Critical Analysis of Energy Input vs. Output in Electrolytic Cells
AUG 1, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Electrolytic Cell Energy Dynamics
Electrolytic cells are fundamental components in various industrial processes, particularly in the production of chemicals and metals. The energy dynamics within these cells play a crucial role in determining their efficiency and overall performance. At the core of electrolytic cell operation is the conversion of electrical energy into chemical energy, a process that involves complex interactions between electrodes, electrolytes, and the target substances.
The energy input in electrolytic cells primarily comes from an external power source, typically in the form of direct current (DC). This electrical energy is used to drive non-spontaneous redox reactions, overcoming the thermodynamic barriers that would otherwise prevent the desired chemical transformations. The magnitude of this energy input is a critical factor in determining the rate and extent of the electrolytic process.
On the output side, the energy manifests in various forms. The primary output is the chemical energy stored in the products of electrolysis. This can include the formation of new chemical bonds, the separation of elements from compounds, or the deposition of metals. Additionally, a portion of the input energy is inevitably lost as heat due to resistive losses in the electrolyte and electrodes, as well as through side reactions that do not contribute to the desired output.
The relationship between energy input and output in electrolytic cells is governed by fundamental principles of electrochemistry, including Faraday's laws of electrolysis. These laws establish the quantitative relationships between the amount of electrical charge passed through the cell and the amount of substance produced or consumed at the electrodes. However, the actual energy efficiency of the process is often less than 100% due to various factors such as overpotential, concentration gradients, and resistive losses.
Understanding and optimizing the energy dynamics in electrolytic cells is crucial for improving their efficiency and reducing operational costs. This involves careful consideration of factors such as electrode materials, electrolyte composition, cell design, and operating conditions. Advanced techniques like pulse electrolysis and the use of catalysts can significantly alter the energy landscape within the cell, potentially leading to improved energy utilization and product yield.
The analysis of energy input versus output in electrolytic cells is not only important for industrial applications but also for emerging technologies such as water electrolysis for hydrogen production and advanced battery systems. As global energy demands continue to rise and environmental concerns become more pressing, the development of more efficient electrolytic processes becomes increasingly critical. This necessitates a deep understanding of the intricate energy dynamics within these systems, paving the way for innovative solutions and technological advancements in the field of electrochemistry.
The energy input in electrolytic cells primarily comes from an external power source, typically in the form of direct current (DC). This electrical energy is used to drive non-spontaneous redox reactions, overcoming the thermodynamic barriers that would otherwise prevent the desired chemical transformations. The magnitude of this energy input is a critical factor in determining the rate and extent of the electrolytic process.
On the output side, the energy manifests in various forms. The primary output is the chemical energy stored in the products of electrolysis. This can include the formation of new chemical bonds, the separation of elements from compounds, or the deposition of metals. Additionally, a portion of the input energy is inevitably lost as heat due to resistive losses in the electrolyte and electrodes, as well as through side reactions that do not contribute to the desired output.
The relationship between energy input and output in electrolytic cells is governed by fundamental principles of electrochemistry, including Faraday's laws of electrolysis. These laws establish the quantitative relationships between the amount of electrical charge passed through the cell and the amount of substance produced or consumed at the electrodes. However, the actual energy efficiency of the process is often less than 100% due to various factors such as overpotential, concentration gradients, and resistive losses.
Understanding and optimizing the energy dynamics in electrolytic cells is crucial for improving their efficiency and reducing operational costs. This involves careful consideration of factors such as electrode materials, electrolyte composition, cell design, and operating conditions. Advanced techniques like pulse electrolysis and the use of catalysts can significantly alter the energy landscape within the cell, potentially leading to improved energy utilization and product yield.
The analysis of energy input versus output in electrolytic cells is not only important for industrial applications but also for emerging technologies such as water electrolysis for hydrogen production and advanced battery systems. As global energy demands continue to rise and environmental concerns become more pressing, the development of more efficient electrolytic processes becomes increasingly critical. This necessitates a deep understanding of the intricate energy dynamics within these systems, paving the way for innovative solutions and technological advancements in the field of electrochemistry.
Market Demand Analysis
The market demand for electrolytic cells and related technologies has been steadily growing, driven by several key factors in the global energy landscape. The increasing focus on renewable energy sources and the need for efficient energy storage solutions have propelled the electrolysis industry into the spotlight. As countries worldwide strive to reduce their carbon footprint and transition towards cleaner energy alternatives, the demand for electrolytic cells has surged, particularly in sectors such as hydrogen production, water treatment, and industrial chemical processes.
In the hydrogen production sector, electrolytic cells play a crucial role in green hydrogen generation, which is seen as a promising clean energy carrier. The global green hydrogen market is expected to expand significantly in the coming years, with projections indicating substantial growth rates. This expansion is fueled by government initiatives, corporate commitments to decarbonization, and the increasing adoption of hydrogen fuel cells in transportation and industrial applications.
The water treatment industry also contributes significantly to the market demand for electrolytic cells. As water scarcity becomes a pressing global issue, advanced water treatment technologies, including electrolytic processes, are gaining traction. Electrolytic cells are utilized in various water treatment applications, such as desalination, wastewater treatment, and the removal of contaminants from drinking water. The growing population and urbanization trends further amplify the need for efficient water treatment solutions, driving the demand for electrolytic technologies.
In the industrial sector, electrolytic cells find applications in numerous chemical processes, including chlor-alkali production, metal refining, and the synthesis of various chemicals. The chemical industry's continuous growth and the push for more sustainable production methods contribute to the increasing adoption of electrolytic technologies. Additionally, the rising demand for high-purity chemicals in electronics manufacturing and other specialized industries further bolsters the market for advanced electrolytic cell systems.
The market demand analysis also reveals a growing interest in improving the energy efficiency of electrolytic cells. As energy costs continue to be a significant factor in industrial operations, there is a strong emphasis on developing electrolytic systems with higher energy conversion efficiencies. This trend aligns with the broader industry focus on sustainability and cost reduction, driving research and development efforts towards more efficient electrolytic cell designs and materials.
Furthermore, the market shows a clear preference for scalable and flexible electrolytic cell technologies that can be integrated into existing industrial processes or renewable energy systems. This demand is particularly evident in the context of grid balancing and energy storage applications, where electrolytic cells can play a vital role in converting excess renewable energy into storable forms like hydrogen.
In the hydrogen production sector, electrolytic cells play a crucial role in green hydrogen generation, which is seen as a promising clean energy carrier. The global green hydrogen market is expected to expand significantly in the coming years, with projections indicating substantial growth rates. This expansion is fueled by government initiatives, corporate commitments to decarbonization, and the increasing adoption of hydrogen fuel cells in transportation and industrial applications.
The water treatment industry also contributes significantly to the market demand for electrolytic cells. As water scarcity becomes a pressing global issue, advanced water treatment technologies, including electrolytic processes, are gaining traction. Electrolytic cells are utilized in various water treatment applications, such as desalination, wastewater treatment, and the removal of contaminants from drinking water. The growing population and urbanization trends further amplify the need for efficient water treatment solutions, driving the demand for electrolytic technologies.
In the industrial sector, electrolytic cells find applications in numerous chemical processes, including chlor-alkali production, metal refining, and the synthesis of various chemicals. The chemical industry's continuous growth and the push for more sustainable production methods contribute to the increasing adoption of electrolytic technologies. Additionally, the rising demand for high-purity chemicals in electronics manufacturing and other specialized industries further bolsters the market for advanced electrolytic cell systems.
The market demand analysis also reveals a growing interest in improving the energy efficiency of electrolytic cells. As energy costs continue to be a significant factor in industrial operations, there is a strong emphasis on developing electrolytic systems with higher energy conversion efficiencies. This trend aligns with the broader industry focus on sustainability and cost reduction, driving research and development efforts towards more efficient electrolytic cell designs and materials.
Furthermore, the market shows a clear preference for scalable and flexible electrolytic cell technologies that can be integrated into existing industrial processes or renewable energy systems. This demand is particularly evident in the context of grid balancing and energy storage applications, where electrolytic cells can play a vital role in converting excess renewable energy into storable forms like hydrogen.
Technical Challenges
Electrolytic cells face several significant technical challenges that impact their energy efficiency and overall performance. One of the primary issues is the high energy input required for the electrolysis process, which often exceeds the energy output in the form of useful products. This energy imbalance is a major hurdle in making electrolytic processes economically viable and environmentally sustainable.
The electrode materials used in electrolytic cells present another critical challenge. These materials must withstand harsh chemical environments while maintaining high conductivity and catalytic activity. Degradation of electrodes over time leads to decreased efficiency and increased operational costs. Developing more durable and efficient electrode materials remains an ongoing research focus in the field.
Membrane technology is another area of concern in electrolytic cells. The selective permeability of membranes is crucial for maintaining separation between the anode and cathode compartments while allowing ion transport. However, current membrane materials often suffer from fouling, degradation, and insufficient selectivity, which can lead to reduced efficiency and product purity.
Heat management in electrolytic cells poses a significant technical challenge. The electrolysis process generates heat, which can lead to energy losses and potentially damage cell components if not properly managed. Developing effective cooling systems and heat recovery mechanisms is essential for improving overall energy efficiency.
The scaling up of electrolytic processes from laboratory to industrial scale introduces additional complexities. Maintaining uniform current distribution and electrolyte concentration across large-scale cells is challenging and can result in reduced efficiency and product quality. Engineering solutions to address these scale-up issues are crucial for the widespread adoption of electrolytic technologies.
Bubble formation and gas evolution at the electrodes present another set of challenges. These phenomena can lead to increased electrical resistance, reduced active electrode surface area, and potential safety hazards. Developing electrode designs and cell configurations that minimize these effects is an important area of research.
Finally, the intermittent nature of renewable energy sources poses challenges for integrating electrolytic processes into sustainable energy systems. Developing flexible and responsive electrolytic cells that can operate efficiently under variable power inputs is crucial for leveraging renewable energy in electrolytic applications.
Addressing these technical challenges requires interdisciplinary research efforts, combining expertise in electrochemistry, materials science, process engineering, and energy systems. Overcoming these hurdles is essential for improving the energy balance of electrolytic cells and realizing their full potential in various industrial and energy applications.
The electrode materials used in electrolytic cells present another critical challenge. These materials must withstand harsh chemical environments while maintaining high conductivity and catalytic activity. Degradation of electrodes over time leads to decreased efficiency and increased operational costs. Developing more durable and efficient electrode materials remains an ongoing research focus in the field.
Membrane technology is another area of concern in electrolytic cells. The selective permeability of membranes is crucial for maintaining separation between the anode and cathode compartments while allowing ion transport. However, current membrane materials often suffer from fouling, degradation, and insufficient selectivity, which can lead to reduced efficiency and product purity.
Heat management in electrolytic cells poses a significant technical challenge. The electrolysis process generates heat, which can lead to energy losses and potentially damage cell components if not properly managed. Developing effective cooling systems and heat recovery mechanisms is essential for improving overall energy efficiency.
The scaling up of electrolytic processes from laboratory to industrial scale introduces additional complexities. Maintaining uniform current distribution and electrolyte concentration across large-scale cells is challenging and can result in reduced efficiency and product quality. Engineering solutions to address these scale-up issues are crucial for the widespread adoption of electrolytic technologies.
Bubble formation and gas evolution at the electrodes present another set of challenges. These phenomena can lead to increased electrical resistance, reduced active electrode surface area, and potential safety hazards. Developing electrode designs and cell configurations that minimize these effects is an important area of research.
Finally, the intermittent nature of renewable energy sources poses challenges for integrating electrolytic processes into sustainable energy systems. Developing flexible and responsive electrolytic cells that can operate efficiently under variable power inputs is crucial for leveraging renewable energy in electrolytic applications.
Addressing these technical challenges requires interdisciplinary research efforts, combining expertise in electrochemistry, materials science, process engineering, and energy systems. Overcoming these hurdles is essential for improving the energy balance of electrolytic cells and realizing their full potential in various industrial and energy applications.
Current Energy Efficiency Solutions
01 Energy efficiency in electrolytic cells
Electrolytic cells are designed to optimize energy input versus output. This involves improving the efficiency of the electrochemical reactions, reducing energy losses, and enhancing overall system performance. Various techniques are employed to minimize energy consumption while maximizing the desired output, such as improved electrode materials, optimized cell design, and advanced control systems.- Energy efficiency in electrolytic cells: Electrolytic cells are designed to optimize energy input versus output. This involves improving the efficiency of the electrochemical reactions, reducing energy losses, and enhancing overall system performance. Various techniques are employed to minimize energy consumption while maximizing the desired output, such as improved electrode materials, optimized cell designs, and advanced control systems.
- Advanced electrode materials for improved energy balance: The development of advanced electrode materials plays a crucial role in enhancing the energy efficiency of electrolytic cells. These materials are designed to facilitate faster electron transfer, reduce overpotential, and increase the active surface area. By optimizing the electrode composition and structure, the energy input required for the electrolytic process can be significantly reduced while maintaining or improving the output.
- Energy recovery and regeneration systems: Implementing energy recovery and regeneration systems in electrolytic cells can significantly improve the overall energy balance. These systems capture and reuse waste heat or byproducts generated during the electrolytic process, converting them back into useful energy. This approach helps to reduce the net energy input required and increases the overall efficiency of the electrolytic cell.
- Optimization of cell design and operating conditions: The design of electrolytic cells and their operating conditions greatly influence the energy input-output relationship. Factors such as cell geometry, electrode spacing, electrolyte composition, and operating temperature are carefully optimized to minimize energy losses and maximize productivity. Advanced modeling and simulation techniques are used to predict and optimize cell performance under various conditions.
- Integration of renewable energy sources: To improve the overall energy balance of electrolytic processes, there is a growing trend towards integrating renewable energy sources. This approach involves coupling electrolytic cells with solar, wind, or other renewable energy systems to provide clean and sustainable power. By utilizing intermittent renewable energy, the net energy input from non-renewable sources can be reduced, improving the overall environmental impact and energy efficiency of the electrolytic process.
02 Novel electrode materials for enhanced performance
The development of advanced electrode materials plays a crucial role in improving the energy efficiency of electrolytic cells. These materials are designed to facilitate faster electron transfer, reduce overpotential, and increase the active surface area. Nanostructured electrodes, composite materials, and catalytic coatings are some examples of innovations in this field that contribute to better energy input-output ratios.Expand Specific Solutions03 Optimization of cell design and configuration
The physical design and configuration of electrolytic cells significantly impact their energy efficiency. This includes factors such as electrode spacing, electrolyte flow patterns, and heat management systems. Innovative cell designs aim to minimize internal resistance, improve mass transfer, and reduce energy losses due to heat generation, ultimately leading to a more favorable energy input-output relationship.Expand Specific Solutions04 Advanced control systems and process monitoring
Implementing sophisticated control systems and real-time process monitoring techniques helps optimize the energy input-output balance in electrolytic cells. These systems can adjust operating parameters dynamically, such as current density, temperature, and electrolyte composition, to maintain optimal conditions. Machine learning algorithms and predictive models are increasingly used to further enhance energy efficiency and process stability.Expand Specific Solutions05 Energy recovery and integration strategies
To improve the overall energy efficiency of electrolytic processes, various energy recovery and integration strategies are employed. These may include heat recovery systems, gas recirculation, and the integration of renewable energy sources. By capturing and reusing waste energy or incorporating intermittent renewable power, the net energy input can be reduced while maintaining or increasing the desired output.Expand Specific Solutions
Key Industry Players
The critical analysis of energy input vs. output in electrolytic cells is currently in a growth phase, with increasing market size driven by the global push for clean energy solutions. The technology's maturity varies across different applications, with some areas more advanced than others. Key players like Industrie De Nora SpA and Hysata Pty Ltd. are pushing the boundaries of efficiency in electrolysis, while established companies such as Siemens Energy and ThyssenKrupp Uhde Chlorine Engineers are leveraging their expertise to improve existing technologies. Research institutions like Huazhong University of Science & Technology and the University of Wollongong are contributing to fundamental advancements, indicating a dynamic and competitive landscape with potential for significant breakthroughs in energy efficiency and cost-effectiveness.
Industrie De Nora SpA
Technical Solution: De Nora specializes in electrode technologies and has applied this expertise to develop advanced electrolyzers. Their approach focuses on optimizing electrode materials and coatings to improve efficiency and durability. De Nora's alkaline electrolyzers achieve efficiencies up to 78% (LHV)[10]. The company has developed proprietary catalysts that reduce overpotential and improve energy efficiency. Their systems incorporate advanced gas separation technologies to ensure high purity hydrogen output. De Nora has also invested in developing chlor-alkali electrolysis technologies, which have synergies with water electrolysis for hydrogen production. Their electrolyzers feature modular designs for easy scaling and maintenance[11].
Strengths: Extensive expertise in electrode technology, high-durability systems, synergies with other electrolysis applications. Weaknesses: Lower efficiency compared to some newer technologies, primarily focused on traditional alkaline electrolysis.
Hysata Pty Ltd.
Technical Solution: Hysata has developed a novel capillary-fed electrolysis cell that achieves 98% system efficiency[1]. This breakthrough design eliminates bubble-related energy losses and enables operation at higher current densities. The cell architecture uses a vertically oriented electrolyzer with electrolyte flowing through capillary feeds, reducing gas bubble obstruction and improving overall energy efficiency. The company claims their technology can produce hydrogen for under $2/kg[2], significantly lower than conventional electrolyzers. Hysata's approach addresses key challenges in water electrolysis by optimizing cell geometry and fluid dynamics to minimize energy losses.
Strengths: Extremely high efficiency, potential for low-cost hydrogen production, innovative cell design. Weaknesses: Technology still in early stages, may face scaling challenges for industrial applications.
Innovative Electrode Materials
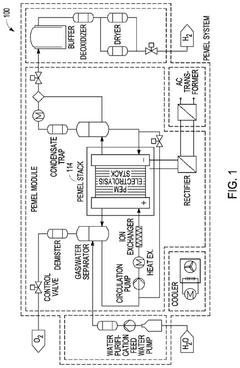
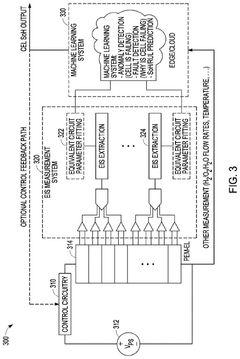
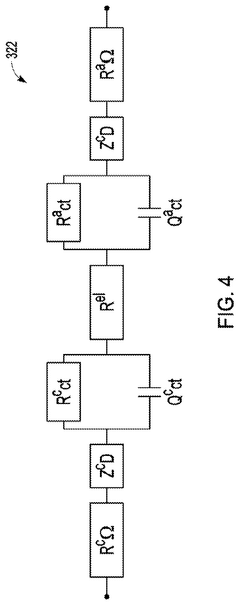
Extracting features for EIS monitoring systems
PatentPendingUS20240337034A1
Innovation
- The implementation of Electrochemical Impedance Spectroscopy (EIS) monitoring techniques to measure impedance variations in electrolyzer cells over time, using machine learning models to track changes and predict normal or abnormal operating conditions, and identify potential faults.
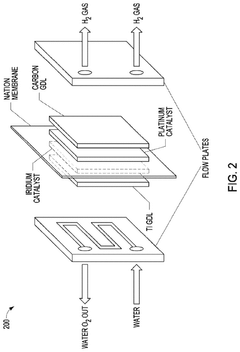
Electrolytic cell
PatentInactiveUS20120012456A1
Innovation
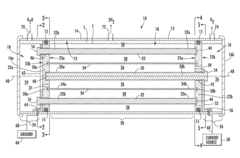
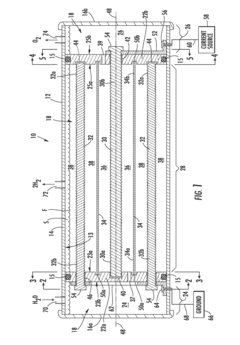
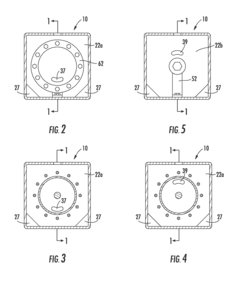
- An improved electrolytic cell design featuring a tubular housing with a novel electrode array, including a centrally disposed anode surrounded by incrementally spaced cathodes and a non-porous sheath that separates oxygen and hydrogen generation chambers, utilizing a unitary inductance-capacitance arrangement and electric pulse generation to enhance electrolysis efficiency.
Environmental Impact Assessment
The environmental impact of electrolytic cells, particularly in the context of energy input versus output, is a critical consideration in the broader discussion of sustainable technology and industrial processes. Electrolytic cells, while essential in various industrial applications, can have significant environmental implications that warrant careful assessment.
One of the primary environmental concerns associated with electrolytic cells is their energy consumption. These cells often require substantial electrical input to drive the desired chemical reactions, which can lead to increased demand for electricity generation. Depending on the source of this electricity, there may be indirect environmental impacts such as greenhouse gas emissions from fossil fuel power plants or land use changes for renewable energy infrastructure.
Water usage is another important factor to consider in the environmental impact assessment of electrolytic cells. Many electrolytic processes require large volumes of water, both as a reactant and for cooling purposes. This can put pressure on local water resources, particularly in water-stressed regions, and may lead to issues of water scarcity or competition with other essential uses.
The production of byproducts and waste materials from electrolytic processes also contributes to their environmental footprint. Some electrolytic reactions generate hazardous substances or gases that require careful handling and disposal. For instance, chlor-alkali electrolysis can produce chlorine gas, which poses risks to human health and the environment if not properly managed.
Furthermore, the materials used in the construction and operation of electrolytic cells can have upstream environmental impacts. The extraction and processing of metals for electrodes, membranes, and other components may involve resource-intensive mining operations and energy-intensive manufacturing processes.
However, it is important to note that the environmental impact of electrolytic cells is not uniformly negative. In many cases, these cells play a crucial role in producing materials and chemicals that enable other environmentally beneficial technologies. For example, electrolytic hydrogen production is seen as a key component in the transition to a low-carbon energy system, potentially offsetting emissions from fossil fuel use in various sectors.
The efficiency of electrolytic cells also plays a significant role in their overall environmental impact. Improvements in cell design, electrode materials, and process optimization can lead to reduced energy consumption and improved output, thereby minimizing the environmental footprint per unit of product. Ongoing research and development in this area hold promise for further enhancing the sustainability of electrolytic processes.
In conclusion, a comprehensive environmental impact assessment of electrolytic cells must consider the balance between their energy input requirements and useful output, as well as their broader implications for resource use, waste generation, and potential contributions to sustainable technologies. This holistic approach is essential for informed decision-making and the development of more environmentally friendly industrial processes.
One of the primary environmental concerns associated with electrolytic cells is their energy consumption. These cells often require substantial electrical input to drive the desired chemical reactions, which can lead to increased demand for electricity generation. Depending on the source of this electricity, there may be indirect environmental impacts such as greenhouse gas emissions from fossil fuel power plants or land use changes for renewable energy infrastructure.
Water usage is another important factor to consider in the environmental impact assessment of electrolytic cells. Many electrolytic processes require large volumes of water, both as a reactant and for cooling purposes. This can put pressure on local water resources, particularly in water-stressed regions, and may lead to issues of water scarcity or competition with other essential uses.
The production of byproducts and waste materials from electrolytic processes also contributes to their environmental footprint. Some electrolytic reactions generate hazardous substances or gases that require careful handling and disposal. For instance, chlor-alkali electrolysis can produce chlorine gas, which poses risks to human health and the environment if not properly managed.
Furthermore, the materials used in the construction and operation of electrolytic cells can have upstream environmental impacts. The extraction and processing of metals for electrodes, membranes, and other components may involve resource-intensive mining operations and energy-intensive manufacturing processes.
However, it is important to note that the environmental impact of electrolytic cells is not uniformly negative. In many cases, these cells play a crucial role in producing materials and chemicals that enable other environmentally beneficial technologies. For example, electrolytic hydrogen production is seen as a key component in the transition to a low-carbon energy system, potentially offsetting emissions from fossil fuel use in various sectors.
The efficiency of electrolytic cells also plays a significant role in their overall environmental impact. Improvements in cell design, electrode materials, and process optimization can lead to reduced energy consumption and improved output, thereby minimizing the environmental footprint per unit of product. Ongoing research and development in this area hold promise for further enhancing the sustainability of electrolytic processes.
In conclusion, a comprehensive environmental impact assessment of electrolytic cells must consider the balance between their energy input requirements and useful output, as well as their broader implications for resource use, waste generation, and potential contributions to sustainable technologies. This holistic approach is essential for informed decision-making and the development of more environmentally friendly industrial processes.
Economic Feasibility Study
The economic feasibility of electrolytic cells is a critical factor in determining their viability for large-scale implementation. This study examines the financial aspects of electrolytic cell technology, focusing on the balance between energy input and output. The capital costs associated with electrolytic cell systems are substantial, primarily due to the expensive materials required for electrodes and membranes. However, these costs have been decreasing over time as manufacturing processes improve and economies of scale are realized.
Operational costs are dominated by electricity consumption, which accounts for a significant portion of the total expenses. The efficiency of energy conversion in electrolytic cells is a key determinant of their economic viability. Current technologies typically achieve energy efficiencies ranging from 60% to 80%, depending on the specific application and operating conditions. Improving this efficiency is crucial for enhancing the economic feasibility of electrolytic cells.
The economic analysis must also consider the value of the products generated by electrolytic cells. In hydrogen production, for instance, the market price of hydrogen and its potential applications in various industries significantly impact the overall economic picture. The growing demand for clean energy solutions and the increasing focus on decarbonization are creating favorable market conditions for electrolytic cell technologies.
Government policies and incentives play a substantial role in the economic feasibility of electrolytic cells. Many countries are implementing supportive measures, such as tax credits, grants, and renewable energy mandates, which can significantly improve the financial outlook for these technologies. These incentives are often designed to bridge the gap between the current cost of electrolytic cell systems and conventional energy technologies.
The long-term economic viability of electrolytic cells is closely tied to advancements in renewable energy sources. As the cost of renewable electricity continues to decline, the operational costs of electrolytic cells are expected to decrease proportionally. This trend could lead to a more favorable energy input-output ratio, enhancing the overall economic feasibility of the technology.
Scalability is another crucial factor in the economic assessment. While small-scale electrolytic cell systems may struggle to achieve profitability, larger installations can benefit from economies of scale, reducing per-unit costs and improving overall economic performance. The potential for integration with existing industrial processes or energy systems can also enhance the economic attractiveness of electrolytic cell technologies.
Operational costs are dominated by electricity consumption, which accounts for a significant portion of the total expenses. The efficiency of energy conversion in electrolytic cells is a key determinant of their economic viability. Current technologies typically achieve energy efficiencies ranging from 60% to 80%, depending on the specific application and operating conditions. Improving this efficiency is crucial for enhancing the economic feasibility of electrolytic cells.
The economic analysis must also consider the value of the products generated by electrolytic cells. In hydrogen production, for instance, the market price of hydrogen and its potential applications in various industries significantly impact the overall economic picture. The growing demand for clean energy solutions and the increasing focus on decarbonization are creating favorable market conditions for electrolytic cell technologies.
Government policies and incentives play a substantial role in the economic feasibility of electrolytic cells. Many countries are implementing supportive measures, such as tax credits, grants, and renewable energy mandates, which can significantly improve the financial outlook for these technologies. These incentives are often designed to bridge the gap between the current cost of electrolytic cell systems and conventional energy technologies.
The long-term economic viability of electrolytic cells is closely tied to advancements in renewable energy sources. As the cost of renewable electricity continues to decline, the operational costs of electrolytic cells are expected to decrease proportionally. This trend could lead to a more favorable energy input-output ratio, enhancing the overall economic feasibility of the technology.
Scalability is another crucial factor in the economic assessment. While small-scale electrolytic cell systems may struggle to achieve profitability, larger installations can benefit from economies of scale, reducing per-unit costs and improving overall economic performance. The potential for integration with existing industrial processes or energy systems can also enhance the economic attractiveness of electrolytic cell technologies.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!