Efficiency Optimization of Electrolytic Cells in Hydrogen Production via Water Electrolysis
AUG 1, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Electrolytic H2 Production: Background and Objectives
Water electrolysis for hydrogen production has emerged as a promising technology in the pursuit of sustainable energy solutions. This process, which involves splitting water molecules into hydrogen and oxygen using electricity, has gained significant attention due to its potential to produce clean, renewable hydrogen fuel. The evolution of this technology can be traced back to the early 19th century when William Nicholson and Anthony Carlisle first demonstrated water electrolysis in 1800.
Over the past two centuries, electrolytic hydrogen production has undergone substantial advancements, driven by the growing demand for clean energy alternatives and the need to reduce greenhouse gas emissions. The technology has progressed from basic laboratory experiments to large-scale industrial applications, with continuous improvements in efficiency, durability, and cost-effectiveness.
In recent years, the focus has shifted towards optimizing the efficiency of electrolytic cells, which are the core components of water electrolysis systems. This optimization is crucial for making hydrogen production more economically viable and environmentally sustainable. The primary goal is to reduce the energy input required to produce a given amount of hydrogen, thereby lowering production costs and increasing the overall efficiency of the process.
The current technological landscape presents several key objectives for efficiency optimization in electrolytic hydrogen production. These include enhancing electrode materials to improve catalytic activity, developing more efficient membranes for ion transport, optimizing cell design to reduce electrical resistance, and improving overall system integration for better heat and mass transfer.
Another important aspect of the technological evolution is the development of different types of electrolyzers, such as alkaline electrolyzers, proton exchange membrane (PEM) electrolyzers, and solid oxide electrolyzers. Each of these technologies has its own set of advantages and challenges, contributing to the diverse landscape of electrolytic hydrogen production methods.
As we look towards the future, the trajectory of electrolytic hydrogen production is closely aligned with global efforts to transition towards a hydrogen-based economy. This transition is driven by the increasing recognition of hydrogen's potential as a versatile energy carrier and its role in decarbonizing various sectors, including transportation, industry, and power generation.
The ongoing research and development in this field aim to address several critical challenges, including reducing the cost of electrolyzers, increasing their lifespan, and improving their ability to operate efficiently with intermittent renewable energy sources. These advancements are essential for scaling up hydrogen production to meet the projected global demand and to make it competitive with conventional fossil fuel-based hydrogen production methods.
Over the past two centuries, electrolytic hydrogen production has undergone substantial advancements, driven by the growing demand for clean energy alternatives and the need to reduce greenhouse gas emissions. The technology has progressed from basic laboratory experiments to large-scale industrial applications, with continuous improvements in efficiency, durability, and cost-effectiveness.
In recent years, the focus has shifted towards optimizing the efficiency of electrolytic cells, which are the core components of water electrolysis systems. This optimization is crucial for making hydrogen production more economically viable and environmentally sustainable. The primary goal is to reduce the energy input required to produce a given amount of hydrogen, thereby lowering production costs and increasing the overall efficiency of the process.
The current technological landscape presents several key objectives for efficiency optimization in electrolytic hydrogen production. These include enhancing electrode materials to improve catalytic activity, developing more efficient membranes for ion transport, optimizing cell design to reduce electrical resistance, and improving overall system integration for better heat and mass transfer.
Another important aspect of the technological evolution is the development of different types of electrolyzers, such as alkaline electrolyzers, proton exchange membrane (PEM) electrolyzers, and solid oxide electrolyzers. Each of these technologies has its own set of advantages and challenges, contributing to the diverse landscape of electrolytic hydrogen production methods.
As we look towards the future, the trajectory of electrolytic hydrogen production is closely aligned with global efforts to transition towards a hydrogen-based economy. This transition is driven by the increasing recognition of hydrogen's potential as a versatile energy carrier and its role in decarbonizing various sectors, including transportation, industry, and power generation.
The ongoing research and development in this field aim to address several critical challenges, including reducing the cost of electrolyzers, increasing their lifespan, and improving their ability to operate efficiently with intermittent renewable energy sources. These advancements are essential for scaling up hydrogen production to meet the projected global demand and to make it competitive with conventional fossil fuel-based hydrogen production methods.
Market Analysis for Green Hydrogen
The green hydrogen market is experiencing rapid growth and transformation, driven by the global push for decarbonization and sustainable energy solutions. As countries and industries seek to reduce their carbon footprint, green hydrogen produced through water electrolysis using renewable energy sources has emerged as a promising alternative to fossil fuels.
The market demand for green hydrogen is expanding across various sectors, including transportation, industry, and power generation. In the transportation sector, hydrogen fuel cell vehicles are gaining traction, particularly for long-haul trucking and public transit. Industries such as steel production, chemical manufacturing, and refining are exploring green hydrogen as a means to reduce their carbon emissions and meet increasingly stringent environmental regulations.
The power generation sector is also showing interest in green hydrogen as a potential solution for long-term energy storage and grid balancing, especially in regions with high renewable energy penetration. This versatility in applications is driving the overall market growth and attracting significant investments from both public and private sectors.
Several key factors are influencing the market dynamics of green hydrogen. The declining costs of renewable energy, particularly solar and wind power, are making green hydrogen production more economically viable. Technological advancements in electrolysis systems, including improved efficiency and durability, are further contributing to cost reductions and scalability.
Government policies and incentives play a crucial role in shaping the green hydrogen market. Many countries have introduced hydrogen strategies and roadmaps, setting ambitious targets for hydrogen production and use. These policies often include financial support mechanisms, research and development funding, and regulatory frameworks to promote the adoption of green hydrogen technologies.
The market size for green hydrogen is projected to grow substantially in the coming years. While current production volumes are relatively small, industry analysts forecast exponential growth as technology matures and infrastructure develops. The potential for green hydrogen to decarbonize hard-to-abate sectors is driving significant interest and investment.
However, challenges remain in scaling up the green hydrogen market. The high cost of production compared to conventional hydrogen and fossil fuels is a significant barrier to widespread adoption. Infrastructure development, including production facilities, transportation networks, and storage systems, requires substantial investment. Additionally, the energy efficiency of the entire hydrogen value chain, from production to end-use, needs improvement to maximize the environmental benefits of green hydrogen.
Despite these challenges, the market outlook for green hydrogen remains optimistic. As technology advances and economies of scale are achieved, the cost of green hydrogen is expected to decrease, making it more competitive with conventional energy sources. The growing emphasis on sustainability and carbon neutrality across industries is likely to drive continued investment and innovation in the green hydrogen sector, positioning it as a key component of the future energy landscape.
The market demand for green hydrogen is expanding across various sectors, including transportation, industry, and power generation. In the transportation sector, hydrogen fuel cell vehicles are gaining traction, particularly for long-haul trucking and public transit. Industries such as steel production, chemical manufacturing, and refining are exploring green hydrogen as a means to reduce their carbon emissions and meet increasingly stringent environmental regulations.
The power generation sector is also showing interest in green hydrogen as a potential solution for long-term energy storage and grid balancing, especially in regions with high renewable energy penetration. This versatility in applications is driving the overall market growth and attracting significant investments from both public and private sectors.
Several key factors are influencing the market dynamics of green hydrogen. The declining costs of renewable energy, particularly solar and wind power, are making green hydrogen production more economically viable. Technological advancements in electrolysis systems, including improved efficiency and durability, are further contributing to cost reductions and scalability.
Government policies and incentives play a crucial role in shaping the green hydrogen market. Many countries have introduced hydrogen strategies and roadmaps, setting ambitious targets for hydrogen production and use. These policies often include financial support mechanisms, research and development funding, and regulatory frameworks to promote the adoption of green hydrogen technologies.
The market size for green hydrogen is projected to grow substantially in the coming years. While current production volumes are relatively small, industry analysts forecast exponential growth as technology matures and infrastructure develops. The potential for green hydrogen to decarbonize hard-to-abate sectors is driving significant interest and investment.
However, challenges remain in scaling up the green hydrogen market. The high cost of production compared to conventional hydrogen and fossil fuels is a significant barrier to widespread adoption. Infrastructure development, including production facilities, transportation networks, and storage systems, requires substantial investment. Additionally, the energy efficiency of the entire hydrogen value chain, from production to end-use, needs improvement to maximize the environmental benefits of green hydrogen.
Despite these challenges, the market outlook for green hydrogen remains optimistic. As technology advances and economies of scale are achieved, the cost of green hydrogen is expected to decrease, making it more competitive with conventional energy sources. The growing emphasis on sustainability and carbon neutrality across industries is likely to drive continued investment and innovation in the green hydrogen sector, positioning it as a key component of the future energy landscape.
Current Challenges in Water Electrolysis Efficiency
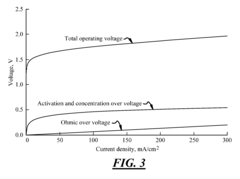
Water electrolysis for hydrogen production faces several significant challenges that hinder its widespread adoption and efficiency. One of the primary issues is the high energy consumption required for the electrolysis process. The theoretical minimum voltage needed for water splitting is 1.23 V, but in practice, higher voltages are necessary to overcome various resistances and kinetic barriers. This overpotential leads to increased energy input and reduced overall efficiency.
Another critical challenge is the durability and stability of electrode materials. The harsh operating conditions, including high pH environments and the presence of oxygen and hydrogen gases, can cause degradation of electrodes over time. This degradation not only reduces the efficiency of the electrolytic cell but also increases maintenance costs and decreases the lifespan of the system.
The choice of electrocatalysts presents another significant hurdle. While noble metals like platinum and iridium offer excellent catalytic activity, their scarcity and high cost limit their large-scale application. Developing efficient, stable, and cost-effective alternatives remains a key research focus in the field.
Water management within the electrolytic cell is also a complex issue. Proper water distribution is crucial for maintaining optimal reaction conditions and preventing dry spots on electrodes. However, excessive water can lead to flooding, which impedes gas evolution and reduces efficiency. Balancing these factors requires sophisticated engineering solutions.
The production of high-purity hydrogen is another challenge. Contamination from oxygen crossover can occur, especially at high current densities, reducing the purity of the hydrogen produced. This necessitates additional purification steps, which add to the overall system complexity and cost.
Scaling up electrolysis systems for industrial-scale hydrogen production presents its own set of challenges. Maintaining uniform current distribution and heat management across large electrode surfaces is difficult, and can lead to localized hotspots and reduced efficiency.
Lastly, the intermittent nature of renewable energy sources, which are often coupled with water electrolysis for green hydrogen production, poses challenges in terms of system operation and efficiency. Fluctuating power inputs can lead to suboptimal operating conditions and reduced overall system performance.
Addressing these challenges requires a multidisciplinary approach, combining advances in materials science, electrochemistry, and process engineering to develop more efficient, durable, and scalable water electrolysis systems for hydrogen production.
Another critical challenge is the durability and stability of electrode materials. The harsh operating conditions, including high pH environments and the presence of oxygen and hydrogen gases, can cause degradation of electrodes over time. This degradation not only reduces the efficiency of the electrolytic cell but also increases maintenance costs and decreases the lifespan of the system.
The choice of electrocatalysts presents another significant hurdle. While noble metals like platinum and iridium offer excellent catalytic activity, their scarcity and high cost limit their large-scale application. Developing efficient, stable, and cost-effective alternatives remains a key research focus in the field.
Water management within the electrolytic cell is also a complex issue. Proper water distribution is crucial for maintaining optimal reaction conditions and preventing dry spots on electrodes. However, excessive water can lead to flooding, which impedes gas evolution and reduces efficiency. Balancing these factors requires sophisticated engineering solutions.
The production of high-purity hydrogen is another challenge. Contamination from oxygen crossover can occur, especially at high current densities, reducing the purity of the hydrogen produced. This necessitates additional purification steps, which add to the overall system complexity and cost.
Scaling up electrolysis systems for industrial-scale hydrogen production presents its own set of challenges. Maintaining uniform current distribution and heat management across large electrode surfaces is difficult, and can lead to localized hotspots and reduced efficiency.
Lastly, the intermittent nature of renewable energy sources, which are often coupled with water electrolysis for green hydrogen production, poses challenges in terms of system operation and efficiency. Fluctuating power inputs can lead to suboptimal operating conditions and reduced overall system performance.
Addressing these challenges requires a multidisciplinary approach, combining advances in materials science, electrochemistry, and process engineering to develop more efficient, durable, and scalable water electrolysis systems for hydrogen production.
Existing Efficiency Enhancement Strategies
01 Electrode material optimization
Improving the efficiency of electrolytic cells can be achieved by optimizing electrode materials. This involves selecting materials with high conductivity, low overpotential, and resistance to corrosion. Advanced materials such as nanostructured electrodes or catalytic coatings can enhance the electrochemical reactions, leading to increased efficiency and reduced energy consumption.- Electrode materials and design: The efficiency of electrolytic cells can be improved by optimizing electrode materials and design. This includes using high-performance catalysts, increasing the surface area of electrodes, and developing novel electrode structures. These enhancements can reduce overpotential, increase reaction rates, and improve overall cell efficiency.
- Membrane technology: Advanced membrane technologies play a crucial role in enhancing electrolytic cell efficiency. Improved ion-exchange membranes can reduce resistance, increase selectivity, and minimize crossover of reactants and products. This leads to higher current efficiency and lower energy consumption in various electrolytic processes.
- Electrolyte composition and optimization: The composition and properties of the electrolyte significantly impact cell efficiency. Optimizing electrolyte concentration, pH, and additives can enhance conductivity, reduce side reactions, and improve overall performance. Advanced electrolyte formulations can also contribute to extended cell life and stability.
- Cell design and configuration: Innovative cell designs and configurations can lead to improved efficiency in electrolytic processes. This includes optimizing cell geometry, flow patterns, and heat management systems. Advanced designs can enhance mass transfer, reduce energy losses, and improve overall system performance.
- Process control and monitoring: Implementing advanced process control and monitoring systems can significantly improve electrolytic cell efficiency. This includes real-time monitoring of key parameters, adaptive control strategies, and predictive maintenance techniques. These approaches can optimize operating conditions, reduce downtime, and maximize overall system efficiency.
02 Cell design and configuration
The design and configuration of electrolytic cells play a crucial role in their efficiency. This includes optimizing factors such as electrode spacing, cell geometry, and electrolyte flow patterns. Improved designs can minimize ohmic losses, enhance mass transfer, and ensure uniform current distribution, resulting in higher overall efficiency of the electrolytic process.Expand Specific Solutions03 Electrolyte composition and management
The composition and management of the electrolyte solution significantly impact cell efficiency. This involves optimizing electrolyte concentration, pH, and temperature, as well as implementing effective electrolyte circulation and purification systems. Proper electrolyte management can reduce side reactions, minimize energy losses, and maintain consistent performance over time.Expand Specific Solutions04 Advanced control systems and monitoring
Implementing advanced control systems and real-time monitoring techniques can enhance electrolytic cell efficiency. This includes using sensors, data analytics, and intelligent control algorithms to optimize operating parameters, detect and prevent performance degradation, and ensure optimal current distribution. Such systems can adapt to changing conditions and maintain peak efficiency throughout the cell's operation.Expand Specific Solutions05 Energy recovery and system integration
Improving overall system efficiency can be achieved through energy recovery techniques and integration with other processes. This may involve capturing and utilizing waste heat, integrating renewable energy sources, or coupling electrolytic processes with other industrial operations. Such approaches can reduce net energy consumption and improve the overall efficiency of the electrolytic cell system.Expand Specific Solutions
Key Players in Electrolysis Industry
The efficiency optimization of electrolytic cells in hydrogen production via water electrolysis is currently in a growth phase, with increasing market size and technological advancements. The global market for water electrolysis is expanding rapidly, driven by the growing demand for clean energy solutions. Companies like GM Global Technology Operations LLC, Umicore SA, and Panasonic Intellectual Property Management Co. Ltd. are investing heavily in research and development to improve electrolysis efficiency. Emerging players such as Verdagy Inc. and Hydrogen Power (Hangzhou) Technology Co., Ltd. are introducing innovative technologies, while established firms like China National Petroleum Corp. and Honda Motor Co., Ltd. are leveraging their resources to scale up production. The technology's maturity is progressing, with ongoing efforts to enhance durability, reduce costs, and increase overall system efficiency.
Panasonic Intellectual Property Management Co. Ltd.
Technical Solution: Panasonic has developed an innovative alkaline water electrolysis technology for efficient hydrogen production. Their system utilizes a proprietary electrode design with a highly porous, three-dimensional structure that significantly increases the active surface area for electrocatalysis[10]. This design allows for higher current densities and improved mass transport, leading to enhanced efficiency. Panasonic's electrolyzers incorporate advanced materials for the diaphragm and electrodes, including non-noble metal catalysts that reduce costs while maintaining high performance. The company has also developed a unique cell stack architecture that minimizes ohmic losses and improves overall system efficiency[11]. Panasonic claims their technology can achieve energy efficiencies of up to 80% (LHV) and has demonstrated stable operation for over 100,000 hours in long-term testing[12].
Strengths: High efficiency and long-term stability. Use of non-noble metal catalysts reduces costs. Alkaline technology is well-established and robust. Weaknesses: Alkaline systems typically have slower response times compared to PEM technology, potentially limiting flexibility for renewable energy integration.
Hydrogen Power (Hangzhou) Technology Co., Ltd.
Technical Solution: Hydrogen Power has developed a novel anion exchange membrane (AEM) electrolysis technology for hydrogen production. Their system operates at near-neutral pH conditions, combining the advantages of both alkaline and PEM electrolysis. The company's proprietary membrane formulation offers high ionic conductivity and chemical stability, addressing key challenges in AEM technology[13]. Hydrogen Power's electrolyzers incorporate advanced non-noble metal catalysts and optimized electrode structures to enhance performance while reducing costs. The system operates at moderate temperatures (40-60°C) and pressures (1-30 bar), balancing efficiency and durability. The company claims their technology can achieve system efficiencies of up to 75% (LHV) and has demonstrated stable operation for over 10,000 hours in pilot-scale testing[14].
Strengths: Near-neutral pH operation reduces material corrosion issues. Use of non-noble metal catalysts lowers costs. Combines advantages of alkaline and PEM technologies. Weaknesses: AEM technology is relatively new and may face long-term stability challenges. Limited track record compared to established technologies.
Innovative Electrode Materials and Catalysts
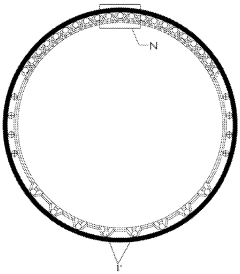
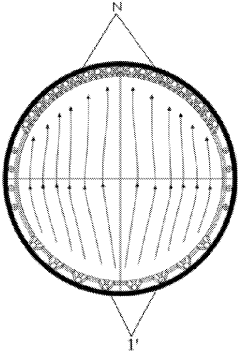
High pressure electrolysis cell for hydrogen production from water
PatentInactiveUS20100276299A1
Innovation
- Optimizing the electrolysis cell geometry by reversing the polarity of the anode and cathode, increasing the anode surface area, and reducing hydrogen permeation through the membrane to decrease current density and overvoltage, thereby enhancing efficiency and hydrogen purity.
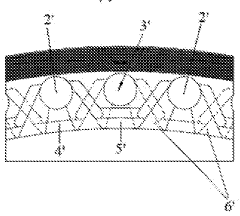
Electrolytic bath pole frame for hydrogen production from water electrolysis and electrolytic bath
PatentWO2024098910A1
Innovation
- Design a pole frame of an electrolytic cell for electrolyzing water to produce hydrogen. The alkali liquid inlet is evenly distributed at the bottom, and the gas-liquid outlet is evenly and symmetrically distributed at the top. The cathode gas-liquid outlet and the anode gas-liquid outlet alternate alternately on the top of the pole frame, using a trumpet-shaped flow channel. Diversion port to improve gas and liquid discharge efficiency.
Economic Feasibility of Improved Electrolysis
The economic feasibility of improved electrolysis in hydrogen production via water electrolysis is a critical factor in determining the viability of large-scale implementation. As efficiency optimization efforts continue to advance, the cost-effectiveness of electrolytic cells becomes increasingly important for commercial adoption.
One of the primary economic considerations is the capital expenditure required for electrolytic cell installations. While improved efficiency can lead to higher hydrogen production rates, the initial investment in advanced electrolytic systems may be substantial. However, these costs are often offset by reduced operational expenses over time, particularly in energy consumption.
Energy costs represent a significant portion of the overall expenses in hydrogen production through electrolysis. Improved efficiency in electrolytic cells directly translates to lower energy requirements per unit of hydrogen produced. This reduction in energy consumption can lead to substantial cost savings, especially in regions with high electricity prices or when utilizing renewable energy sources.
The durability and lifespan of electrolytic cells also play a crucial role in economic feasibility. Enhanced efficiency often correlates with improved cell designs and materials, potentially extending the operational life of the system. Longer-lasting components reduce maintenance and replacement costs, contributing to better long-term economic performance.
Market demand for green hydrogen is another key factor influencing economic feasibility. As industries and governments increasingly prioritize decarbonization, the demand for clean hydrogen is expected to grow significantly. This rising demand could justify higher initial investments in efficient electrolysis technologies, as the potential for long-term profitability increases.
Scalability is an essential aspect of economic feasibility. Improved electrolysis techniques that can be effectively scaled up to industrial levels are more likely to achieve economic viability. Economies of scale can lead to reduced production costs per unit of hydrogen, making the technology more competitive with traditional hydrogen production methods.
Government policies and incentives also play a crucial role in the economic landscape of hydrogen production. Subsidies, tax breaks, or carbon pricing mechanisms can significantly impact the financial attractiveness of investing in improved electrolysis technologies. These policy instruments can help bridge the gap between current costs and market competitiveness.
In conclusion, the economic feasibility of improved electrolysis in hydrogen production is a complex interplay of various factors. While the initial investment may be higher, the potential for reduced operational costs, increased durability, growing market demand, and supportive policies create a promising outlook for the economic viability of advanced electrolytic cell technologies in the hydrogen production sector.
One of the primary economic considerations is the capital expenditure required for electrolytic cell installations. While improved efficiency can lead to higher hydrogen production rates, the initial investment in advanced electrolytic systems may be substantial. However, these costs are often offset by reduced operational expenses over time, particularly in energy consumption.
Energy costs represent a significant portion of the overall expenses in hydrogen production through electrolysis. Improved efficiency in electrolytic cells directly translates to lower energy requirements per unit of hydrogen produced. This reduction in energy consumption can lead to substantial cost savings, especially in regions with high electricity prices or when utilizing renewable energy sources.
The durability and lifespan of electrolytic cells also play a crucial role in economic feasibility. Enhanced efficiency often correlates with improved cell designs and materials, potentially extending the operational life of the system. Longer-lasting components reduce maintenance and replacement costs, contributing to better long-term economic performance.
Market demand for green hydrogen is another key factor influencing economic feasibility. As industries and governments increasingly prioritize decarbonization, the demand for clean hydrogen is expected to grow significantly. This rising demand could justify higher initial investments in efficient electrolysis technologies, as the potential for long-term profitability increases.
Scalability is an essential aspect of economic feasibility. Improved electrolysis techniques that can be effectively scaled up to industrial levels are more likely to achieve economic viability. Economies of scale can lead to reduced production costs per unit of hydrogen, making the technology more competitive with traditional hydrogen production methods.
Government policies and incentives also play a crucial role in the economic landscape of hydrogen production. Subsidies, tax breaks, or carbon pricing mechanisms can significantly impact the financial attractiveness of investing in improved electrolysis technologies. These policy instruments can help bridge the gap between current costs and market competitiveness.
In conclusion, the economic feasibility of improved electrolysis in hydrogen production is a complex interplay of various factors. While the initial investment may be higher, the potential for reduced operational costs, increased durability, growing market demand, and supportive policies create a promising outlook for the economic viability of advanced electrolytic cell technologies in the hydrogen production sector.
Environmental Impact of Optimized H2 Production
The optimization of electrolytic cells in hydrogen production through water electrolysis not only enhances efficiency but also significantly impacts the environmental footprint of the process. As the global demand for clean energy solutions grows, the environmental implications of optimized hydrogen production become increasingly crucial.
One of the primary environmental benefits of improved electrolytic cell efficiency is the reduction in energy consumption. By enhancing the electrochemical reactions and minimizing energy losses, optimized cells require less electrical input to produce the same amount of hydrogen. This decreased energy demand translates directly into reduced greenhouse gas emissions, particularly in regions where the electricity grid still relies heavily on fossil fuels.
Water usage is another critical environmental factor affected by electrolytic cell optimization. More efficient cells can produce hydrogen with less water input, addressing concerns about water scarcity in many parts of the world. Additionally, advanced electrolysis technologies may enable the use of seawater or wastewater as feedstock, further reducing the strain on freshwater resources.
The materials used in electrolytic cells also play a role in their environmental impact. Optimized designs often incorporate more sustainable and recyclable materials, reducing the need for rare earth elements and minimizing the environmental damage associated with mining and processing these resources. Furthermore, improved durability and longevity of electrolytic cells result in less frequent replacements, reducing waste generation and the overall lifecycle environmental impact.
Optimized hydrogen production can also contribute to the circular economy. By-products of the electrolysis process, such as oxygen, can be captured and utilized in various industrial applications, maximizing resource efficiency. Moreover, the integration of renewable energy sources with optimized electrolytic cells creates a synergistic effect, enabling the storage of excess renewable energy in the form of hydrogen and providing a clean energy carrier for various sectors.
The environmental benefits extend beyond the production phase. As hydrogen becomes more efficiently produced, it becomes a more viable alternative to fossil fuels in transportation, industry, and heating. This transition can lead to significant reductions in air pollution and greenhouse gas emissions across multiple sectors of the economy.
However, it is essential to consider the potential environmental trade-offs. While optimized electrolytic cells offer numerous benefits, the increased production of hydrogen may lead to new challenges in storage, transportation, and end-use technologies. Addressing these challenges will be crucial to ensure that the entire hydrogen value chain remains environmentally sustainable.
One of the primary environmental benefits of improved electrolytic cell efficiency is the reduction in energy consumption. By enhancing the electrochemical reactions and minimizing energy losses, optimized cells require less electrical input to produce the same amount of hydrogen. This decreased energy demand translates directly into reduced greenhouse gas emissions, particularly in regions where the electricity grid still relies heavily on fossil fuels.
Water usage is another critical environmental factor affected by electrolytic cell optimization. More efficient cells can produce hydrogen with less water input, addressing concerns about water scarcity in many parts of the world. Additionally, advanced electrolysis technologies may enable the use of seawater or wastewater as feedstock, further reducing the strain on freshwater resources.
The materials used in electrolytic cells also play a role in their environmental impact. Optimized designs often incorporate more sustainable and recyclable materials, reducing the need for rare earth elements and minimizing the environmental damage associated with mining and processing these resources. Furthermore, improved durability and longevity of electrolytic cells result in less frequent replacements, reducing waste generation and the overall lifecycle environmental impact.
Optimized hydrogen production can also contribute to the circular economy. By-products of the electrolysis process, such as oxygen, can be captured and utilized in various industrial applications, maximizing resource efficiency. Moreover, the integration of renewable energy sources with optimized electrolytic cells creates a synergistic effect, enabling the storage of excess renewable energy in the form of hydrogen and providing a clean energy carrier for various sectors.
The environmental benefits extend beyond the production phase. As hydrogen becomes more efficiently produced, it becomes a more viable alternative to fossil fuels in transportation, industry, and heating. This transition can lead to significant reductions in air pollution and greenhouse gas emissions across multiple sectors of the economy.
However, it is essential to consider the potential environmental trade-offs. While optimized electrolytic cells offer numerous benefits, the increased production of hydrogen may lead to new challenges in storage, transportation, and end-use technologies. Addressing these challenges will be crucial to ensure that the entire hydrogen value chain remains environmentally sustainable.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!