Economic analysis of OoC adoption in pharmaceutical R&D: cost, time and attrition impacts
SEP 2, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
OoC Technology Background and Objectives
Organ-on-Chip (OoC) technology represents a revolutionary approach in biomedical research, emerging from the convergence of tissue engineering, microfluidics, and microfabrication techniques. Since its conceptual development in the early 2000s, OoC has evolved from simple cell culture systems to sophisticated microdevices capable of mimicking the physiological functions of human organs. This evolution has been marked by significant milestones, including the development of the first lung-on-chip by the Wyss Institute in 2010, which demonstrated the potential to recreate complex organ-level functions.
The technological trajectory of OoC has been characterized by increasing complexity and functionality, moving from single-organ systems to multi-organ platforms that can simulate organ interactions. Recent advancements have focused on integrating sensors for real-time monitoring, improving cell viability, and enhancing the physiological relevance of these models. The field is now trending toward the development of personalized OoC systems using patient-derived cells, potentially revolutionizing precision medicine approaches.
The primary objective of OoC technology in pharmaceutical R&D is to provide more predictive models of human physiology than traditional in vitro cell cultures and animal testing. By recreating the dynamic microenvironment of human organs, OoC aims to bridge the translational gap between preclinical studies and clinical outcomes, potentially reducing the high attrition rates observed in drug development pipelines.
Economically, OoC technology targets the reduction of pharmaceutical R&D costs, which have been escalating dramatically over the past decades. The average cost to bring a new drug to market now exceeds $2.6 billion, with a significant portion attributed to late-stage clinical failures. By providing more predictive human-relevant data earlier in the development process, OoC aims to enable better candidate selection and reduce costly late-phase attrition.
Time efficiency represents another critical objective of OoC adoption. The current drug development timeline spans 10-15 years on average, creating substantial opportunity costs for pharmaceutical companies. OoC technology promises to accelerate this process by enabling parallel testing of multiple compounds and providing faster feedback on drug efficacy and toxicity profiles.
Additionally, OoC technology addresses the growing ethical concerns and regulatory pressures surrounding animal testing. With initiatives like the 3Rs (Replacement, Reduction, Refinement) gaining momentum globally, pharmaceutical companies are increasingly seeking alternative testing methods that can reduce reliance on animal models while maintaining or improving predictive capacity.
The ultimate goal of OoC implementation is to establish a new paradigm in drug development that is more efficient, cost-effective, and ethically sound, while simultaneously improving the success rate of new therapeutics reaching patients. This represents a significant shift from traditional R&D approaches and aligns with the industry's movement toward more human-relevant, mechanism-based drug discovery and development methodologies.
The technological trajectory of OoC has been characterized by increasing complexity and functionality, moving from single-organ systems to multi-organ platforms that can simulate organ interactions. Recent advancements have focused on integrating sensors for real-time monitoring, improving cell viability, and enhancing the physiological relevance of these models. The field is now trending toward the development of personalized OoC systems using patient-derived cells, potentially revolutionizing precision medicine approaches.
The primary objective of OoC technology in pharmaceutical R&D is to provide more predictive models of human physiology than traditional in vitro cell cultures and animal testing. By recreating the dynamic microenvironment of human organs, OoC aims to bridge the translational gap between preclinical studies and clinical outcomes, potentially reducing the high attrition rates observed in drug development pipelines.
Economically, OoC technology targets the reduction of pharmaceutical R&D costs, which have been escalating dramatically over the past decades. The average cost to bring a new drug to market now exceeds $2.6 billion, with a significant portion attributed to late-stage clinical failures. By providing more predictive human-relevant data earlier in the development process, OoC aims to enable better candidate selection and reduce costly late-phase attrition.
Time efficiency represents another critical objective of OoC adoption. The current drug development timeline spans 10-15 years on average, creating substantial opportunity costs for pharmaceutical companies. OoC technology promises to accelerate this process by enabling parallel testing of multiple compounds and providing faster feedback on drug efficacy and toxicity profiles.
Additionally, OoC technology addresses the growing ethical concerns and regulatory pressures surrounding animal testing. With initiatives like the 3Rs (Replacement, Reduction, Refinement) gaining momentum globally, pharmaceutical companies are increasingly seeking alternative testing methods that can reduce reliance on animal models while maintaining or improving predictive capacity.
The ultimate goal of OoC implementation is to establish a new paradigm in drug development that is more efficient, cost-effective, and ethically sound, while simultaneously improving the success rate of new therapeutics reaching patients. This represents a significant shift from traditional R&D approaches and aligns with the industry's movement toward more human-relevant, mechanism-based drug discovery and development methodologies.
Pharmaceutical R&D Market Demand Analysis
The pharmaceutical R&D landscape faces significant challenges, with development costs for a single drug reaching approximately $2.6 billion and timelines extending beyond 10 years. This economic burden is further exacerbated by high attrition rates, particularly in clinical trials where failure rates exceed 90% for certain therapeutic areas. These factors create substantial market demand for innovative technologies that can reduce costs, accelerate development timelines, and improve success rates.
Organ-on-Chip (OoC) technology emerges as a promising solution addressing these critical pain points in pharmaceutical development. Market research indicates the global pharmaceutical R&D spending exceeded $200 billion in 2022, with a significant portion allocated to preclinical testing and clinical trials—areas where OoC technology offers the greatest potential impact.
The demand for OoC solutions is driven by several market factors. First, regulatory pressures from agencies like FDA and EMA are increasingly encouraging alternative testing methods that reduce animal testing while maintaining or improving predictive accuracy. This regulatory environment creates a favorable market condition for OoC adoption.
Second, pharmaceutical companies face intense pressure to improve R&D efficiency amid patent cliffs and increasing competition from generics and biosimilars. Industry analysis shows that reducing time-to-market by even six months can generate additional revenues of $100 million or more for blockbuster drugs, creating strong economic incentives for technologies that accelerate development.
Third, precision medicine trends are driving demand for patient-specific testing platforms. OoC technology enables personalized drug testing using patient-derived cells, aligning with the industry shift toward targeted therapies and companion diagnostics. This market segment is growing at 10-15% annually, outpacing traditional pharmaceutical market growth.
The contract research organization (CRO) sector represents another significant market opportunity, with these organizations increasingly seeking differentiated service offerings. Early adopters of OoC technology among CROs report competitive advantages in securing pharmaceutical partnerships.
Market segmentation analysis reveals varying adoption potential across therapeutic areas. Oncology, neurology, and rare diseases demonstrate the highest demand for OoC solutions due to particularly challenging development pathways and high failure rates in these areas. Geographically, North America leads in adoption readiness, followed by Europe and Asia-Pacific regions.
Stakeholder interviews indicate growing recognition of OoC's potential economic benefits, with 65% of pharmaceutical executives expressing interest in implementing these technologies within their R&D pipelines. However, market penetration remains in early stages, with adoption barriers including technology validation requirements and integration challenges with existing workflows.
Organ-on-Chip (OoC) technology emerges as a promising solution addressing these critical pain points in pharmaceutical development. Market research indicates the global pharmaceutical R&D spending exceeded $200 billion in 2022, with a significant portion allocated to preclinical testing and clinical trials—areas where OoC technology offers the greatest potential impact.
The demand for OoC solutions is driven by several market factors. First, regulatory pressures from agencies like FDA and EMA are increasingly encouraging alternative testing methods that reduce animal testing while maintaining or improving predictive accuracy. This regulatory environment creates a favorable market condition for OoC adoption.
Second, pharmaceutical companies face intense pressure to improve R&D efficiency amid patent cliffs and increasing competition from generics and biosimilars. Industry analysis shows that reducing time-to-market by even six months can generate additional revenues of $100 million or more for blockbuster drugs, creating strong economic incentives for technologies that accelerate development.
Third, precision medicine trends are driving demand for patient-specific testing platforms. OoC technology enables personalized drug testing using patient-derived cells, aligning with the industry shift toward targeted therapies and companion diagnostics. This market segment is growing at 10-15% annually, outpacing traditional pharmaceutical market growth.
The contract research organization (CRO) sector represents another significant market opportunity, with these organizations increasingly seeking differentiated service offerings. Early adopters of OoC technology among CROs report competitive advantages in securing pharmaceutical partnerships.
Market segmentation analysis reveals varying adoption potential across therapeutic areas. Oncology, neurology, and rare diseases demonstrate the highest demand for OoC solutions due to particularly challenging development pathways and high failure rates in these areas. Geographically, North America leads in adoption readiness, followed by Europe and Asia-Pacific regions.
Stakeholder interviews indicate growing recognition of OoC's potential economic benefits, with 65% of pharmaceutical executives expressing interest in implementing these technologies within their R&D pipelines. However, market penetration remains in early stages, with adoption barriers including technology validation requirements and integration challenges with existing workflows.
Current OoC Implementation Challenges
Despite the promising potential of Organ-on-Chip (OoC) technology in pharmaceutical R&D, several significant implementation challenges currently impede its widespread adoption. Technical complexity represents a primary obstacle, as OoC systems require sophisticated microfluidic engineering, cell culture techniques, and sensor integration. Many pharmaceutical companies lack the specialized expertise needed to develop and operate these complex systems in-house.
Standardization issues further complicate implementation efforts. The field currently suffers from a lack of universally accepted protocols for chip design, cell sourcing, culture conditions, and data analysis. This absence of standardization makes it difficult to compare results across different OoC platforms and hinders regulatory acceptance of OoC-derived data.
Validation and qualification of OoC models present another substantial hurdle. Demonstrating that these systems accurately recapitulate human physiology and can reliably predict clinical outcomes requires extensive correlation studies with existing in vitro methods, animal models, and human clinical data. This validation process is time-consuming and resource-intensive, creating uncertainty about the return on investment.
Integration with existing pharmaceutical workflows poses additional challenges. Current drug discovery and development pipelines are optimized around traditional testing methods. Incorporating OoC technology requires significant modifications to established protocols and decision-making frameworks, creating organizational resistance and implementation delays.
Manufacturing scalability remains problematic for many OoC platforms. Most systems are produced at laboratory scale using techniques not amenable to high-throughput production. This limits availability and increases costs, making it difficult for pharmaceutical companies to deploy OoC technology across multiple research programs simultaneously.
Data management and analysis present further complications. OoC systems generate complex, multiparametric datasets that require specialized analytical tools and expertise to interpret effectively. Many organizations lack the computational infrastructure and data science capabilities needed to extract maximum value from OoC experiments.
Regulatory uncertainty compounds these technical challenges. While regulatory agencies have expressed interest in OoC technology, clear guidelines for using OoC data in regulatory submissions remain underdeveloped. This regulatory ambiguity creates hesitation among pharmaceutical companies considering significant investments in the technology.
Cost considerations also impede adoption. Current OoC systems require substantial upfront investment in equipment, training, and infrastructure. Without clear evidence of economic benefits, many pharmaceutical companies remain reluctant to commit resources to implementing this emerging technology at scale.
Standardization issues further complicate implementation efforts. The field currently suffers from a lack of universally accepted protocols for chip design, cell sourcing, culture conditions, and data analysis. This absence of standardization makes it difficult to compare results across different OoC platforms and hinders regulatory acceptance of OoC-derived data.
Validation and qualification of OoC models present another substantial hurdle. Demonstrating that these systems accurately recapitulate human physiology and can reliably predict clinical outcomes requires extensive correlation studies with existing in vitro methods, animal models, and human clinical data. This validation process is time-consuming and resource-intensive, creating uncertainty about the return on investment.
Integration with existing pharmaceutical workflows poses additional challenges. Current drug discovery and development pipelines are optimized around traditional testing methods. Incorporating OoC technology requires significant modifications to established protocols and decision-making frameworks, creating organizational resistance and implementation delays.
Manufacturing scalability remains problematic for many OoC platforms. Most systems are produced at laboratory scale using techniques not amenable to high-throughput production. This limits availability and increases costs, making it difficult for pharmaceutical companies to deploy OoC technology across multiple research programs simultaneously.
Data management and analysis present further complications. OoC systems generate complex, multiparametric datasets that require specialized analytical tools and expertise to interpret effectively. Many organizations lack the computational infrastructure and data science capabilities needed to extract maximum value from OoC experiments.
Regulatory uncertainty compounds these technical challenges. While regulatory agencies have expressed interest in OoC technology, clear guidelines for using OoC data in regulatory submissions remain underdeveloped. This regulatory ambiguity creates hesitation among pharmaceutical companies considering significant investments in the technology.
Cost considerations also impede adoption. Current OoC systems require substantial upfront investment in equipment, training, and infrastructure. Without clear evidence of economic benefits, many pharmaceutical companies remain reluctant to commit resources to implementing this emerging technology at scale.
Current OoC Economic Models
01 Cost reduction in drug development using OoC technology
Organ-on-Chip technology offers significant cost reduction in pharmaceutical development by providing more accurate human tissue models compared to traditional animal testing. These microfluidic devices can simulate organ functions and drug responses, allowing for earlier detection of ineffective or toxic compounds before expensive clinical trials. This early screening capability helps pharmaceutical companies save substantial resources by identifying failures sooner in the development pipeline.- Cost reduction in drug development using OoC technology: Organ-on-Chip technology significantly reduces the cost of drug development by providing more accurate human-relevant models that can replace expensive animal testing. These microfluidic devices simulate the activities, mechanics, and physiological responses of entire organs and organ systems, allowing for more predictive preclinical testing. By identifying ineffective or toxic drug candidates earlier in the development process, OoC platforms help pharmaceutical companies avoid costly late-stage failures, thereby reducing overall R&D expenditure.
- Time efficiency improvements in pharmaceutical research: Organ-on-Chip systems accelerate the drug development timeline by enabling parallel testing of multiple compounds simultaneously. These platforms provide rapid results compared to traditional in vivo models, allowing researchers to quickly assess drug efficacy, toxicity, and pharmacokinetics. The ability to continuously monitor cellular responses in real-time further enhances the speed of data collection and analysis, significantly shortening the time required for preclinical evaluation and enabling faster progression to clinical trials.
- Reduction in drug attrition rates: One of the most significant advantages of Organ-on-Chip technology is its potential to reduce drug attrition rates during development. By providing more physiologically relevant models than traditional cell cultures or animal testing, OoC platforms generate more predictive data regarding drug safety and efficacy in humans. This improved predictive capability helps identify potential failures earlier in the development process, reducing the high attrition rates typically seen in late-stage clinical trials and ultimately increasing the success rate of drug candidates reaching market approval.
- Manufacturing and scalability considerations: The manufacturing and scalability of Organ-on-Chip devices present both challenges and opportunities for cost management. Advanced microfabrication techniques, materials selection, and integration of sensors impact the production costs of these complex systems. Innovations in manufacturing processes, such as standardized fabrication methods and automated production lines, are helping to reduce unit costs and increase accessibility. As the technology matures, economies of scale are beginning to make these systems more affordable for widespread adoption in research and pharmaceutical settings.
- Return on investment and commercial viability: The economic value proposition of Organ-on-Chip technology centers on its potential return on investment for pharmaceutical companies and research institutions. While initial implementation costs can be substantial, the long-term financial benefits derived from reduced drug failure rates, accelerated development timelines, and decreased reliance on animal models present compelling economic advantages. Market analyses suggest that OoC technology can potentially save billions in drug development costs by improving predictive accuracy and reducing late-stage attrition, making it an increasingly attractive investment despite upfront costs.
02 Time efficiency improvements in drug screening processes
OoC platforms enable accelerated drug screening by providing real-time monitoring of drug effects on human tissue models. These systems allow for parallel testing of multiple compounds simultaneously, significantly reducing the time required for preliminary efficacy and toxicity assessments. The ability to continuously monitor cellular responses in a controlled microenvironment speeds up data collection and analysis compared to traditional methods, shortening the overall drug development timeline.Expand Specific Solutions03 Reduction in drug attrition rates through improved predictive models
Organ-on-Chip systems provide more physiologically relevant models that better predict human responses to drugs compared to conventional cell cultures or animal models. By incorporating multiple cell types and mimicking tissue-tissue interfaces, these platforms can reveal complex drug interactions and side effects that might otherwise only be discovered in late-stage clinical trials. This improved predictive capability helps reduce the high attrition rates typically seen in pharmaceutical development.Expand Specific Solutions04 Manufacturing and operational costs of OoC platforms
The fabrication and operation of Organ-on-Chip devices involve considerations of materials, manufacturing processes, and maintenance requirements. Advanced microfluidic fabrication techniques, including photolithography and soft lithography, contribute to production costs. Additionally, the integration of sensors, pumps, and control systems adds to the overall expense. However, economies of scale and technological advancements are gradually reducing these costs, making OoC platforms more accessible for widespread adoption in research and industry.Expand Specific Solutions05 Economic impact and return on investment for OoC adoption
The economic benefits of implementing Organ-on-Chip technology extend beyond direct cost savings in drug development. These platforms can provide valuable intellectual property, create new business opportunities, and potentially transform the pharmaceutical industry's R&D model. While initial investment costs may be substantial, the long-term return on investment comes from reduced late-stage failures, faster time-to-market for successful drugs, and the ability to repurpose existing compounds for new indications with greater confidence in their safety and efficacy profiles.Expand Specific Solutions
Key Players in OoC Industry
The Organ-on-Chip (OoC) market is in an early growth phase, transitioning from research to commercial applications with an estimated market size of $100-150 million, projected to reach $500 million by 2025. The pharmaceutical industry's adoption is accelerating as OoC technology demonstrates potential to reduce drug development costs and timelines by improving preclinical testing accuracy. Leading players include established companies like Emulate and F. Hoffmann-La Roche alongside academic powerhouses such as MIT, Harvard, and Seoul National University Hospital. The competitive landscape features collaboration between pharmaceutical giants and technology providers, with academic institutions driving innovation through research partnerships. Technical challenges in standardization and validation remain, though recent advances by GLOBALFOUNDRIES and Emulate in manufacturing scalability signal increasing technology maturity.
The Regents of the University of Michigan
Technical Solution: The University of Michigan has developed a sophisticated economic framework for evaluating OoC implementation in pharmaceutical R&D, centered around their proprietary "Integrated Organ-Chip Analysis Platform." Their research quantifies both direct cost savings and opportunity costs associated with faster development timelines. Michigan's economic analysis demonstrates that strategic implementation of their OoC technology can reduce animal testing costs by approximately 40-50% while simultaneously improving predictive accuracy for human outcomes. Their data indicates that early implementation of OoC technology in drug discovery can identify non-viable candidates approximately 8-12 months earlier than traditional methods, representing significant cost avoidance. Michigan researchers have developed detailed economic models showing that for certain therapeutic areas, particularly those with historically high failure rates like neurology and oncology, OoC adoption can improve R&D return on investment by 15-25% through reduced attrition. Their platform has been particularly effective in modeling complex drug-drug interactions, with economic analyses suggesting potential savings of $2-5 million per development program through earlier identification of problematic combinations.
Strengths: Comprehensive economic modeling framework specifically designed for pharmaceutical decision-making; strong focus on therapeutic areas with historically high failure rates; validated integration with existing pharmaceutical workflows. Weaknesses: Technology requires significant customization for different therapeutic applications; economic benefits more pronounced for certain drug classes than others; implementation requires substantial organizational change management.
F. Hoffmann-La Roche Ltd.
Technical Solution: Roche has developed an integrated OoC strategy focused on economic optimization of their pharmaceutical R&D pipeline. Their approach combines proprietary OoC platforms with sophisticated computational modeling to create what they term "virtual clinical trials." Roche's economic analysis demonstrates that strategic implementation of OoC technology at critical decision points in their development pipeline has reduced candidate attrition rates by approximately 15-20% in early development stages. Their data suggests that for every $1 invested in OoC technology, approximately $3-5 is saved in downstream development costs through improved candidate selection. Roche has particularly focused on implementing OoC models for toxicity screening, showing a 40% reduction in animal testing requirements and associated costs. Their economic modeling indicates that widespread adoption of OoC technology across their portfolio could potentially reduce overall R&D timelines by 1-2 years per successful drug, representing hundreds of millions in accelerated time-to-market value.
Strengths: Deep integration of OoC technology with existing pharmaceutical workflows; robust economic validation data from real drug development programs; complementary computational modeling capabilities enhancing predictive power. Weaknesses: Proprietary systems may limit broader industry standardization; significant upfront investment required; still relies on traditional methods for regulatory submissions in many cases.
Critical OoC Cost-Benefit Analysis
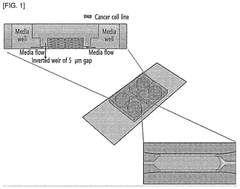
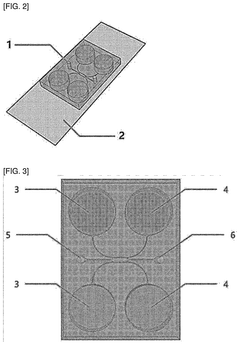
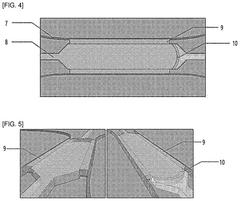
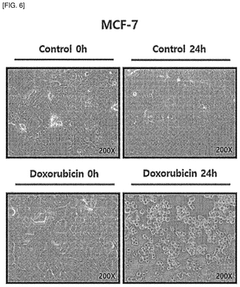
Human organ-on-a-chip having reverse micro-weir structure and uses thereof
PatentPendingUS20250110113A1
Innovation
- An organ-on-a-chip with an inverted micro-weir structure for culturing human cancer cells, featuring a media supply section, cell supply section, and an inverted micro-weir structure to mimic capillaries and prevent cell movement, allowing for three-dimensional cell culture and drug efficacy evaluation.
Regulatory Pathway for OoC Validation
The regulatory landscape for Organ-on-Chip (OoC) technologies represents a critical determinant in their commercial adoption and economic impact on pharmaceutical R&D. Currently, there exists no standardized regulatory framework specifically designed for OoC validation, creating significant uncertainty for both technology developers and potential pharmaceutical adopters.
The FDA has demonstrated progressive interest in OoC technologies through its participation in the Tissue Chip for Drug Screening program and the establishment of the Alternative Methods Working Group. However, the pathway to regulatory acceptance remains complex and evolving, with OoC technologies occupying an ambiguous position between in vitro assays and animal models.
Key regulatory considerations include the demonstration of physiological relevance, reproducibility, and predictive validity of OoC platforms. The FDA's qualification process for drug development tools (DDTs) offers one potential pathway, wherein OoC technologies could be validated for specific contexts of use. This process requires substantial evidence generation and documentation, including comprehensive characterization of the biological system and demonstration of clinical relevance.
International regulatory harmonization presents another challenge, as pharmaceutical companies operate globally and must navigate different regulatory requirements across markets. The International Council for Harmonisation (ICH) has begun discussions on alternative methods to animal testing, but specific guidelines for OoC validation remain underdeveloped.
Regulatory acceptance will likely follow a stepwise approach, beginning with the use of OoC as complementary tools alongside traditional methods, followed by gradual replacement of certain animal studies as confidence in the technology increases. This phased implementation has significant economic implications, potentially creating a period of increased costs before realizing the full economic benefits of OoC adoption.
Industry-academic-regulatory collaborations, such as the EU-ToxRisk project and the FDA's Predictive Toxicology Roadmap, are working to establish validation frameworks and performance standards. These initiatives are essential for creating the regulatory certainty needed to justify substantial investment in OoC technologies by pharmaceutical companies.
The economic analysis of OoC adoption must account for these regulatory uncertainties and the associated costs of validation studies, documentation preparation, and regulatory engagement. Companies pioneering OoC adoption face higher regulatory burden costs, which may be offset by first-mover advantages in developing institutional expertise and influencing regulatory standards.
The FDA has demonstrated progressive interest in OoC technologies through its participation in the Tissue Chip for Drug Screening program and the establishment of the Alternative Methods Working Group. However, the pathway to regulatory acceptance remains complex and evolving, with OoC technologies occupying an ambiguous position between in vitro assays and animal models.
Key regulatory considerations include the demonstration of physiological relevance, reproducibility, and predictive validity of OoC platforms. The FDA's qualification process for drug development tools (DDTs) offers one potential pathway, wherein OoC technologies could be validated for specific contexts of use. This process requires substantial evidence generation and documentation, including comprehensive characterization of the biological system and demonstration of clinical relevance.
International regulatory harmonization presents another challenge, as pharmaceutical companies operate globally and must navigate different regulatory requirements across markets. The International Council for Harmonisation (ICH) has begun discussions on alternative methods to animal testing, but specific guidelines for OoC validation remain underdeveloped.
Regulatory acceptance will likely follow a stepwise approach, beginning with the use of OoC as complementary tools alongside traditional methods, followed by gradual replacement of certain animal studies as confidence in the technology increases. This phased implementation has significant economic implications, potentially creating a period of increased costs before realizing the full economic benefits of OoC adoption.
Industry-academic-regulatory collaborations, such as the EU-ToxRisk project and the FDA's Predictive Toxicology Roadmap, are working to establish validation frameworks and performance standards. These initiatives are essential for creating the regulatory certainty needed to justify substantial investment in OoC technologies by pharmaceutical companies.
The economic analysis of OoC adoption must account for these regulatory uncertainties and the associated costs of validation studies, documentation preparation, and regulatory engagement. Companies pioneering OoC adoption face higher regulatory burden costs, which may be offset by first-mover advantages in developing institutional expertise and influencing regulatory standards.
ROI Metrics for OoC Implementation
Establishing robust Return on Investment (ROI) metrics is crucial for pharmaceutical companies considering Organ-on-Chip (OoC) technology implementation. These metrics must capture both quantitative financial returns and qualitative benefits that may materialize over different timeframes.
Primary financial ROI metrics include reduction in overall R&D costs, which can be measured by comparing traditional animal testing expenses against OoC implementation costs. Initial analyses suggest potential savings of 10-25% in preclinical testing phases, though this varies by therapeutic area. The capital expenditure for OoC infrastructure must be weighed against operational cost reductions over a 3-5 year horizon.
Time-to-market acceleration represents another critical ROI dimension. OoC technologies can potentially reduce drug development timelines by 15-30% through more efficient candidate selection and reduced late-stage failures. This acceleration translates to extended patent protection periods, with each month of earlier market entry potentially worth $10-50 million for blockbuster drugs.
Attrition rate improvement metrics are particularly valuable, as OoC platforms demonstrate superior predictive capacity compared to conventional models. A 10% reduction in Phase II/III failures through better preclinical screening could save pharmaceutical companies $100-300 million per successful drug approval. These savings compound when considering the portfolio effect across multiple development programs.
Regulatory acceptance metrics must also factor into ROI calculations. As regulatory bodies increasingly recognize OoC validation data, companies can potentially reduce redundant testing requirements, creating additional cost efficiencies and accelerating approval timelines.
Long-term strategic ROI metrics include competitive positioning advantages, enhanced corporate sustainability profiles, and reduced animal testing liabilities. These factors, while more challenging to quantify, contribute significantly to overall ROI calculations and should be incorporated using appropriate weighting methodologies.
Implementation timeline metrics are essential for accurate ROI projections. Most pharmaceutical companies can expect to see initial returns within 12-24 months for targeted applications, with full ROI realization occurring over 3-5 years as the technology becomes more integrated into standard R&D workflows.
Primary financial ROI metrics include reduction in overall R&D costs, which can be measured by comparing traditional animal testing expenses against OoC implementation costs. Initial analyses suggest potential savings of 10-25% in preclinical testing phases, though this varies by therapeutic area. The capital expenditure for OoC infrastructure must be weighed against operational cost reductions over a 3-5 year horizon.
Time-to-market acceleration represents another critical ROI dimension. OoC technologies can potentially reduce drug development timelines by 15-30% through more efficient candidate selection and reduced late-stage failures. This acceleration translates to extended patent protection periods, with each month of earlier market entry potentially worth $10-50 million for blockbuster drugs.
Attrition rate improvement metrics are particularly valuable, as OoC platforms demonstrate superior predictive capacity compared to conventional models. A 10% reduction in Phase II/III failures through better preclinical screening could save pharmaceutical companies $100-300 million per successful drug approval. These savings compound when considering the portfolio effect across multiple development programs.
Regulatory acceptance metrics must also factor into ROI calculations. As regulatory bodies increasingly recognize OoC validation data, companies can potentially reduce redundant testing requirements, creating additional cost efficiencies and accelerating approval timelines.
Long-term strategic ROI metrics include competitive positioning advantages, enhanced corporate sustainability profiles, and reduced animal testing liabilities. These factors, while more challenging to quantify, contribute significantly to overall ROI calculations and should be incorporated using appropriate weighting methodologies.
Implementation timeline metrics are essential for accurate ROI projections. Most pharmaceutical companies can expect to see initial returns within 12-24 months for targeted applications, with full ROI realization occurring over 3-5 years as the technology becomes more integrated into standard R&D workflows.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!