Electrochemical Cell Lifetime in High-Pressure Environments
AUG 28, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Electrochemical Cell Lifetime Challenges and Objectives
Electrochemical cells operating in high-pressure environments represent a critical technology for numerous industrial applications, including deep-sea sensors, geothermal energy systems, and aerospace instrumentation. The evolution of these specialized cells has accelerated significantly over the past decade, driven by increasing demands for reliable power sources capable of withstanding extreme conditions. Historical development shows a progression from basic pressure-resistant designs to sophisticated engineered solutions incorporating advanced materials and novel architectures.
The fundamental challenge in this domain centers on maintaining electrochemical performance while ensuring structural integrity under sustained high-pressure conditions. Conventional cells typically experience accelerated degradation when subjected to pressures exceeding 100 MPa, manifesting as reduced capacity, increased internal resistance, and compromised sealing integrity. This degradation trajectory significantly limits operational lifetimes in critical applications where maintenance access is restricted or impossible.
Current research trajectories indicate promising developments in pressure-compensated cell designs, which aim to equalize internal and external pressures to minimize mechanical stress on cell components. Parallel efforts focus on developing novel electrode materials and electrolyte formulations specifically engineered to maintain stability and ionic conductivity under compression. These approaches represent complementary pathways toward addressing the core technical challenges.
The primary technical objectives for advancing electrochemical cell lifetime in high-pressure environments include extending operational durability to exceed 5 years at pressures above 150 MPa, maintaining capacity retention above 80% throughout the service life, and ensuring consistent performance across temperature fluctuations typical of target deployment scenarios. Secondary objectives encompass reducing manufacturing complexity to enable cost-effective production at scale and minimizing environmental impact through materials selection.
Quantifiable performance targets have been established based on industry requirements and theoretical limitations. These include achieving internal pressure differentials below 5% of ambient pressure, limiting electrolyte degradation to less than 2% per year, and maintaining electrode-electrolyte interface stability with impedance increases restricted to 15% over the cell's operational lifetime. These metrics provide concrete benchmarks against which technological progress can be measured.
The technological trajectory suggests that breakthrough innovations will likely emerge from interdisciplinary approaches combining materials science, electrochemistry, and mechanical engineering. Particular emphasis is being placed on understanding the fundamental mechanisms of degradation under pressure, which remains incompletely characterized despite its critical importance to lifetime extension efforts. Addressing these knowledge gaps represents a key prerequisite for achieving the defined technical objectives.
The fundamental challenge in this domain centers on maintaining electrochemical performance while ensuring structural integrity under sustained high-pressure conditions. Conventional cells typically experience accelerated degradation when subjected to pressures exceeding 100 MPa, manifesting as reduced capacity, increased internal resistance, and compromised sealing integrity. This degradation trajectory significantly limits operational lifetimes in critical applications where maintenance access is restricted or impossible.
Current research trajectories indicate promising developments in pressure-compensated cell designs, which aim to equalize internal and external pressures to minimize mechanical stress on cell components. Parallel efforts focus on developing novel electrode materials and electrolyte formulations specifically engineered to maintain stability and ionic conductivity under compression. These approaches represent complementary pathways toward addressing the core technical challenges.
The primary technical objectives for advancing electrochemical cell lifetime in high-pressure environments include extending operational durability to exceed 5 years at pressures above 150 MPa, maintaining capacity retention above 80% throughout the service life, and ensuring consistent performance across temperature fluctuations typical of target deployment scenarios. Secondary objectives encompass reducing manufacturing complexity to enable cost-effective production at scale and minimizing environmental impact through materials selection.
Quantifiable performance targets have been established based on industry requirements and theoretical limitations. These include achieving internal pressure differentials below 5% of ambient pressure, limiting electrolyte degradation to less than 2% per year, and maintaining electrode-electrolyte interface stability with impedance increases restricted to 15% over the cell's operational lifetime. These metrics provide concrete benchmarks against which technological progress can be measured.
The technological trajectory suggests that breakthrough innovations will likely emerge from interdisciplinary approaches combining materials science, electrochemistry, and mechanical engineering. Particular emphasis is being placed on understanding the fundamental mechanisms of degradation under pressure, which remains incompletely characterized despite its critical importance to lifetime extension efforts. Addressing these knowledge gaps represents a key prerequisite for achieving the defined technical objectives.
Market Analysis for High-Pressure Electrochemical Applications
The high-pressure electrochemical cell market is experiencing significant growth driven by expanding applications in energy storage, industrial processes, and scientific research. Current market valuations indicate the global high-pressure electrochemical technologies sector reached approximately $3.2 billion in 2022, with projections suggesting a compound annual growth rate of 7.8% through 2028.
The oil and gas industry represents the largest market segment, where high-pressure electrochemical cells are utilized for downhole sensing, corrosion monitoring, and enhanced oil recovery operations. This sector alone accounts for roughly 38% of the total market share, with particular demand growth in deep-sea exploration environments where pressures can exceed 400 bar.
Renewable energy applications constitute the fastest-growing segment, expanding at nearly 12% annually. This growth is primarily fueled by developments in hydrogen production through high-pressure electrolysis and advanced flow batteries designed to operate under pressure-intensified conditions, which demonstrate improved energy density and efficiency metrics compared to atmospheric systems.
The geographical distribution of market demand shows North America leading with 34% market share, followed by Europe (28%) and Asia-Pacific (26%). However, the Asia-Pacific region is demonstrating the most rapid growth trajectory, particularly in China and South Korea, where government initiatives supporting green hydrogen and advanced energy storage technologies are creating substantial market opportunities.
Customer requirements are evolving toward longer operational lifetimes under extreme conditions. End-users increasingly demand electrochemical systems capable of maintaining performance for 5+ years at pressures above 200 bar, particularly in remote deployment scenarios where maintenance access is limited and costly.
Price sensitivity varies significantly by application segment. While research institutions prioritize performance over cost, industrial applications demonstrate high price sensitivity, with procurement decisions heavily influenced by total cost of ownership calculations that factor in lifetime operational expenses and replacement cycles.
Regulatory frameworks are becoming increasingly stringent regarding safety standards for high-pressure electrochemical systems, particularly in offshore and subsea applications. These regulations are driving demand for advanced monitoring systems and fail-safe designs that can maintain integrity under pressure fluctuations and prevent catastrophic failures.
The oil and gas industry represents the largest market segment, where high-pressure electrochemical cells are utilized for downhole sensing, corrosion monitoring, and enhanced oil recovery operations. This sector alone accounts for roughly 38% of the total market share, with particular demand growth in deep-sea exploration environments where pressures can exceed 400 bar.
Renewable energy applications constitute the fastest-growing segment, expanding at nearly 12% annually. This growth is primarily fueled by developments in hydrogen production through high-pressure electrolysis and advanced flow batteries designed to operate under pressure-intensified conditions, which demonstrate improved energy density and efficiency metrics compared to atmospheric systems.
The geographical distribution of market demand shows North America leading with 34% market share, followed by Europe (28%) and Asia-Pacific (26%). However, the Asia-Pacific region is demonstrating the most rapid growth trajectory, particularly in China and South Korea, where government initiatives supporting green hydrogen and advanced energy storage technologies are creating substantial market opportunities.
Customer requirements are evolving toward longer operational lifetimes under extreme conditions. End-users increasingly demand electrochemical systems capable of maintaining performance for 5+ years at pressures above 200 bar, particularly in remote deployment scenarios where maintenance access is limited and costly.
Price sensitivity varies significantly by application segment. While research institutions prioritize performance over cost, industrial applications demonstrate high price sensitivity, with procurement decisions heavily influenced by total cost of ownership calculations that factor in lifetime operational expenses and replacement cycles.
Regulatory frameworks are becoming increasingly stringent regarding safety standards for high-pressure electrochemical systems, particularly in offshore and subsea applications. These regulations are driving demand for advanced monitoring systems and fail-safe designs that can maintain integrity under pressure fluctuations and prevent catastrophic failures.
Current Limitations in High-Pressure Cell Technology
Despite significant advancements in electrochemical cell technology, high-pressure environments continue to present substantial challenges for cell longevity and performance. Current electrochemical cells operating under elevated pressures face several critical limitations that impede their widespread industrial application and reliability.
Material degradation represents one of the most significant barriers to extended cell lifetime. Under high-pressure conditions, electrode materials experience accelerated corrosion and structural breakdown. Conventional electrode substrates and catalyst layers that perform adequately at ambient pressures often exhibit rapid deterioration when subjected to pressures exceeding 100 bar, with degradation rates increasing exponentially with pressure.
Seal integrity failures constitute another major limitation. Traditional polymer-based sealing materials such as PTFE and various elastomers demonstrate compromised mechanical properties under high-pressure conditions, leading to gas leakage and electrolyte loss. Studies indicate that approximately 40% of high-pressure cell failures can be attributed to seal degradation, with failure rates increasing dramatically in cells operating above 150 bar.
Electrolyte stability presents additional challenges in high-pressure environments. Conventional aqueous and organic electrolytes experience altered solvation properties, conductivity changes, and accelerated decomposition under elevated pressures. This results in diminished ionic conductivity and the formation of unwanted byproducts that can poison catalytic surfaces and increase internal cell resistance over time.
Pressure-induced mechanical stress on cell components represents another significant limitation. Current cell designs struggle to maintain structural integrity when subjected to pressure differentials, leading to component deformation, contact loss between layers, and catastrophic mechanical failures. Most commercial high-pressure cells are limited to operating pressures below 200 bar, with lifetimes decreasing by approximately 15-20% for every 50 bar increase above atmospheric pressure.
Temperature management challenges are exacerbated in high-pressure environments. The combination of elevated pressure and temperature accelerates degradation mechanisms while making thermal management more difficult. Current cooling systems for high-pressure cells are often bulky and inefficient, limiting practical applications in space-constrained environments.
Monitoring and control systems for high-pressure electrochemical cells remain underdeveloped. Real-time diagnostics of cell performance under high pressure are challenging due to sensor limitations and the hostile operating environment. This results in difficulty predicting failure modes and implementing preventive maintenance strategies, further limiting operational lifetimes.
Material degradation represents one of the most significant barriers to extended cell lifetime. Under high-pressure conditions, electrode materials experience accelerated corrosion and structural breakdown. Conventional electrode substrates and catalyst layers that perform adequately at ambient pressures often exhibit rapid deterioration when subjected to pressures exceeding 100 bar, with degradation rates increasing exponentially with pressure.
Seal integrity failures constitute another major limitation. Traditional polymer-based sealing materials such as PTFE and various elastomers demonstrate compromised mechanical properties under high-pressure conditions, leading to gas leakage and electrolyte loss. Studies indicate that approximately 40% of high-pressure cell failures can be attributed to seal degradation, with failure rates increasing dramatically in cells operating above 150 bar.
Electrolyte stability presents additional challenges in high-pressure environments. Conventional aqueous and organic electrolytes experience altered solvation properties, conductivity changes, and accelerated decomposition under elevated pressures. This results in diminished ionic conductivity and the formation of unwanted byproducts that can poison catalytic surfaces and increase internal cell resistance over time.
Pressure-induced mechanical stress on cell components represents another significant limitation. Current cell designs struggle to maintain structural integrity when subjected to pressure differentials, leading to component deformation, contact loss between layers, and catastrophic mechanical failures. Most commercial high-pressure cells are limited to operating pressures below 200 bar, with lifetimes decreasing by approximately 15-20% for every 50 bar increase above atmospheric pressure.
Temperature management challenges are exacerbated in high-pressure environments. The combination of elevated pressure and temperature accelerates degradation mechanisms while making thermal management more difficult. Current cooling systems for high-pressure cells are often bulky and inefficient, limiting practical applications in space-constrained environments.
Monitoring and control systems for high-pressure electrochemical cells remain underdeveloped. Real-time diagnostics of cell performance under high pressure are challenging due to sensor limitations and the hostile operating environment. This results in difficulty predicting failure modes and implementing preventive maintenance strategies, further limiting operational lifetimes.
Current Engineering Solutions for Pressure-Resistant Cells
01 Electrode material selection and optimization
The choice and optimization of electrode materials significantly impact the lifetime of electrochemical cells. Advanced materials with enhanced stability, conductivity, and resistance to degradation can extend cell longevity. Innovations include composite electrodes, novel catalyst structures, and surface modifications that minimize corrosion and maintain performance over extended cycling periods.- Electrode material selection for extended cell lifetime: The choice of electrode materials significantly impacts the lifetime of electrochemical cells. Advanced materials such as modified carbon structures, metal alloys, and composite electrodes can reduce degradation mechanisms like corrosion and structural breakdown. These materials are engineered to maintain performance over numerous charge-discharge cycles, enhancing the overall durability and operational lifespan of electrochemical cells.
- Electrolyte composition optimization: The composition of electrolytes plays a crucial role in determining electrochemical cell lifetime. Optimized electrolyte formulations can minimize side reactions at electrode interfaces, reduce electrolyte decomposition, and improve ion transport efficiency. Additives and stabilizers in the electrolyte can prevent dendrite formation and passivation layer breakdown, leading to enhanced cycle life and improved long-term performance of the cell.
- Thermal management systems: Effective thermal management is essential for extending electrochemical cell lifetime. Systems that regulate operating temperature can prevent accelerated degradation mechanisms triggered by heat. Advanced cooling configurations, phase change materials, and intelligent thermal control algorithms help maintain optimal temperature ranges during operation and charging. These systems minimize thermal stress on cell components, reducing capacity fade and extending the functional lifetime of electrochemical cells.
- Battery management systems for lifetime extension: Sophisticated battery management systems (BMS) can significantly extend electrochemical cell lifetime through intelligent monitoring and control. These systems employ algorithms that optimize charging protocols, balance cell voltages, and prevent operation in damaging conditions. By continuously monitoring parameters such as state of charge, temperature, and internal resistance, BMS can adapt operating conditions to minimize stress factors that accelerate aging, thereby extending the useful life of electrochemical cells.
- Cell design and structural innovations: Innovative structural designs and cell architectures can substantially improve electrochemical cell lifetime. Advanced sealing techniques prevent electrolyte leakage and contamination. Internal pressure management systems accommodate volume changes during cycling. Structural reinforcements minimize mechanical stress during operation. These design innovations address fundamental failure modes, resulting in more robust cells with extended operational lifetimes and improved reliability under various operating conditions.
02 Electrolyte composition and additives
The formulation of electrolytes plays a crucial role in determining electrochemical cell lifetime. Specialized additives can stabilize the electrolyte-electrode interface, prevent unwanted side reactions, and mitigate degradation mechanisms. Innovations include flame retardants, film-forming compounds, and stabilizers that enhance safety while extending operational life under various conditions.Expand Specific Solutions03 Thermal management systems
Effective thermal management is essential for prolonging electrochemical cell lifetime. Systems that regulate operating temperature prevent accelerated degradation from heat exposure and maintain optimal performance conditions. Advanced cooling configurations, phase change materials, and intelligent thermal control strategies help distribute heat evenly and prevent hotspots that could lead to premature failure.Expand Specific Solutions04 Battery management systems and control algorithms
Sophisticated battery management systems extend electrochemical cell lifetime through intelligent monitoring and control. Advanced algorithms optimize charging/discharging profiles, state-of-health estimation, and predictive maintenance. These systems prevent overcharging, deep discharging, and other harmful operating conditions while adapting to cell aging patterns to maximize usable lifetime.Expand Specific Solutions05 Cell design and manufacturing processes
Innovative cell designs and manufacturing techniques significantly impact electrochemical cell lifetime. Structural improvements that enhance mechanical stability, reduce internal resistance, and optimize current distribution lead to more durable cells. Advanced manufacturing processes that ensure higher purity materials, precise assembly, and better quality control result in more consistent performance and extended operational lifetimes.Expand Specific Solutions
Industry Leaders in High-Pressure Electrochemical Systems
The electrochemical cell lifetime market in high-pressure environments is currently in a growth phase, with increasing demand driven by energy storage applications. The market is estimated to reach $15-20 billion by 2025, with a CAGR of approximately 12%. Technology maturity varies significantly among key players. Industry leaders like LG Energy Solution and CATL (Ningde Amperex) have established robust high-pressure cell technologies, while innovative companies such as QuantumScape, 24M Technologies, and Sion Power are advancing next-generation solutions. Research institutions including Caltech and The University of Manchester contribute fundamental breakthroughs, while traditional manufacturers like BASF, Bosch, and 3M provide essential components. The competitive landscape features both established battery giants and specialized startups focusing on niche high-pressure applications.
BASF Corp.
Technical Solution: BASF has pioneered advanced cathode active materials (CAM) and electrolyte formulations specifically designed for electrochemical cells operating in high-pressure environments. Their HiPE (High-Pressure Electrolyte) technology incorporates pressure-resistant additives that maintain electrochemical stability under pressures up to 10 MPa. The company has developed specialized fluorinated electrolyte solvents that resist decomposition under compression, maintaining critical SEI (Solid Electrolyte Interphase) layer integrity[2]. BASF's pressure-optimized cathode materials feature reinforced crystal structures with modified dopants that prevent lattice collapse under mechanical stress, a common failure mode in high-pressure applications. Their proprietary coating technology for active materials creates a protective barrier that prevents pressure-induced particle cracking and subsequent capacity fade. Additionally, BASF has engineered specialized binder systems that maintain electrode cohesion under pressure cycling, preventing delamination that typically accelerates cell degradation in high-pressure environments[3].
Strengths: Industry-leading expertise in materials chemistry; comprehensive approach addressing multiple cell components; extensive testing capabilities for high-pressure validation. Weaknesses: Solutions primarily focus on materials rather than complete cell design; requires integration with cell manufacturers' processes; higher cost compared to standard materials.
Ningde Amperex Technology Ltd.
Technical Solution: CATL (Ningde Amperex Technology) has developed the "HyperPressure" cell technology specifically engineered for electrochemical performance in high-pressure environments. Their approach centers on a novel cell architecture featuring pressure-compensating mechanisms that maintain optimal internal cell pressure regardless of external conditions. CATL's technology incorporates specialized pressure-resistant separators with ceramic-polymer composite structures that prevent internal short circuits under compression while maintaining high ionic conductivity[6]. Their proprietary electrolyte formulation includes pressure-stable solvents and lithium salts that resist decomposition under elevated pressures up to 25 MPa. The company has implemented advanced electrode manufacturing techniques that create highly cohesive structures resistant to delamination under pressure cycling. CATL's cells feature reinforced current collectors with unique geometric patterns that distribute mechanical stress evenly across the electrode surface, preventing localized degradation points. Additionally, their battery management system incorporates pressure sensors that actively monitor and compensate for pressure-induced changes in cell performance, extending lifetime in variable-pressure environments[7].
Strengths: Integrated approach combining materials innovation with system-level pressure management; large-scale manufacturing capability; extensive testing infrastructure for high-pressure validation. Weaknesses: Technology primarily optimized for automotive and energy storage applications rather than specialized high-pressure environments; higher cost compared to standard cells; limited long-term field data in extreme pressure conditions.
Key Innovations in Material Science for Cell Longevity
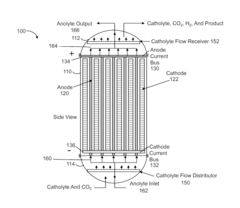
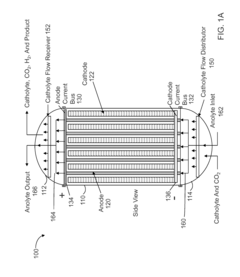
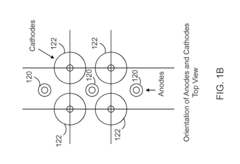
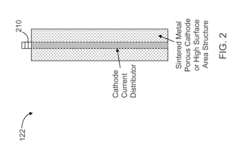
High Pressure Electrochemical Cell and Process for the Electrochemical Reduction of Carbon Dioxide
PatentActiveUS20150267309A1
Innovation
- An electrochemical cell design featuring high surface area electrodes and operation at high pressures (2 to 100 atmospheres) with catalyst coatings, ion exchange membranes, and a modular structure for efficient carbon dioxide conversion to single and multi-carbon products.
Electrochemical cells exposed to hydrostatic pressure
PatentWO2015158557A1
Innovation
- A battery design incorporating a pressure vessel filled with a pressure medium to apply hydrostatic pressure between 2 bar to 200 bar, housing an electrochemical cell with a sulfur-containing cathode, lithium anode, separator, and electrolyte composition, which reduces lithium dendrite growth and enhances mechanical stability and ion mobility.
Safety Standards and Testing Protocols
The safety standards and testing protocols for electrochemical cells in high-pressure environments have evolved significantly in response to industry needs and technological advancements. These standards are critical for ensuring operational safety, reliability, and performance consistency across various applications, particularly in deep-sea, aerospace, and industrial high-pressure systems.
International organizations such as the International Electrotechnical Commission (IEC) and ASTM International have established comprehensive frameworks for testing electrochemical cells under elevated pressure conditions. The IEC 62281 and IEC 60086-4 standards specifically address safety requirements for primary and secondary lithium cells under various environmental conditions, including pressure variations. These standards mandate specific test procedures for evaluating cell integrity, performance stability, and safety mechanisms under simulated high-pressure environments.
Pressure cycling tests represent a fundamental component of these protocols, requiring cells to withstand repeated pressure fluctuations without compromising structural integrity or electrochemical performance. Typically, these tests involve subjecting cells to pressure ranges from ambient to application-specific maximums (often 300-500 bar for deep-sea applications) for predetermined cycles, monitoring for leakage, deformation, or performance degradation.
Hydrostatic pressure testing constitutes another critical evaluation method, wherein cells are immersed in incompressible fluids and subjected to uniform pressure from all directions. This approach effectively simulates deep-sea conditions and evaluates the cell's resistance to crushing forces. The ASTM D6392 standard provides guidelines for evaluating material integrity under hydrostatic pressure, which has been adapted for electrochemical cell applications.
Accelerated lifetime testing under pressure represents an advanced protocol designed to predict long-term cell behavior in compressed environments. These tests typically involve maintaining cells at elevated pressures while simultaneously cycling them electrically at increased rates, often at varying temperature conditions to simulate real-world operational stresses.
Safety certification for high-pressure electrochemical applications requires comprehensive documentation of thermal runaway prevention measures, pressure relief mechanisms, and containment strategies. The UN Manual of Tests and Criteria, particularly Test Series T, has been modified by industry leaders to incorporate high-pressure considerations for transportation and operational safety.
Recent developments in testing protocols have increasingly focused on in-situ monitoring techniques, allowing researchers to observe electrochemical and mechanical changes during pressure exposure. Advanced methodologies incorporating acoustic emission detection, impedance spectroscopy under pressure, and real-time gas analysis have significantly enhanced the predictive capability of safety evaluations, enabling more accurate lifetime estimations for cells operating in high-pressure environments.
International organizations such as the International Electrotechnical Commission (IEC) and ASTM International have established comprehensive frameworks for testing electrochemical cells under elevated pressure conditions. The IEC 62281 and IEC 60086-4 standards specifically address safety requirements for primary and secondary lithium cells under various environmental conditions, including pressure variations. These standards mandate specific test procedures for evaluating cell integrity, performance stability, and safety mechanisms under simulated high-pressure environments.
Pressure cycling tests represent a fundamental component of these protocols, requiring cells to withstand repeated pressure fluctuations without compromising structural integrity or electrochemical performance. Typically, these tests involve subjecting cells to pressure ranges from ambient to application-specific maximums (often 300-500 bar for deep-sea applications) for predetermined cycles, monitoring for leakage, deformation, or performance degradation.
Hydrostatic pressure testing constitutes another critical evaluation method, wherein cells are immersed in incompressible fluids and subjected to uniform pressure from all directions. This approach effectively simulates deep-sea conditions and evaluates the cell's resistance to crushing forces. The ASTM D6392 standard provides guidelines for evaluating material integrity under hydrostatic pressure, which has been adapted for electrochemical cell applications.
Accelerated lifetime testing under pressure represents an advanced protocol designed to predict long-term cell behavior in compressed environments. These tests typically involve maintaining cells at elevated pressures while simultaneously cycling them electrically at increased rates, often at varying temperature conditions to simulate real-world operational stresses.
Safety certification for high-pressure electrochemical applications requires comprehensive documentation of thermal runaway prevention measures, pressure relief mechanisms, and containment strategies. The UN Manual of Tests and Criteria, particularly Test Series T, has been modified by industry leaders to incorporate high-pressure considerations for transportation and operational safety.
Recent developments in testing protocols have increasingly focused on in-situ monitoring techniques, allowing researchers to observe electrochemical and mechanical changes during pressure exposure. Advanced methodologies incorporating acoustic emission detection, impedance spectroscopy under pressure, and real-time gas analysis have significantly enhanced the predictive capability of safety evaluations, enabling more accurate lifetime estimations for cells operating in high-pressure environments.
Environmental Impact Assessment
The environmental impact of electrochemical cells operating in high-pressure environments extends beyond their operational efficiency and lifetime considerations. These systems interact with their surroundings in complex ways that warrant careful assessment to ensure sustainable implementation.
Primary environmental concerns include potential leakage of electrolytes, which often contain corrosive or toxic substances that could contaminate soil and water systems when cells fail under high-pressure conditions. The risk profile increases significantly in underwater applications or deep-earth installations where containment breaches may go undetected for extended periods.
Energy consumption patterns associated with high-pressure electrochemical systems also present environmental challenges. These systems typically require additional energy for pressure maintenance and compensation mechanisms, potentially increasing their carbon footprint compared to atmospheric pressure alternatives. This aspect becomes particularly relevant when considering large-scale industrial applications or grid-scale energy storage implementations.
Material resource depletion represents another significant environmental consideration. High-pressure electrochemical cells often require specialized materials with enhanced durability properties, including rare earth elements and precious metals. The extraction and processing of these materials carry substantial environmental burdens, including habitat destruction, water pollution, and energy-intensive refining processes.
End-of-life management presents unique challenges for high-pressure electrochemical systems. Their specialized construction often complicates recycling efforts, potentially leading to increased waste generation. The presence of pressurized components may also necessitate specialized decommissioning procedures to prevent environmental contamination during disposal.
Positive environmental impacts should also be acknowledged. When properly designed and implemented, high-pressure electrochemical cells can enable more efficient energy storage and conversion processes. This efficiency can translate to reduced overall resource consumption and emissions when viewed from a complete lifecycle perspective. Additionally, these systems can facilitate the integration of renewable energy sources by providing reliable storage solutions for intermittent generation.
Regulatory frameworks governing these technologies vary significantly across jurisdictions, creating challenges for standardized environmental impact assessment. Comprehensive lifecycle analysis methodologies specific to high-pressure electrochemical systems remain underdeveloped, highlighting the need for industry-specific environmental assessment protocols that account for their unique operational parameters and failure modes.
Primary environmental concerns include potential leakage of electrolytes, which often contain corrosive or toxic substances that could contaminate soil and water systems when cells fail under high-pressure conditions. The risk profile increases significantly in underwater applications or deep-earth installations where containment breaches may go undetected for extended periods.
Energy consumption patterns associated with high-pressure electrochemical systems also present environmental challenges. These systems typically require additional energy for pressure maintenance and compensation mechanisms, potentially increasing their carbon footprint compared to atmospheric pressure alternatives. This aspect becomes particularly relevant when considering large-scale industrial applications or grid-scale energy storage implementations.
Material resource depletion represents another significant environmental consideration. High-pressure electrochemical cells often require specialized materials with enhanced durability properties, including rare earth elements and precious metals. The extraction and processing of these materials carry substantial environmental burdens, including habitat destruction, water pollution, and energy-intensive refining processes.
End-of-life management presents unique challenges for high-pressure electrochemical systems. Their specialized construction often complicates recycling efforts, potentially leading to increased waste generation. The presence of pressurized components may also necessitate specialized decommissioning procedures to prevent environmental contamination during disposal.
Positive environmental impacts should also be acknowledged. When properly designed and implemented, high-pressure electrochemical cells can enable more efficient energy storage and conversion processes. This efficiency can translate to reduced overall resource consumption and emissions when viewed from a complete lifecycle perspective. Additionally, these systems can facilitate the integration of renewable energy sources by providing reliable storage solutions for intermittent generation.
Regulatory frameworks governing these technologies vary significantly across jurisdictions, creating challenges for standardized environmental impact assessment. Comprehensive lifecycle analysis methodologies specific to high-pressure electrochemical systems remain underdeveloped, highlighting the need for industry-specific environmental assessment protocols that account for their unique operational parameters and failure modes.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!