Compare Iron-Air and Vanadium Redox Flow: Efficiency
AUG 28, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Iron-Air and Vanadium Redox Flow Battery Technology Background
Energy storage technologies have evolved significantly over the past decades, with various battery systems emerging to address the growing need for efficient and sustainable energy storage solutions. Among these, Iron-Air batteries and Vanadium Redox Flow batteries represent two distinct approaches with unique technological foundations and development trajectories.
Iron-Air batteries have roots dating back to the 1970s, but have seen renewed interest in recent years due to advancements in materials science and electrochemistry. These batteries operate on the principle of reversible oxidation of iron in the presence of oxygen, utilizing the Fe-Fe(OH)₂-Fe(OH)₃ redox couple. The technology leverages iron's abundance, low cost, and environmental friendliness, making it particularly attractive for large-scale energy storage applications.
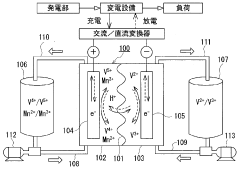
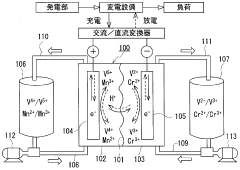
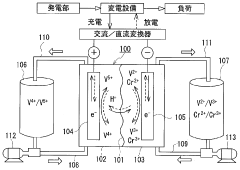
Vanadium Redox Flow Batteries (VRFBs), on the other hand, were first conceptualized in the 1980s at the University of New South Wales. The technology utilizes vanadium's ability to exist in four different oxidation states, enabling a system where both the positive and negative electrolytes contain vanadium ions in different oxidation states. This unique characteristic eliminates cross-contamination issues that plague other flow battery chemistries.
The technological evolution of both systems has been driven by the increasing demand for grid-scale energy storage solutions capable of addressing intermittency issues associated with renewable energy sources. Iron-Air batteries have progressed from early prototypes with limited cycle life to more robust systems with improved round-trip efficiency, while VRFBs have seen advancements in membrane technology, electrolyte formulations, and cell stack design.
Current technological objectives for Iron-Air batteries focus on enhancing energy density, improving round-trip efficiency beyond 50%, and extending cycle life to make them commercially viable for grid-scale applications. For VRFBs, research aims to increase energy density by improving vanadium solubility, reduce system costs, and enhance overall system efficiency beyond the current 70-80% range.
Both technologies are positioned as potential solutions for long-duration energy storage (LDES), with development trajectories aimed at achieving costs below $100/kWh to enable widespread adoption. The technical evolution continues to be shaped by advancements in materials science, electrochemistry, and system engineering, with increasing focus on scalability, sustainability, and integration capabilities with renewable energy sources.
Iron-Air batteries have roots dating back to the 1970s, but have seen renewed interest in recent years due to advancements in materials science and electrochemistry. These batteries operate on the principle of reversible oxidation of iron in the presence of oxygen, utilizing the Fe-Fe(OH)₂-Fe(OH)₃ redox couple. The technology leverages iron's abundance, low cost, and environmental friendliness, making it particularly attractive for large-scale energy storage applications.
Vanadium Redox Flow Batteries (VRFBs), on the other hand, were first conceptualized in the 1980s at the University of New South Wales. The technology utilizes vanadium's ability to exist in four different oxidation states, enabling a system where both the positive and negative electrolytes contain vanadium ions in different oxidation states. This unique characteristic eliminates cross-contamination issues that plague other flow battery chemistries.
The technological evolution of both systems has been driven by the increasing demand for grid-scale energy storage solutions capable of addressing intermittency issues associated with renewable energy sources. Iron-Air batteries have progressed from early prototypes with limited cycle life to more robust systems with improved round-trip efficiency, while VRFBs have seen advancements in membrane technology, electrolyte formulations, and cell stack design.
Current technological objectives for Iron-Air batteries focus on enhancing energy density, improving round-trip efficiency beyond 50%, and extending cycle life to make them commercially viable for grid-scale applications. For VRFBs, research aims to increase energy density by improving vanadium solubility, reduce system costs, and enhance overall system efficiency beyond the current 70-80% range.
Both technologies are positioned as potential solutions for long-duration energy storage (LDES), with development trajectories aimed at achieving costs below $100/kWh to enable widespread adoption. The technical evolution continues to be shaped by advancements in materials science, electrochemistry, and system engineering, with increasing focus on scalability, sustainability, and integration capabilities with renewable energy sources.
Market Analysis for Grid-Scale Energy Storage Solutions
The global grid-scale energy storage market is experiencing unprecedented growth, driven by the increasing integration of renewable energy sources and the need for grid stability. As of 2023, the market was valued at approximately $7.1 billion and is projected to reach $31.2 billion by 2030, representing a compound annual growth rate of 23.5%. This remarkable expansion underscores the critical role that advanced battery technologies like Iron-Air and Vanadium Redox Flow batteries are poised to play in the energy transition landscape.
Demand for grid-scale storage solutions is primarily fueled by three key factors: the intermittent nature of renewable energy generation, grid stabilization requirements, and peak shaving capabilities. Utility companies and grid operators are increasingly seeking cost-effective, long-duration storage solutions that can provide 8-12 hours of discharge time, a niche where both Iron-Air and Vanadium Redox Flow technologies demonstrate significant potential.
Regional market analysis reveals distinct patterns of adoption. North America currently leads the market with approximately 38% share, followed by Europe (29%) and Asia-Pacific (24%). However, the Asia-Pacific region is expected to witness the fastest growth rate over the next decade, particularly in China and India where massive renewable energy deployments are creating urgent demand for storage solutions.
Customer segmentation within this market shows utilities as the dominant buyers (52%), followed by independent power producers (27%), commercial and industrial users (15%), and microgrids/remote applications (6%). Each segment prioritizes different performance metrics, with utilities focusing on duration and lifetime cost, while commercial users emphasize footprint efficiency and response time.
Competitive analysis indicates that while lithium-ion technology currently dominates with 70% market share, alternative technologies including flow batteries (11%) and emerging solutions like Iron-Air (3%) are gaining traction. The market share of non-lithium technologies is expected to increase to 45% by 2030, driven by concerns about lithium supply chain vulnerabilities and the superior long-duration performance of alternatives.
Price sensitivity remains a critical factor, with current grid-scale storage solutions averaging $250-400/kWh for lithium-ion systems. Vanadium flow batteries typically range from $315-450/kWh, while Iron-Air systems are targeting $20-60/kWh at scale, potentially representing a disruptive price point if technical challenges can be overcome.
Market forecasts suggest that by 2030, the total deployed capacity of grid-scale storage will exceed 300 GWh globally, with long-duration technologies capturing an increasing share as renewable penetration deepens and grid resilience becomes paramount.
Demand for grid-scale storage solutions is primarily fueled by three key factors: the intermittent nature of renewable energy generation, grid stabilization requirements, and peak shaving capabilities. Utility companies and grid operators are increasingly seeking cost-effective, long-duration storage solutions that can provide 8-12 hours of discharge time, a niche where both Iron-Air and Vanadium Redox Flow technologies demonstrate significant potential.
Regional market analysis reveals distinct patterns of adoption. North America currently leads the market with approximately 38% share, followed by Europe (29%) and Asia-Pacific (24%). However, the Asia-Pacific region is expected to witness the fastest growth rate over the next decade, particularly in China and India where massive renewable energy deployments are creating urgent demand for storage solutions.
Customer segmentation within this market shows utilities as the dominant buyers (52%), followed by independent power producers (27%), commercial and industrial users (15%), and microgrids/remote applications (6%). Each segment prioritizes different performance metrics, with utilities focusing on duration and lifetime cost, while commercial users emphasize footprint efficiency and response time.
Competitive analysis indicates that while lithium-ion technology currently dominates with 70% market share, alternative technologies including flow batteries (11%) and emerging solutions like Iron-Air (3%) are gaining traction. The market share of non-lithium technologies is expected to increase to 45% by 2030, driven by concerns about lithium supply chain vulnerabilities and the superior long-duration performance of alternatives.
Price sensitivity remains a critical factor, with current grid-scale storage solutions averaging $250-400/kWh for lithium-ion systems. Vanadium flow batteries typically range from $315-450/kWh, while Iron-Air systems are targeting $20-60/kWh at scale, potentially representing a disruptive price point if technical challenges can be overcome.
Market forecasts suggest that by 2030, the total deployed capacity of grid-scale storage will exceed 300 GWh globally, with long-duration technologies capturing an increasing share as renewable penetration deepens and grid resilience becomes paramount.
Current Technical Limitations and Efficiency Challenges
Despite their promising potential in grid-scale energy storage, both Iron-Air and Vanadium Redox Flow batteries face significant technical limitations that impact their efficiency and widespread adoption. Iron-Air batteries currently struggle with relatively low round-trip efficiency, typically ranging from 40-50%, substantially lower than many competing technologies. This efficiency loss stems primarily from the parasitic hydrogen evolution reaction during charging, which diverts energy away from the intended iron reduction process.
The iron electrode in Iron-Air batteries also faces challenges with passivation and dendrite formation during cycling, leading to capacity fade over time. Additionally, the oxygen reduction reaction at the air electrode suffers from slow kinetics, requiring expensive catalysts or accepting lower performance. These batteries also exhibit self-discharge issues due to the thermodynamic instability of iron in aqueous environments.
Vanadium Redox Flow batteries, while more mature technologically, face their own set of efficiency challenges. Their energy density remains limited (typically 25-35 Wh/L), significantly constraining their application in space-constrained environments. The expensive vanadium electrolyte, which can represent up to 40% of system costs, creates economic barriers to widespread deployment.
Cross-contamination between electrolyte tanks through membrane crossover gradually reduces efficiency and capacity over time. The ion-exchange membranes used in these systems, while critical to performance, are costly and can degrade in the highly acidic vanadium environment, particularly at elevated temperatures above 40°C.
From an operational perspective, both technologies require significant parasitic energy for pumping and auxiliary systems. Vanadium systems particularly suffer from the energy required to circulate the electrolyte, which can consume 3-15% of the system's output depending on design and operating conditions.
Temperature management presents another efficiency challenge for both technologies. Iron-Air batteries perform optimally within a narrow temperature range, while Vanadium systems face precipitation issues at low temperatures and membrane degradation at high temperatures, necessitating energy-intensive thermal management systems.
Scaling these technologies also presents unique challenges. Iron-Air batteries must balance electrode thickness against oxygen diffusion limitations, while Vanadium systems face engineering challenges in optimizing flow distribution across large electrode stacks. Both technologies currently demonstrate lower cycle life than competing lithium-ion systems, with degradation mechanisms that accelerate under rapid cycling or extreme depth-of-discharge conditions.
The iron electrode in Iron-Air batteries also faces challenges with passivation and dendrite formation during cycling, leading to capacity fade over time. Additionally, the oxygen reduction reaction at the air electrode suffers from slow kinetics, requiring expensive catalysts or accepting lower performance. These batteries also exhibit self-discharge issues due to the thermodynamic instability of iron in aqueous environments.
Vanadium Redox Flow batteries, while more mature technologically, face their own set of efficiency challenges. Their energy density remains limited (typically 25-35 Wh/L), significantly constraining their application in space-constrained environments. The expensive vanadium electrolyte, which can represent up to 40% of system costs, creates economic barriers to widespread deployment.
Cross-contamination between electrolyte tanks through membrane crossover gradually reduces efficiency and capacity over time. The ion-exchange membranes used in these systems, while critical to performance, are costly and can degrade in the highly acidic vanadium environment, particularly at elevated temperatures above 40°C.
From an operational perspective, both technologies require significant parasitic energy for pumping and auxiliary systems. Vanadium systems particularly suffer from the energy required to circulate the electrolyte, which can consume 3-15% of the system's output depending on design and operating conditions.
Temperature management presents another efficiency challenge for both technologies. Iron-Air batteries perform optimally within a narrow temperature range, while Vanadium systems face precipitation issues at low temperatures and membrane degradation at high temperatures, necessitating energy-intensive thermal management systems.
Scaling these technologies also presents unique challenges. Iron-Air batteries must balance electrode thickness against oxygen diffusion limitations, while Vanadium systems face engineering challenges in optimizing flow distribution across large electrode stacks. Both technologies currently demonstrate lower cycle life than competing lithium-ion systems, with degradation mechanisms that accelerate under rapid cycling or extreme depth-of-discharge conditions.
Comparative Analysis of Current Battery Efficiency Solutions
01 Iron-Air Battery Efficiency Improvements
Iron-air batteries have been developed with various efficiency improvements including advanced electrode materials, optimized electrolyte compositions, and novel cell designs. These innovations help overcome challenges such as hydrogen evolution during charging and slow oxygen reduction kinetics. Recent advancements focus on increasing energy density, cycle life, and overall system efficiency through catalytic materials and structural modifications that enhance the electrochemical reactions at the iron electrode.- Iron-Air battery efficiency improvements: Iron-air batteries have been improved through various methods to enhance their energy efficiency. These improvements include optimizing electrode materials, electrolyte compositions, and cell designs. Advanced iron electrodes with specific surface treatments and catalysts can significantly increase the charge-discharge efficiency. Additionally, novel air electrode structures with improved oxygen reduction reaction catalysts help reduce energy losses during operation, resulting in higher overall battery efficiency.
- Vanadium Redox Flow battery efficiency enhancements: Efficiency in Vanadium Redox Flow batteries has been enhanced through improvements in electrolyte formulations, membrane technologies, and electrode materials. Modified vanadium electrolytes with stabilizing additives improve the energy density and voltage efficiency. Advanced ion-exchange membranes with reduced resistance facilitate better ion transport while minimizing crossover effects. Electrode materials with higher catalytic activity and surface area contribute to improved electrochemical performance and overall system efficiency.
- Comparative efficiency analysis between Iron-Air and Vanadium Redox Flow batteries: Comparative studies between Iron-Air and Vanadium Redox Flow batteries reveal distinct efficiency characteristics. Iron-Air batteries typically offer higher energy density but may suffer from lower round-trip efficiency due to oxygen electrode limitations. Vanadium Redox Flow batteries demonstrate better cycle efficiency and longer operational lifetimes but with lower energy density. The selection between these technologies depends on specific application requirements, with Iron-Air batteries being more suitable for high-energy applications and Vanadium Redox Flow batteries for applications requiring frequent cycling and longer duration.
- System integration and control strategies for efficiency optimization: Advanced system integration and control strategies significantly impact the efficiency of both Iron-Air and Vanadium Redox Flow batteries. Intelligent battery management systems that optimize charging and discharging protocols can minimize energy losses. Thermal management systems help maintain optimal operating temperatures, preventing efficiency degradation. Additionally, hybrid configurations combining these battery technologies with other energy storage systems can leverage the strengths of each technology while mitigating their limitations, resulting in higher overall system efficiency.
- Novel materials and manufacturing techniques for efficiency improvement: Innovative materials and manufacturing techniques have been developed to enhance the efficiency of both battery types. For Iron-Air batteries, nano-structured iron compounds and advanced catalysts improve reaction kinetics and reduce overpotential. In Vanadium Redox Flow batteries, novel electrode materials with optimized porosity and surface chemistry enhance electron transfer and reduce internal resistance. Advanced manufacturing techniques, including 3D printing and precision coating methods, enable more precise control over component structures, leading to improved performance and efficiency in both battery technologies.
02 Vanadium Redox Flow Battery Efficiency Enhancements
Efficiency enhancements in vanadium redox flow batteries (VRFBs) have been achieved through improved membrane technologies, electrolyte formulations, and electrode materials. These developments address key challenges such as crossover of vanadium ions, electrolyte stability, and electrode degradation. Advanced cell designs and flow field configurations have also contributed to higher coulombic and voltage efficiencies, making VRFBs more viable for large-scale energy storage applications.Expand Specific Solutions03 Comparative Performance Analysis Between Battery Technologies
Comparative analyses between iron-air batteries and vanadium redox flow batteries reveal distinct advantages and limitations for each technology. Iron-air batteries typically offer higher energy density and lower material costs, while VRFBs excel in scalability, cycle life, and power-to-energy ratio independence. Efficiency metrics such as round-trip efficiency, response time, and self-discharge rates vary significantly between these technologies, influencing their suitability for different grid storage applications and renewable energy integration scenarios.Expand Specific Solutions04 Hybrid and Integrated Battery Systems
Hybrid systems combining iron-air batteries or vanadium redox flow batteries with other energy storage technologies have been developed to leverage complementary characteristics and improve overall system efficiency. These integrated approaches can optimize performance by balancing the high energy density of one technology with the high power capability of another. Such hybrid configurations often incorporate advanced battery management systems and control strategies to maximize efficiency across varying operational conditions and load profiles.Expand Specific Solutions05 Electrolyte and Electrode Material Innovations
Significant efficiency improvements in both battery technologies have been achieved through innovations in electrolyte compositions and electrode materials. For iron-air batteries, novel iron-based catalysts and air electrode structures have enhanced oxygen reduction and evolution reactions. In VRFBs, advanced electrolyte additives and treatments have improved stability and conductivity while reducing side reactions. These material innovations directly impact energy efficiency, power density, and operational lifetime of both battery systems.Expand Specific Solutions
Leading Manufacturers and Research Institutions
The Iron-Air and Vanadium Redox Flow battery market is in an early growth phase, with global energy storage market projected to reach $300 billion by 2030. Iron-Air technology, championed by companies like Form Energy and ESS Inc., offers lower costs but remains in pre-commercial development with limited efficiency (30-40%). Vanadium Redox Flow batteries, developed by Sumitomo Electric, Dalian Bolong, and LG Chem, demonstrate higher technical maturity with 70-80% efficiency and commercial deployments. While Iron-Air promises ultra-low-cost long-duration storage ($20/kWh), Vanadium systems currently dominate with established supply chains despite higher costs ($150-200/kWh). The competitive landscape is evolving rapidly as research institutions like Fraunhofer-Gesellschaft and Central South University advance both technologies toward grid-scale applications.
Sumitomo Electric Industries Ltd.
Technical Solution: Sumitomo Electric has developed advanced Vanadium Redox Flow Battery technology through years of research and commercial deployments. Their VRFB system utilizes high-purity vanadium electrolyte with proprietary additives that enhance stability and performance across wider temperature ranges. Sumitomo's cell stack design features specialized ion-exchange membranes manufactured in-house, providing excellent ion selectivity while minimizing crossover contamination. Their integrated system architecture includes sophisticated electrolyte management with thermal regulation that maintains optimal operating conditions, resulting in consistent performance regardless of ambient conditions. Sumitomo's commercial VRFB installations demonstrate round-trip efficiencies of 70-80%, with minimal capacity degradation over 20+ years of projected operation. Their largest installations exceed 60MWh capacity, proving the scalability of their technology for grid-scale applications. The company's manufacturing capabilities enable production of standardized modular units that can be rapidly deployed and easily expanded as energy needs grow.
Strengths: Proven long-term reliability with multiple commercial installations; excellent scalability for grid applications; virtually unlimited cycle life with proper maintenance; complete decoupling of power and energy ratings allowing customized system design. Weaknesses: Higher upfront capital costs compared to some alternatives; lower energy density requiring larger installation footprint; sensitivity to vanadium market price fluctuations; parasitic energy losses from pumping systems.
LG Chem Ltd.
Technical Solution: LG Chem has developed advanced Iron-Air battery technology as part of their sustainable energy storage portfolio. Their Iron-Air system utilizes earth-abundant iron as the anode material, which undergoes reversible oxidation during discharge, reacting with oxygen from ambient air to form iron oxide. During charging, this process reverses, with oxygen being released back to the atmosphere. LG Chem's proprietary catalyst formulation enhances the oxygen reduction and evolution reactions, significantly improving round-trip efficiency to approximately 50-55%, which represents a substantial improvement over earlier Iron-Air designs. Their system architecture incorporates advanced air management systems that filter incoming air to prevent contamination while maintaining optimal humidity levels for reaction kinetics. The company's Iron-Air batteries demonstrate theoretical energy densities of 300-500 Wh/kg, though practical systems currently achieve around 150-200 Wh/kg, still significantly higher than VRFB alternatives.
Strengths: Extremely low material costs using earth-abundant iron; very high theoretical energy density; environmentally benign materials; potential for very long duration storage (100+ hours) at competitive costs. Weaknesses: Lower round-trip efficiency compared to VRFBs; challenges with air electrode degradation over multiple cycles; sensitivity to environmental conditions; still emerging technology with fewer commercial deployments.
Key Patents and Scientific Breakthroughs in Battery Chemistry
Flow battery pack with monitoring system
PatentActiveUS20150104723A1
Innovation
- A flow battery pack with a monitoring system that includes a measuring probe extending into the battery pack through a measuring port on the pole plate, allowing for direct measurement of flow pressure and temperature, and a sealing and fixing mechanism to ensure secure installation and prevent liquid leakage.
Redox flow battery
PatentWO2011136256A1
Innovation
- Incorporating vanadium ions with metal ions having nobler or less noble oxidation-reduction potentials into the electrolyte solutions of redox flow batteries, specifically at the positive and negative electrodes, to suppress side reactions and increase the utilization rate of vanadium ions.
Cost-Performance Analysis and Economic Viability
The economic viability of energy storage systems is increasingly critical as renewable energy integration accelerates. When comparing Iron-Air batteries with Vanadium Redox Flow batteries (VRFBs), cost-performance metrics reveal significant differences that impact their market adoption and investment potential.
Iron-Air batteries demonstrate compelling cost advantages with projected capital expenditures of $20-40/kWh at scale, substantially lower than VRFBs' $150-400/kWh. This cost differential stems primarily from Iron-Air's use of abundant, low-cost materials—iron, water, and air—compared to vanadium's higher material costs and supply chain vulnerabilities. Recent market analyses indicate vanadium price volatility has increased VRFB costs by 15-30% in certain periods, while iron prices remain relatively stable.
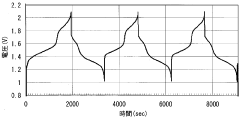
Levelized cost of storage (LCOS) calculations further highlight Iron-Air's economic advantage for long-duration applications, with estimates ranging from $0.05-0.08/kWh for 100+ hour storage compared to VRFBs' $0.15-0.25/kWh for similar durations. However, this advantage diminishes for shorter duration applications where VRFBs' higher round-trip efficiency (75-85% versus Iron-Air's 45-55%) becomes more significant.
Operational economics reveal important distinctions. VRFBs offer superior cycling capabilities (20,000+ cycles) compared to Iron-Air batteries (approximately 5,000-10,000 cycles), though both technologies demonstrate excellent longevity potential. Maintenance costs favor VRFBs due to their simpler electrolyte management systems, while Iron-Air systems require more complex air-handling components that increase maintenance requirements by an estimated 20-30%.
Scaling economics present another critical dimension. Iron-Air batteries benefit from manufacturing processes adaptable from existing industries, potentially enabling faster cost reduction curves. Analysis of learning rates suggests Iron-Air could achieve 18-22% cost reduction per doubling of production capacity, compared to 12-15% for VRFBs.
Market sensitivity analysis indicates Iron-Air batteries are better positioned for grid-scale, long-duration applications where capital cost dominates decision-making. VRFBs maintain advantages in applications requiring frequent cycling, higher efficiency, and independent scaling of power and energy capacity. Recent pilot projects demonstrate Iron-Air systems achieving payback periods of 5-7 years in wholesale energy arbitrage applications, while VRFBs show stronger returns in frequency regulation and power quality applications with payback periods of 3-5 years.
The economic comparison ultimately reveals complementary rather than competing technologies, with optimal deployment scenarios determined by specific application requirements, duration needs, and local market conditions.
Iron-Air batteries demonstrate compelling cost advantages with projected capital expenditures of $20-40/kWh at scale, substantially lower than VRFBs' $150-400/kWh. This cost differential stems primarily from Iron-Air's use of abundant, low-cost materials—iron, water, and air—compared to vanadium's higher material costs and supply chain vulnerabilities. Recent market analyses indicate vanadium price volatility has increased VRFB costs by 15-30% in certain periods, while iron prices remain relatively stable.
Levelized cost of storage (LCOS) calculations further highlight Iron-Air's economic advantage for long-duration applications, with estimates ranging from $0.05-0.08/kWh for 100+ hour storage compared to VRFBs' $0.15-0.25/kWh for similar durations. However, this advantage diminishes for shorter duration applications where VRFBs' higher round-trip efficiency (75-85% versus Iron-Air's 45-55%) becomes more significant.
Operational economics reveal important distinctions. VRFBs offer superior cycling capabilities (20,000+ cycles) compared to Iron-Air batteries (approximately 5,000-10,000 cycles), though both technologies demonstrate excellent longevity potential. Maintenance costs favor VRFBs due to their simpler electrolyte management systems, while Iron-Air systems require more complex air-handling components that increase maintenance requirements by an estimated 20-30%.
Scaling economics present another critical dimension. Iron-Air batteries benefit from manufacturing processes adaptable from existing industries, potentially enabling faster cost reduction curves. Analysis of learning rates suggests Iron-Air could achieve 18-22% cost reduction per doubling of production capacity, compared to 12-15% for VRFBs.
Market sensitivity analysis indicates Iron-Air batteries are better positioned for grid-scale, long-duration applications where capital cost dominates decision-making. VRFBs maintain advantages in applications requiring frequent cycling, higher efficiency, and independent scaling of power and energy capacity. Recent pilot projects demonstrate Iron-Air systems achieving payback periods of 5-7 years in wholesale energy arbitrage applications, while VRFBs show stronger returns in frequency regulation and power quality applications with payback periods of 3-5 years.
The economic comparison ultimately reveals complementary rather than competing technologies, with optimal deployment scenarios determined by specific application requirements, duration needs, and local market conditions.
Environmental Impact and Sustainability Considerations
The environmental impact of energy storage technologies has become a critical consideration in the transition to sustainable energy systems. Iron-Air batteries and Vanadium Redox Flow batteries (VRFBs) present distinct environmental profiles that warrant thorough examination when evaluating their overall efficiency and sustainability.
Iron-Air batteries utilize earth-abundant materials, with iron being the fourth most common element in the Earth's crust. This abundance translates to lower environmental impact from mining operations compared to batteries requiring rare earth elements or precious metals. The manufacturing process for Iron-Air batteries generally involves lower energy consumption and produces fewer toxic byproducts than many alternative battery technologies. Additionally, these batteries demonstrate excellent recyclability, with iron components being nearly 100% recyclable at end-of-life.
Conversely, VRFBs rely on vanadium, which has a more complex environmental footprint. Vanadium mining and processing can generate significant environmental impacts, including habitat disruption, water pollution, and energy-intensive refining processes. However, the environmental advantage of VRFBs lies in their longevity and operational characteristics. The electrolyte in VRFBs does not degrade over time, potentially lasting for decades with proper maintenance, which significantly reduces lifecycle waste generation.
Water consumption represents another important environmental consideration. VRFBs require substantial amounts of water for electrolyte preparation and cooling systems, potentially straining water resources in water-scarce regions. Iron-Air batteries, by comparison, have minimal water requirements during operation, though their manufacturing process does involve water usage.
Carbon footprint analysis reveals that both technologies offer significant improvements over fossil fuel alternatives. Iron-Air batteries generally demonstrate lower embodied carbon during manufacturing, while VRFBs may achieve lower lifecycle emissions due to their exceptional longevity and reliability. The carbon intensity of electricity used during manufacturing and operation remains a critical factor for both technologies.
Safety considerations also factor into environmental impact assessments. Iron-Air batteries present minimal risk of toxic leakage or fire hazards, reducing potential environmental contamination risks. VRFBs contain acidic electrolytes that require proper containment systems to prevent environmental damage in case of leakage, though their non-flammable nature eliminates fire-related environmental risks.
Land use efficiency differs significantly between these technologies. VRFBs typically require larger physical footprints for equivalent energy storage capacity compared to Iron-Air batteries, potentially increasing habitat disruption and land transformation impacts when deployed at utility scale.
Iron-Air batteries utilize earth-abundant materials, with iron being the fourth most common element in the Earth's crust. This abundance translates to lower environmental impact from mining operations compared to batteries requiring rare earth elements or precious metals. The manufacturing process for Iron-Air batteries generally involves lower energy consumption and produces fewer toxic byproducts than many alternative battery technologies. Additionally, these batteries demonstrate excellent recyclability, with iron components being nearly 100% recyclable at end-of-life.
Conversely, VRFBs rely on vanadium, which has a more complex environmental footprint. Vanadium mining and processing can generate significant environmental impacts, including habitat disruption, water pollution, and energy-intensive refining processes. However, the environmental advantage of VRFBs lies in their longevity and operational characteristics. The electrolyte in VRFBs does not degrade over time, potentially lasting for decades with proper maintenance, which significantly reduces lifecycle waste generation.
Water consumption represents another important environmental consideration. VRFBs require substantial amounts of water for electrolyte preparation and cooling systems, potentially straining water resources in water-scarce regions. Iron-Air batteries, by comparison, have minimal water requirements during operation, though their manufacturing process does involve water usage.
Carbon footprint analysis reveals that both technologies offer significant improvements over fossil fuel alternatives. Iron-Air batteries generally demonstrate lower embodied carbon during manufacturing, while VRFBs may achieve lower lifecycle emissions due to their exceptional longevity and reliability. The carbon intensity of electricity used during manufacturing and operation remains a critical factor for both technologies.
Safety considerations also factor into environmental impact assessments. Iron-Air batteries present minimal risk of toxic leakage or fire hazards, reducing potential environmental contamination risks. VRFBs contain acidic electrolytes that require proper containment systems to prevent environmental damage in case of leakage, though their non-flammable nature eliminates fire-related environmental risks.
Land use efficiency differs significantly between these technologies. VRFBs typically require larger physical footprints for equivalent energy storage capacity compared to Iron-Air batteries, potentially increasing habitat disruption and land transformation impacts when deployed at utility scale.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!