technology while respecting diverse ethical perspectives.
Informed consent represents a primary ethical concern in this domain. Research participants must fully understand the potential long-term consequences of phosphorylation modifications, which may persist beyond the study period. The current limitations in predicting all possible outcomes of such modifications necessitates transparent communication about known and unknown risks.
The principle of non-maleficence becomes particularly relevant when considering the potential for unintended consequences. Phosphorylation pathways interact with numerous cellular processes, and alterations may trigger cascading effects throughout biological systems. Scientists must establish robust safety protocols and monitoring mechanisms to prevent harm to research subjects and eventual therapeutic recipients.
Questions of equitable access emerge as phosphorylation-based technologies demonstrate promising applications in treating protein deficiency disorders. If successful, these technologies could create new therapeutic options, but their complexity may result in prohibitively expensive treatments accessible only to privileged populations, potentially exacerbating existing healthcare disparities.
The dual-use potential of phosphorylation enhancement technologies presents another ethical dimension. While developed for legitimate medical applications, these techniques could be repurposed for non-therapeutic enhancements, raising concerns about the creation of biological advantages for select individuals. This necessitates careful regulatory frameworks that distinguish between therapeutic applications and enhancement purposes.
Environmental considerations must also be addressed, as modified organisms containing enhanced phosphorylation pathways could potentially interact with natural ecosystems if containment measures fail. The ecological impact of such interactions remains largely unknown and requires thorough risk assessment protocols.
The governance of phosphorylation research demands international coordination to establish consistent ethical standards. Different cultural and regulatory approaches to biotechnology could lead to regulatory arbitrage, where research migrates to jurisdictions with less stringent oversight. Harmonized global frameworks would help ensure responsible advancement of this promising technology while respecting diverse ethical perspectives.
Translational Applications in Drug Discovery and Disease Treatment
The measurement of phosphorylation-induced protein synthesis boosts offers significant translational applications in drug discovery and disease treatment. This technology enables researchers to identify compounds that modulate protein synthesis through phosphorylation pathways, creating new avenues for therapeutic intervention in various diseases characterized by dysregulated protein synthesis.
In oncology, this measurement technique has facilitated the development of targeted therapies that inhibit aberrant protein synthesis in cancer cells. For instance, mTOR inhibitors like rapamycin and its analogs were developed based on understanding phosphorylation-dependent protein synthesis regulation. These drugs have shown efficacy in treating certain types of cancers, including renal cell carcinoma and breast cancer, by targeting the hyperactivated mTOR pathway that drives excessive protein synthesis.
Neurodegenerative disorders represent another promising application area. Alzheimer's, Parkinson's, and Huntington's diseases are characterized by protein misfolding and aggregation. By measuring phosphorylation-induced protein synthesis changes, researchers have identified potential drug targets that can modulate the synthesis of disease-associated proteins. This has led to clinical trials of compounds that regulate protein synthesis through kinase inhibition or phosphatase activation.
Metabolic diseases, particularly diabetes, benefit from this technology through the development of drugs targeting insulin signaling pathways. Insulin activates protein synthesis via phosphorylation cascades, and measuring these effects has enabled the identification of compounds that enhance insulin sensitivity. Several antidiabetic drugs now in development aim to normalize protein synthesis rates in insulin-resistant tissues.
The technology also accelerates personalized medicine approaches. Patient-derived cells can be analyzed for phosphorylation-dependent protein synthesis responses to potential therapeutics, allowing for treatment customization. This approach has shown promise in selecting optimal treatments for cancer patients based on their unique cellular signaling profiles.
In infectious disease treatment, measuring phosphorylation-induced protein synthesis has revealed how pathogens hijack host translation machinery. This understanding has led to the development of novel antivirals and antibiotics that selectively target pathogen-specific protein synthesis mechanisms without disrupting normal host cell functions.
The pharmaceutical industry has incorporated this measurement technology into high-throughput screening platforms, significantly accelerating the drug discovery process. Compounds that modulate phosphorylation-dependent protein synthesis can be rapidly identified and optimized, reducing the time and cost of bringing new therapeutics to market.
In oncology, this measurement technique has facilitated the development of targeted therapies that inhibit aberrant protein synthesis in cancer cells. For instance, mTOR inhibitors like rapamycin and its analogs were developed based on understanding phosphorylation-dependent protein synthesis regulation. These drugs have shown efficacy in treating certain types of cancers, including renal cell carcinoma and breast cancer, by targeting the hyperactivated mTOR pathway that drives excessive protein synthesis.
Neurodegenerative disorders represent another promising application area. Alzheimer's, Parkinson's, and Huntington's diseases are characterized by protein misfolding and aggregation. By measuring phosphorylation-induced protein synthesis changes, researchers have identified potential drug targets that can modulate the synthesis of disease-associated proteins. This has led to clinical trials of compounds that regulate protein synthesis through kinase inhibition or phosphatase activation.
Metabolic diseases, particularly diabetes, benefit from this technology through the development of drugs targeting insulin signaling pathways. Insulin activates protein synthesis via phosphorylation cascades, and measuring these effects has enabled the identification of compounds that enhance insulin sensitivity. Several antidiabetic drugs now in development aim to normalize protein synthesis rates in insulin-resistant tissues.
The technology also accelerates personalized medicine approaches. Patient-derived cells can be analyzed for phosphorylation-dependent protein synthesis responses to potential therapeutics, allowing for treatment customization. This approach has shown promise in selecting optimal treatments for cancer patients based on their unique cellular signaling profiles.
In infectious disease treatment, measuring phosphorylation-induced protein synthesis has revealed how pathogens hijack host translation machinery. This understanding has led to the development of novel antivirals and antibiotics that selectively target pathogen-specific protein synthesis mechanisms without disrupting normal host cell functions.
The pharmaceutical industry has incorporated this measurement technology into high-throughput screening platforms, significantly accelerating the drug discovery process. Compounds that modulate phosphorylation-dependent protein synthesis can be rapidly identified and optimized, reducing the time and cost of bringing new therapeutics to market.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!