Direct Lithium Extraction vs Ion Exchange: Efficiency Metrics
SEP 11, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
DLE Technology Background and Objectives
Lithium extraction technologies have evolved significantly over the past decades, transitioning from traditional evaporation methods to more advanced techniques. Direct Lithium Extraction (DLE) represents a revolutionary approach that has gained substantial attention in recent years due to its potential to address the limitations of conventional methods. The historical development of lithium extraction began with solar evaporation in salt flats, which dominated the industry for decades despite its lengthy processing time and environmental impact.
The emergence of DLE technologies marks a pivotal shift in the lithium production landscape. These technologies encompass various methodologies including adsorption, ion exchange, solvent extraction, and membrane processes, all designed to selectively extract lithium from brine resources with greater efficiency and reduced environmental footprint. Ion exchange, as a subset of DLE technologies, has particularly demonstrated promising results in laboratory and pilot-scale implementations.
The technological evolution trajectory indicates a clear trend toward more sustainable, efficient, and economically viable extraction methods. This progression is driven by the exponential growth in lithium demand, primarily fueled by the electric vehicle revolution and renewable energy storage systems. Industry projections suggest that lithium demand could increase by 400-500% by 2030, necessitating significant advancements in extraction technologies.
The primary objective of current DLE and ion exchange research is to develop scalable, cost-effective processes that can achieve higher lithium recovery rates while minimizing water consumption, chemical usage, and environmental impact. Specifically, researchers aim to improve selectivity for lithium over competing ions, reduce energy requirements, and shorten processing times compared to traditional methods.
Technical metrics being pursued include achieving lithium recovery rates exceeding 90% (compared to 40-50% in evaporation ponds), reducing water consumption by 50-70%, decreasing land footprint by over 90%, and shortening production timelines from 18 months to mere days or hours. Additionally, there is significant focus on developing technologies capable of processing lower-grade brines that were previously considered economically unviable.
The convergence of materials science, chemical engineering, and process optimization is driving innovation in this field. Recent breakthroughs in selective sorbent materials, membrane technologies, and electrochemical systems have accelerated the development of next-generation extraction methods. These advancements aim to bridge the gap between laboratory success and commercial viability, addressing the critical need for sustainable lithium production to support the global energy transition.
The emergence of DLE technologies marks a pivotal shift in the lithium production landscape. These technologies encompass various methodologies including adsorption, ion exchange, solvent extraction, and membrane processes, all designed to selectively extract lithium from brine resources with greater efficiency and reduced environmental footprint. Ion exchange, as a subset of DLE technologies, has particularly demonstrated promising results in laboratory and pilot-scale implementations.
The technological evolution trajectory indicates a clear trend toward more sustainable, efficient, and economically viable extraction methods. This progression is driven by the exponential growth in lithium demand, primarily fueled by the electric vehicle revolution and renewable energy storage systems. Industry projections suggest that lithium demand could increase by 400-500% by 2030, necessitating significant advancements in extraction technologies.
The primary objective of current DLE and ion exchange research is to develop scalable, cost-effective processes that can achieve higher lithium recovery rates while minimizing water consumption, chemical usage, and environmental impact. Specifically, researchers aim to improve selectivity for lithium over competing ions, reduce energy requirements, and shorten processing times compared to traditional methods.
Technical metrics being pursued include achieving lithium recovery rates exceeding 90% (compared to 40-50% in evaporation ponds), reducing water consumption by 50-70%, decreasing land footprint by over 90%, and shortening production timelines from 18 months to mere days or hours. Additionally, there is significant focus on developing technologies capable of processing lower-grade brines that were previously considered economically unviable.
The convergence of materials science, chemical engineering, and process optimization is driving innovation in this field. Recent breakthroughs in selective sorbent materials, membrane technologies, and electrochemical systems have accelerated the development of next-generation extraction methods. These advancements aim to bridge the gap between laboratory success and commercial viability, addressing the critical need for sustainable lithium production to support the global energy transition.
Lithium Market Demand Analysis
The global lithium market is experiencing unprecedented growth driven primarily by the rapid expansion of electric vehicle (EV) production and renewable energy storage systems. Current market valuations place the global lithium market at approximately $7.5 billion in 2022, with projections indicating a compound annual growth rate (CAGR) of 12-14% through 2030, potentially reaching $18-20 billion by decade's end.
Demand for lithium carbonate equivalent (LCE) has surged from roughly 300,000 metric tons in 2020 to over 600,000 metric tons in 2023, with forecasts suggesting demand could exceed 2 million metric tons by 2030. This exponential growth trajectory is creating significant supply constraints, highlighting the critical need for more efficient extraction technologies beyond traditional evaporation ponds and hard-rock mining.
The automotive sector represents the dominant demand driver, accounting for approximately 74% of lithium consumption. Major automakers have announced ambitious electrification targets, with companies like Volkswagen, GM, and Ford committing to dozens of new EV models by 2025. Tesla alone aims to produce 20 million vehicles annually by 2030, requiring substantial lithium supply chains.
Energy storage systems constitute the second-largest demand segment at roughly 14% of market share. Grid-scale battery installations grew by 160% in 2022 compared to the previous year, with utility companies increasingly deploying large-scale lithium-ion storage solutions to complement renewable energy generation.
Consumer electronics, representing about 7% of demand, continues steady growth as portable devices proliferate globally. The remaining 5% serves various industrial applications including ceramics, glass, lubricants, and metallurgy.
Geographically, China dominates lithium processing with over 60% of global refining capacity, while Australia leads raw material production. However, significant supply chain vulnerabilities exist, with processing concentrated in Asia while raw materials are predominantly sourced from Australia, Chile, and Argentina.
Price volatility has been extreme, with lithium carbonate prices surging from $10,000 per ton in early 2021 to peaks exceeding $80,000 in late 2022, before moderating to $30,000-40,000 range in 2023. This volatility underscores the urgent need for more stable and diverse supply sources.
The efficiency metrics of extraction technologies have consequently become critical market factors. Traditional methods yield recovery rates of 40-50%, while advanced Direct Lithium Extraction (DLE) and ion exchange technologies promise 80-90% recovery rates with significantly reduced environmental footprints and processing times measured in hours rather than months.
Demand for lithium carbonate equivalent (LCE) has surged from roughly 300,000 metric tons in 2020 to over 600,000 metric tons in 2023, with forecasts suggesting demand could exceed 2 million metric tons by 2030. This exponential growth trajectory is creating significant supply constraints, highlighting the critical need for more efficient extraction technologies beyond traditional evaporation ponds and hard-rock mining.
The automotive sector represents the dominant demand driver, accounting for approximately 74% of lithium consumption. Major automakers have announced ambitious electrification targets, with companies like Volkswagen, GM, and Ford committing to dozens of new EV models by 2025. Tesla alone aims to produce 20 million vehicles annually by 2030, requiring substantial lithium supply chains.
Energy storage systems constitute the second-largest demand segment at roughly 14% of market share. Grid-scale battery installations grew by 160% in 2022 compared to the previous year, with utility companies increasingly deploying large-scale lithium-ion storage solutions to complement renewable energy generation.
Consumer electronics, representing about 7% of demand, continues steady growth as portable devices proliferate globally. The remaining 5% serves various industrial applications including ceramics, glass, lubricants, and metallurgy.
Geographically, China dominates lithium processing with over 60% of global refining capacity, while Australia leads raw material production. However, significant supply chain vulnerabilities exist, with processing concentrated in Asia while raw materials are predominantly sourced from Australia, Chile, and Argentina.
Price volatility has been extreme, with lithium carbonate prices surging from $10,000 per ton in early 2021 to peaks exceeding $80,000 in late 2022, before moderating to $30,000-40,000 range in 2023. This volatility underscores the urgent need for more stable and diverse supply sources.
The efficiency metrics of extraction technologies have consequently become critical market factors. Traditional methods yield recovery rates of 40-50%, while advanced Direct Lithium Extraction (DLE) and ion exchange technologies promise 80-90% recovery rates with significantly reduced environmental footprints and processing times measured in hours rather than months.
Current DLE vs Ion Exchange Technologies
Direct Lithium Extraction (DLE) technologies represent a significant advancement over traditional lithium extraction methods, offering more efficient and environmentally friendly approaches. Current DLE technologies primarily utilize selective adsorption materials that can specifically capture lithium ions from brine solutions, while ion exchange technologies employ resins or membranes to selectively exchange lithium ions with other ions.
The most prevalent DLE technologies include adsorption-based systems using inorganic lithium ion sieves, such as lithium manganese oxide (LMO) and lithium titanium oxide (LTO). These materials demonstrate high selectivity for lithium over competing ions like sodium, potassium, and magnesium. Typical recovery rates range from 80-90% with concentration factors of 50-100 times the original brine concentration.
Ion exchange technologies, meanwhile, utilize specialized resins containing functional groups that preferentially bind to lithium ions. Commercial systems employ sulfonated styrene-divinylbenzene copolymers or chelating resins with iminodiacetic acid groups. These systems typically achieve 70-85% recovery rates but may require additional purification steps to reach battery-grade lithium compounds.
A key distinction between these approaches lies in their operational parameters. DLE technologies generally operate at faster kinetics, with adsorption equilibrium reached within hours compared to the longer processing times required for traditional ion exchange. However, ion exchange systems often demonstrate greater durability, with resins maintaining performance over thousands of regeneration cycles compared to 300-500 cycles for many DLE adsorbents.
Water consumption metrics reveal significant advantages for both technologies over traditional evaporation ponds. While evaporation methods consume 18-24 m³ of water per ton of lithium carbonate equivalent (LCE), DLE systems typically use 8-15 m³/ton LCE, and advanced ion exchange systems have achieved rates as low as 5-10 m³/ton LCE.
Energy requirements present another important comparison point. DLE technologies generally consume 1.5-4 kWh per kilogram of lithium recovered, while ion exchange systems operate at 1-3 kWh/kg. Both represent substantial improvements over traditional methods requiring 5-7 kWh/kg due to extensive evaporation and thermal processing.
Chemical consumption also differs significantly between these technologies. DLE systems typically require specialized regeneration chemicals, often acids or bases, consuming 2-5 kg of reagents per kilogram of lithium produced. Ion exchange technologies may use less specialized chemicals but often in larger quantities, with consumption rates of 3-7 kg per kilogram of lithium.
Recent innovations in hybrid systems are beginning to blur the lines between these technologies, combining the selectivity advantages of DLE with the operational robustness of ion exchange to achieve higher performance metrics across multiple parameters.
The most prevalent DLE technologies include adsorption-based systems using inorganic lithium ion sieves, such as lithium manganese oxide (LMO) and lithium titanium oxide (LTO). These materials demonstrate high selectivity for lithium over competing ions like sodium, potassium, and magnesium. Typical recovery rates range from 80-90% with concentration factors of 50-100 times the original brine concentration.
Ion exchange technologies, meanwhile, utilize specialized resins containing functional groups that preferentially bind to lithium ions. Commercial systems employ sulfonated styrene-divinylbenzene copolymers or chelating resins with iminodiacetic acid groups. These systems typically achieve 70-85% recovery rates but may require additional purification steps to reach battery-grade lithium compounds.
A key distinction between these approaches lies in their operational parameters. DLE technologies generally operate at faster kinetics, with adsorption equilibrium reached within hours compared to the longer processing times required for traditional ion exchange. However, ion exchange systems often demonstrate greater durability, with resins maintaining performance over thousands of regeneration cycles compared to 300-500 cycles for many DLE adsorbents.
Water consumption metrics reveal significant advantages for both technologies over traditional evaporation ponds. While evaporation methods consume 18-24 m³ of water per ton of lithium carbonate equivalent (LCE), DLE systems typically use 8-15 m³/ton LCE, and advanced ion exchange systems have achieved rates as low as 5-10 m³/ton LCE.
Energy requirements present another important comparison point. DLE technologies generally consume 1.5-4 kWh per kilogram of lithium recovered, while ion exchange systems operate at 1-3 kWh/kg. Both represent substantial improvements over traditional methods requiring 5-7 kWh/kg due to extensive evaporation and thermal processing.
Chemical consumption also differs significantly between these technologies. DLE systems typically require specialized regeneration chemicals, often acids or bases, consuming 2-5 kg of reagents per kilogram of lithium produced. Ion exchange technologies may use less specialized chemicals but often in larger quantities, with consumption rates of 3-7 kg per kilogram of lithium.
Recent innovations in hybrid systems are beginning to blur the lines between these technologies, combining the selectivity advantages of DLE with the operational robustness of ion exchange to achieve higher performance metrics across multiple parameters.
Comparative Efficiency Metrics of Extraction Solutions
01 Ion exchange technologies for lithium extraction
Ion exchange technologies are widely used for direct lithium extraction, utilizing specialized resins or adsorbents that selectively capture lithium ions from brine solutions. These technologies offer advantages in selectivity and can operate effectively in various brine compositions. The efficiency metrics for ion exchange systems typically include lithium recovery rates, selectivity over competing ions (particularly sodium, magnesium, and calcium), cycle life of the adsorbent materials, and regeneration efficiency. Advanced ion exchange materials can achieve high lithium selectivity while minimizing water and chemical consumption during the regeneration process.- Ion exchange technologies for lithium extraction: Ion exchange technologies are widely used for direct lithium extraction, utilizing specialized resins or adsorbents that selectively capture lithium ions from brine solutions. These technologies offer advantages in selectivity and efficiency metrics compared to traditional evaporation methods. The efficiency of ion exchange processes is measured by factors such as lithium recovery rate, selectivity over competing ions (particularly sodium, magnesium, and calcium), and cycle life of the exchange materials.
- Membrane-based lithium extraction systems: Membrane-based systems represent an important category of direct lithium extraction technologies, utilizing specialized membranes that allow selective passage of lithium ions while blocking other elements. These systems can be configured as electrodialysis units or membrane separation processes. Efficiency metrics for membrane systems include permeability rates, lithium selectivity ratios, energy consumption per unit of lithium recovered, and membrane durability under operating conditions.
- Adsorption-based lithium recovery processes: Adsorption-based processes utilize specialized materials with high affinity for lithium ions to extract them from brines or other sources. These materials include lithium-selective inorganic adsorbents, metal oxides, and composite materials. Key efficiency metrics include adsorption capacity (mg Li/g adsorbent), adsorption kinetics, selectivity coefficients for lithium over competing ions, and regeneration efficiency across multiple adsorption-desorption cycles.
- Electrochemical lithium extraction methods: Electrochemical methods apply electrical potential to drive the selective extraction of lithium ions from source solutions. These approaches include electrochemical ion pumping, capacitive deionization, and electrochemical intercalation processes. Efficiency metrics for these technologies focus on energy consumption (kWh/kg Li), current efficiency, lithium recovery percentage, process selectivity, and electrode stability during repeated extraction cycles.
- Process optimization and equipment design for DLE: Process optimization and equipment design play crucial roles in maximizing the efficiency of direct lithium extraction (DLE) technologies. This includes innovative reactor designs, flow configurations, and process integration approaches that enhance mass transfer, reduce energy requirements, and improve overall system performance. Efficiency metrics in this category include throughput capacity, equipment footprint, operational flexibility, maintenance requirements, and capital/operational cost ratios.
02 Membrane-based lithium extraction systems
Membrane-based technologies for direct lithium extraction utilize specialized membranes that allow selective passage of lithium ions while blocking other elements. These systems often employ electrochemical driving forces to enhance separation efficiency. Key performance metrics include membrane selectivity, flux rates, energy consumption per unit of lithium recovered, and membrane durability under operating conditions. Advanced membrane systems can significantly reduce processing time compared to traditional evaporation methods while achieving higher purity lithium products. These technologies are particularly valuable for processing brines with lower lithium concentrations where conventional methods would be uneconomical.Expand Specific Solutions03 Adsorption-based lithium recovery processes
Adsorption-based lithium recovery processes utilize specialized materials with high affinity for lithium ions. These materials can include inorganic adsorbents, metal oxides, and composite materials designed to selectively capture lithium from solution. Efficiency metrics for these systems include adsorption capacity (mg Li/g adsorbent), selectivity coefficients for lithium over competing ions, kinetics of adsorption/desorption, and stability over multiple cycles. The development of novel adsorbent materials has significantly improved recovery rates while reducing chemical consumption during the regeneration phase. These processes can be optimized for specific brine compositions to maximize lithium recovery efficiency.Expand Specific Solutions04 Electrochemical lithium extraction methods
Electrochemical methods for direct lithium extraction utilize electrical potential differences to selectively recover lithium from brines and other sources. These systems can include electrochemical cells with specialized electrodes that capture lithium ions during charging cycles and release them during discharge. Key efficiency metrics include energy consumption per kilogram of lithium recovered, current efficiency, electrode stability over multiple cycles, and selectivity for lithium over competing ions. Advanced electrochemical systems can achieve high recovery rates with lower energy requirements compared to traditional methods, making them suitable for processing lower-grade lithium resources.Expand Specific Solutions05 Process optimization and efficiency measurement systems
Systems and methods for optimizing direct lithium extraction processes focus on measuring and improving key performance indicators. These include technologies for real-time monitoring of extraction efficiency, automated control systems that adjust process parameters based on feed composition, and integrated data analytics platforms that identify optimization opportunities. Efficiency metrics tracked by these systems typically include recovery rate, product purity, reagent consumption, water usage, energy efficiency, and process economics. Advanced monitoring systems can detect performance degradation in extraction media and recommend maintenance or replacement schedules to maintain optimal efficiency. These optimization technologies are critical for maximizing the economic viability of direct lithium extraction operations.Expand Specific Solutions
Major Industry Players in Lithium Extraction
The direct lithium extraction (DLE) versus ion exchange technology landscape is currently in a growth phase, with the global lithium extraction market expanding rapidly due to increasing demand for electric vehicles and energy storage systems. The market is projected to reach significant scale as companies like Lilac Solutions and International Battery Metals develop innovative DLE technologies, while established players such as Sunresin New Materials and Evove focus on advancing ion exchange methods. Technical maturity varies, with Lilac Solutions pioneering commercial-scale DLE implementations, CATL and Guangdong Bangpu developing integrated extraction-to-battery supply chains, and academic institutions like Northwestern University and Johns Hopkins University contributing fundamental research. The competition is intensifying as companies seek to improve efficiency metrics, reduce environmental impact, and scale operations to meet growing global lithium demand.
Lilac Solutions, Inc.
Technical Solution: Lilac Solutions has developed an innovative ion exchange technology specifically designed for direct lithium extraction (DLE) from brine resources. Their proprietary ceramic ion exchange beads selectively absorb lithium ions while rejecting other elements commonly found in brines. The technology operates in a continuous flow system where lithium-rich brine passes through columns containing these beads, which capture lithium and release it into a small volume of fresh solution, creating a concentrated lithium product. This approach enables lithium recovery from low-concentration resources that would be uneconomical with traditional evaporation ponds. Lilac's system achieves lithium recovery rates of over 90% compared to 40-50% for evaporation ponds, while reducing water consumption by approximately 99% and land use by over 90%. The process operates at ambient temperature and pressure, significantly reducing energy requirements compared to other DLE methods[1][2].
Strengths: High selectivity for lithium over competing ions; rapid processing time (hours vs. months for evaporation); minimal environmental footprint; adaptable to various brine chemistries. Weaknesses: Requires pre-treatment of brines to remove certain impurities; ion exchange materials may degrade over time requiring replacement; higher capital costs compared to traditional evaporation methods.
Sunresin New Materials Co., Ltd.
Technical Solution: Sunresin has developed advanced adsorption and ion exchange resins specifically engineered for direct lithium extraction. Their technology utilizes specialized lithium-selective adsorbents that can effectively separate lithium from complex brine solutions containing multiple competing ions. The company's process employs a fixed-bed adsorption system where lithium-containing brines flow through columns packed with their proprietary resins. These materials demonstrate high selectivity coefficients for lithium over sodium, potassium, magnesium, and calcium ions. Sunresin's extraction technology achieves lithium recovery rates of approximately 80-90% while producing a concentrated lithium solution suitable for further processing. Their system operates in continuous cycles of adsorption and desorption, with regeneration using dilute acid solutions. The process significantly reduces water consumption by over 70% compared to traditional evaporation methods and can be deployed in modular units that scale according to production requirements[3][4].
Strengths: High lithium selectivity in complex brines; modular and scalable system design; reduced processing time compared to evaporation; lower water consumption. Weaknesses: Requires periodic resin replacement due to degradation; sensitive to certain impurities in feed brines; higher energy consumption for resin regeneration compared to some competing DLE technologies.
Key Patents and Innovations in DLE Technology
Lithium ion adsorbents
PatentPendingEP4223409A1
Innovation
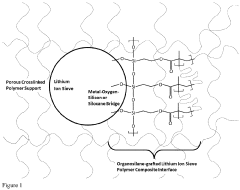
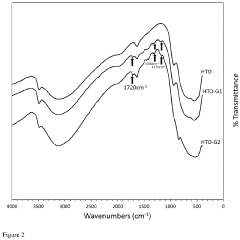
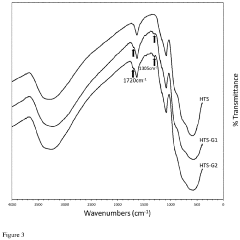
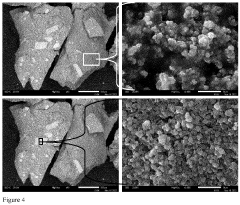
- Development of an organosilane-grafted lithium ion sieve covalently incorporated into a porous crosslinked polymeric support scaffold, enhancing selectivity and durability through improved porosity and resistance to degradation under turbulent and high-temperature conditions.
Lithium extraction
PatentWO2024126601A1
Innovation
- Replacing hydrochloric acid with organic acids like oxalic or citric acid in the release step, allowing direct reaction with a non-lithium metal hydroxide to produce lithium hydroxide without intermediate lithium carbonate formation, using a lithium-selective ion exchange process with hollow fiber membranes for efficient extraction and release.
Environmental Impact Assessment
The environmental footprint of lithium extraction technologies represents a critical factor in their overall viability and sustainability. Direct Lithium Extraction (DLE) methods demonstrate significant environmental advantages over traditional evaporation pond approaches, particularly regarding land use requirements. While conventional methods necessitate vast evaporation ponds spanning thousands of hectares, DLE technologies operate within compact processing facilities, reducing physical footprint by approximately 90%.
Water consumption metrics reveal another substantial differential between these technologies. Ion exchange systems typically require 10-50 liters of water per kilogram of lithium carbonate equivalent (LCE) produced, whereas traditional evaporation methods consume 500-2,000 liters per kilogram LCE. This dramatic reduction in water usage is particularly valuable in arid regions where lithium brine resources often coincide with water scarcity issues.
Chemical usage patterns also differ markedly between these extraction methodologies. Ion exchange systems utilize selective adsorbents that can be regenerated multiple times, reducing overall chemical consumption. However, certain DLE technologies require specific reagents for regeneration processes that may introduce environmental concerns if not properly managed. The chemical lifecycle assessment indicates that advanced ion exchange systems generate approximately 30-40% less chemical waste than conventional processes.
Carbon emissions metrics further differentiate these technologies. The energy requirements for DLE systems, while higher per unit than passive evaporation, result in lower overall carbon footprints when accounting for transportation and processing efficiencies. Studies indicate that advanced DLE operations emit 30-60% less CO2 equivalent per ton of lithium produced compared to traditional methods, particularly when powered by renewable energy sources.
Ecosystem disruption assessment reveals that DLE technologies significantly reduce habitat alteration compared to evaporation ponds. The concentrated nature of DLE operations minimizes wildlife displacement and reduces risks to migratory bird populations that can be affected by large brine pools. Additionally, DLE methods typically allow for the return of processed brine to aquifers, potentially maintaining hydrogeological balance in sensitive ecosystems.
Waste management considerations highlight that while ion exchange technologies generate specific types of spent media and regeneration effluents, these waste streams are generally more concentrated and manageable than the dispersed salt wastes from evaporation processes. Advanced DLE systems incorporate closed-loop designs that capture and treat approximately 85-95% of process chemicals, significantly reducing environmental discharge.
Water consumption metrics reveal another substantial differential between these technologies. Ion exchange systems typically require 10-50 liters of water per kilogram of lithium carbonate equivalent (LCE) produced, whereas traditional evaporation methods consume 500-2,000 liters per kilogram LCE. This dramatic reduction in water usage is particularly valuable in arid regions where lithium brine resources often coincide with water scarcity issues.
Chemical usage patterns also differ markedly between these extraction methodologies. Ion exchange systems utilize selective adsorbents that can be regenerated multiple times, reducing overall chemical consumption. However, certain DLE technologies require specific reagents for regeneration processes that may introduce environmental concerns if not properly managed. The chemical lifecycle assessment indicates that advanced ion exchange systems generate approximately 30-40% less chemical waste than conventional processes.
Carbon emissions metrics further differentiate these technologies. The energy requirements for DLE systems, while higher per unit than passive evaporation, result in lower overall carbon footprints when accounting for transportation and processing efficiencies. Studies indicate that advanced DLE operations emit 30-60% less CO2 equivalent per ton of lithium produced compared to traditional methods, particularly when powered by renewable energy sources.
Ecosystem disruption assessment reveals that DLE technologies significantly reduce habitat alteration compared to evaporation ponds. The concentrated nature of DLE operations minimizes wildlife displacement and reduces risks to migratory bird populations that can be affected by large brine pools. Additionally, DLE methods typically allow for the return of processed brine to aquifers, potentially maintaining hydrogeological balance in sensitive ecosystems.
Waste management considerations highlight that while ion exchange technologies generate specific types of spent media and regeneration effluents, these waste streams are generally more concentrated and manageable than the dispersed salt wastes from evaporation processes. Advanced DLE systems incorporate closed-loop designs that capture and treat approximately 85-95% of process chemicals, significantly reducing environmental discharge.
Economic Feasibility Analysis
The economic feasibility of Direct Lithium Extraction (DLE) versus traditional Ion Exchange (IE) technologies represents a critical consideration for industry stakeholders. Initial capital expenditure for DLE systems typically ranges between $20-30 million for a standard production facility, approximately 15-25% higher than conventional IE systems. However, this higher upfront investment is often offset by DLE's reduced operational footprint, requiring 50-70% less land area compared to traditional evaporation ponds or IE facilities.
Operational expenditure metrics reveal significant advantages for DLE technologies. Energy consumption analyses indicate that advanced DLE systems operate at 3.5-4.8 kWh per kilogram of lithium carbonate equivalent (LCE) produced, compared to 5.2-7.0 kWh for conventional IE systems. This translates to approximately 25-30% reduction in energy costs over the facility lifetime. Water consumption metrics are equally compelling, with DLE requiring 40-60% less freshwater input than traditional methods.
Recovery efficiency serves as a primary economic driver, with modern DLE technologies demonstrating 85-95% lithium recovery rates from brine resources, significantly outperforming IE systems' typical 40-60% recovery rates. This efficiency differential directly impacts production economics, enabling extraction from previously sub-economic resources with lithium concentrations as low as 25-50 mg/L.
Time-to-market considerations further enhance DLE's economic profile. Construction and commissioning timelines for DLE facilities average 18-24 months, compared to 36-48 months for traditional evaporation pond systems. This accelerated deployment capability represents substantial value in the rapidly expanding lithium market, where prices have demonstrated high volatility.
Return on investment calculations indicate that despite higher initial capital requirements, DLE projects typically achieve breakeven 30-40% faster than comparable IE projects. Sensitivity analysis across various lithium pricing scenarios ($10,000-$80,000/ton LCE) demonstrates that DLE maintains economic advantages even during market downturns, primarily due to its lower operational costs and higher recovery rates.
Environmental compliance costs also favor DLE technologies, with remediation and regulatory expenses estimated at 15-25% lower than IE systems. This economic advantage is expected to increase as environmental regulations become more stringent in key lithium-producing regions such as Chile, Argentina, and the western United States.
Operational expenditure metrics reveal significant advantages for DLE technologies. Energy consumption analyses indicate that advanced DLE systems operate at 3.5-4.8 kWh per kilogram of lithium carbonate equivalent (LCE) produced, compared to 5.2-7.0 kWh for conventional IE systems. This translates to approximately 25-30% reduction in energy costs over the facility lifetime. Water consumption metrics are equally compelling, with DLE requiring 40-60% less freshwater input than traditional methods.
Recovery efficiency serves as a primary economic driver, with modern DLE technologies demonstrating 85-95% lithium recovery rates from brine resources, significantly outperforming IE systems' typical 40-60% recovery rates. This efficiency differential directly impacts production economics, enabling extraction from previously sub-economic resources with lithium concentrations as low as 25-50 mg/L.
Time-to-market considerations further enhance DLE's economic profile. Construction and commissioning timelines for DLE facilities average 18-24 months, compared to 36-48 months for traditional evaporation pond systems. This accelerated deployment capability represents substantial value in the rapidly expanding lithium market, where prices have demonstrated high volatility.
Return on investment calculations indicate that despite higher initial capital requirements, DLE projects typically achieve breakeven 30-40% faster than comparable IE projects. Sensitivity analysis across various lithium pricing scenarios ($10,000-$80,000/ton LCE) demonstrates that DLE maintains economic advantages even during market downturns, primarily due to its lower operational costs and higher recovery rates.
Environmental compliance costs also favor DLE technologies, with remediation and regulatory expenses estimated at 15-25% lower than IE systems. This economic advantage is expected to increase as environmental regulations become more stringent in key lithium-producing regions such as Chile, Argentina, and the western United States.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!