Electrochemical Cell Vs Alkaline Battery: Lifespan Metrics
AUG 28, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Battery Technology Evolution and Performance Goals
Battery technology has evolved significantly since the invention of the first electrochemical cell by Alessandro Volta in 1800. The journey from primitive zinc-copper cells to today's sophisticated energy storage solutions represents over two centuries of continuous innovation driven by increasing energy demands and application requirements. Early batteries were primarily simple primary cells with limited capacity and short lifespans, whereas modern batteries incorporate advanced materials science and electrochemistry to achieve superior performance metrics.
The evolution of alkaline batteries began in the 1950s when Lewis Urry at Eveready Battery Company (now Energizer) developed the first commercially viable alkaline battery. This innovation represented a significant improvement over the zinc-carbon batteries prevalent at that time, offering higher energy density and longer shelf life. The alkaline battery technology has since undergone numerous refinements to enhance its performance characteristics, particularly in terms of lifespan and reliability.
Electrochemical cells, the broader category encompassing various battery types including alkaline batteries, have diversified into numerous specialized forms. The technological trajectory has been marked by the development of rechargeable variants, miniaturization for portable electronics, and the pursuit of higher energy densities and longer operational lifespans. This evolution reflects the industry's response to changing consumer needs and environmental considerations.
Performance goals in battery technology are increasingly focused on several key metrics: energy density (both volumetric and gravimetric), cycle life, calendar life, charge/discharge efficiency, temperature tolerance, and environmental impact. For alkaline batteries specifically, the industry has established benchmarks for shelf life (typically 5-10 years), continuous discharge performance, and capacity retention under various load conditions. These metrics serve as critical indicators of battery quality and suitability for different applications.
The comparison between general electrochemical cells and alkaline batteries specifically reveals distinct performance trajectories. While alkaline batteries have reached a relatively mature state with incremental improvements in lifespan and capacity, newer electrochemical cell technologies continue to demonstrate substantial growth potential. The industry aims to develop batteries that can deliver thousands of charge-discharge cycles while maintaining 80% or more of original capacity, representing a significant advancement over traditional alkaline batteries.
Current technological goals include developing batteries with self-healing capabilities to extend lifespan, improving low-temperature performance, and reducing internal resistance to enhance power delivery. Additionally, there is growing emphasis on sustainability metrics, including recyclability, reduced toxic materials, and lower carbon footprint throughout the battery lifecycle. These evolving performance targets reflect both market demands and regulatory pressures shaping the future of battery technology.
The evolution of alkaline batteries began in the 1950s when Lewis Urry at Eveready Battery Company (now Energizer) developed the first commercially viable alkaline battery. This innovation represented a significant improvement over the zinc-carbon batteries prevalent at that time, offering higher energy density and longer shelf life. The alkaline battery technology has since undergone numerous refinements to enhance its performance characteristics, particularly in terms of lifespan and reliability.
Electrochemical cells, the broader category encompassing various battery types including alkaline batteries, have diversified into numerous specialized forms. The technological trajectory has been marked by the development of rechargeable variants, miniaturization for portable electronics, and the pursuit of higher energy densities and longer operational lifespans. This evolution reflects the industry's response to changing consumer needs and environmental considerations.
Performance goals in battery technology are increasingly focused on several key metrics: energy density (both volumetric and gravimetric), cycle life, calendar life, charge/discharge efficiency, temperature tolerance, and environmental impact. For alkaline batteries specifically, the industry has established benchmarks for shelf life (typically 5-10 years), continuous discharge performance, and capacity retention under various load conditions. These metrics serve as critical indicators of battery quality and suitability for different applications.
The comparison between general electrochemical cells and alkaline batteries specifically reveals distinct performance trajectories. While alkaline batteries have reached a relatively mature state with incremental improvements in lifespan and capacity, newer electrochemical cell technologies continue to demonstrate substantial growth potential. The industry aims to develop batteries that can deliver thousands of charge-discharge cycles while maintaining 80% or more of original capacity, representing a significant advancement over traditional alkaline batteries.
Current technological goals include developing batteries with self-healing capabilities to extend lifespan, improving low-temperature performance, and reducing internal resistance to enhance power delivery. Additionally, there is growing emphasis on sustainability metrics, including recyclability, reduced toxic materials, and lower carbon footprint throughout the battery lifecycle. These evolving performance targets reflect both market demands and regulatory pressures shaping the future of battery technology.
Market Demand Analysis for Battery Solutions
The global battery market has witnessed significant growth in recent years, driven by increasing demand for portable electronic devices, electric vehicles, and renewable energy storage solutions. The market for battery solutions is expected to reach $310 billion by 2027, with a compound annual growth rate of 14.1% from 2020 to 2027. Within this expanding market, electrochemical cells and alkaline batteries represent two distinct yet important segments with different performance characteristics, particularly regarding lifespan metrics.
Consumer electronics continue to be a major driver for battery demand, with approximately 1.5 billion smartphones sold annually worldwide. These devices predominantly use lithium-ion electrochemical cells due to their superior energy density and rechargeability. Market research indicates that consumers increasingly prioritize battery life when purchasing electronic devices, with 78% of smartphone users citing battery performance as a critical factor in their buying decisions.
The industrial sector presents another significant market for battery solutions, particularly for applications requiring long-term reliability. Industrial equipment manufacturers report that maintenance costs related to battery replacement can account for up to 15% of total operational expenses. This has created a growing demand for batteries with extended lifespan metrics, where the performance differences between electrochemical cells and alkaline batteries become particularly relevant.
Renewable energy storage represents the fastest-growing segment for advanced battery solutions, expanding at 22% annually. Grid-scale energy storage installations increased by 62% in 2022 compared to the previous year. This sector predominantly utilizes sophisticated electrochemical cells rather than traditional alkaline batteries due to their cycle life advantages and deeper discharge capabilities.
Consumer awareness regarding battery disposal and environmental impact has also shaped market demand. Surveys indicate that 65% of consumers in developed markets express concern about battery waste. This has created a market premium for rechargeable electrochemical cells, which can reduce waste by replacing hundreds of single-use alkaline batteries over their lifespan.
Regional analysis reveals varying demand patterns, with North America and Europe showing stronger preference for high-performance electrochemical cells despite higher initial costs, while developing markets maintain significant demand for lower-cost alkaline batteries. However, this gap is narrowing as manufacturing efficiencies reduce the cost differential between these technologies.
The medical device sector represents a specialized market segment where battery reliability is critical. Medical equipment manufacturers report that battery failure accounts for approximately 17% of device malfunctions, creating demand for solutions with predictable lifespan metrics and clear end-of-life indicators.
Consumer electronics continue to be a major driver for battery demand, with approximately 1.5 billion smartphones sold annually worldwide. These devices predominantly use lithium-ion electrochemical cells due to their superior energy density and rechargeability. Market research indicates that consumers increasingly prioritize battery life when purchasing electronic devices, with 78% of smartphone users citing battery performance as a critical factor in their buying decisions.
The industrial sector presents another significant market for battery solutions, particularly for applications requiring long-term reliability. Industrial equipment manufacturers report that maintenance costs related to battery replacement can account for up to 15% of total operational expenses. This has created a growing demand for batteries with extended lifespan metrics, where the performance differences between electrochemical cells and alkaline batteries become particularly relevant.
Renewable energy storage represents the fastest-growing segment for advanced battery solutions, expanding at 22% annually. Grid-scale energy storage installations increased by 62% in 2022 compared to the previous year. This sector predominantly utilizes sophisticated electrochemical cells rather than traditional alkaline batteries due to their cycle life advantages and deeper discharge capabilities.
Consumer awareness regarding battery disposal and environmental impact has also shaped market demand. Surveys indicate that 65% of consumers in developed markets express concern about battery waste. This has created a market premium for rechargeable electrochemical cells, which can reduce waste by replacing hundreds of single-use alkaline batteries over their lifespan.
Regional analysis reveals varying demand patterns, with North America and Europe showing stronger preference for high-performance electrochemical cells despite higher initial costs, while developing markets maintain significant demand for lower-cost alkaline batteries. However, this gap is narrowing as manufacturing efficiencies reduce the cost differential between these technologies.
The medical device sector represents a specialized market segment where battery reliability is critical. Medical equipment manufacturers report that battery failure accounts for approximately 17% of device malfunctions, creating demand for solutions with predictable lifespan metrics and clear end-of-life indicators.
Current State and Challenges in Battery Technology
The global battery technology landscape is currently experiencing unprecedented growth, with the market expected to reach $310 billion by 2027. This expansion is driven primarily by increasing demand for portable electronics, electric vehicles, and renewable energy storage solutions. Within this context, electrochemical cells and alkaline batteries represent two distinct technological approaches with significant differences in their lifespan metrics and performance characteristics.
Alkaline batteries, which dominate the consumer disposable market, have reached a technological plateau in recent years. While improvements in manufacturing processes have increased their shelf life to approximately 10 years, their operational lifespan remains limited to 4-8 hours of continuous use for high-drain applications. This technology faces fundamental chemical constraints that limit further significant advancements in energy density and discharge characteristics.
In contrast, rechargeable electrochemical cells continue to evolve rapidly. Lithium-ion technology, the most prominent electrochemical cell type, has seen energy density improvements of approximately 5-8% annually over the past decade. Modern lithium-ion cells can achieve 500-1000 complete charge cycles while maintaining 80% of their original capacity, translating to a functional lifespan of 2-5 years depending on usage patterns.
A significant challenge facing both technologies is the trade-off between energy density and lifespan. Engineering solutions that maximize one metric typically compromise the other. For alkaline batteries, increasing manganese dioxide concentration can improve capacity but accelerates internal corrosion, reducing shelf life. Similarly, for lithium-ion cells, higher energy densities often correlate with faster capacity degradation over repeated charge cycles.
Temperature sensitivity remains a critical limitation for both technologies. Alkaline batteries lose up to 60% of their capacity at 0°C, while lithium-ion cells experience accelerated aging at temperatures above 30°C, with capacity degradation rates doubling with each 10°C increase. This thermal vulnerability presents significant challenges for applications in extreme environments.
Safety concerns continue to constrain development, particularly for high-energy-density electrochemical cells. Thermal runaway risks in lithium-ion batteries have necessitated sophisticated battery management systems, adding complexity and cost. Meanwhile, alkaline batteries face environmental challenges due to their disposal impact, despite improvements in reducing mercury and other toxic components.
Resource constraints represent another growing challenge, with critical materials like cobalt, nickel, and lithium facing supply limitations. Price volatility for these materials has increased by over 200% in the past five years, creating uncertainty for manufacturers and potentially limiting production scaling capabilities for next-generation battery technologies.
Alkaline batteries, which dominate the consumer disposable market, have reached a technological plateau in recent years. While improvements in manufacturing processes have increased their shelf life to approximately 10 years, their operational lifespan remains limited to 4-8 hours of continuous use for high-drain applications. This technology faces fundamental chemical constraints that limit further significant advancements in energy density and discharge characteristics.
In contrast, rechargeable electrochemical cells continue to evolve rapidly. Lithium-ion technology, the most prominent electrochemical cell type, has seen energy density improvements of approximately 5-8% annually over the past decade. Modern lithium-ion cells can achieve 500-1000 complete charge cycles while maintaining 80% of their original capacity, translating to a functional lifespan of 2-5 years depending on usage patterns.
A significant challenge facing both technologies is the trade-off between energy density and lifespan. Engineering solutions that maximize one metric typically compromise the other. For alkaline batteries, increasing manganese dioxide concentration can improve capacity but accelerates internal corrosion, reducing shelf life. Similarly, for lithium-ion cells, higher energy densities often correlate with faster capacity degradation over repeated charge cycles.
Temperature sensitivity remains a critical limitation for both technologies. Alkaline batteries lose up to 60% of their capacity at 0°C, while lithium-ion cells experience accelerated aging at temperatures above 30°C, with capacity degradation rates doubling with each 10°C increase. This thermal vulnerability presents significant challenges for applications in extreme environments.
Safety concerns continue to constrain development, particularly for high-energy-density electrochemical cells. Thermal runaway risks in lithium-ion batteries have necessitated sophisticated battery management systems, adding complexity and cost. Meanwhile, alkaline batteries face environmental challenges due to their disposal impact, despite improvements in reducing mercury and other toxic components.
Resource constraints represent another growing challenge, with critical materials like cobalt, nickel, and lithium facing supply limitations. Price volatility for these materials has increased by over 200% in the past five years, creating uncertainty for manufacturers and potentially limiting production scaling capabilities for next-generation battery technologies.
Comparative Analysis of Battery Lifespan Metrics
01 Electrode material composition for extended battery life
The composition of electrode materials significantly impacts alkaline battery lifespan. Advanced materials such as modified zinc anodes with reduced corrosion properties and improved manganese dioxide cathodes can enhance battery performance. Incorporating specific additives and optimizing particle size distribution in electrode materials helps maintain capacity during discharge cycles and prevents internal degradation mechanisms, resulting in longer-lasting batteries with improved shelf life.- Electrode materials and compositions for extended battery life: Advanced electrode materials and compositions can significantly enhance the lifespan of alkaline batteries and electrochemical cells. By optimizing the composition of cathode and anode materials, incorporating specific additives, and improving the structural integrity of electrodes, battery performance can be extended. These innovations reduce degradation during charge-discharge cycles and enhance the overall energy density and longevity of the battery systems.
- Electrolyte formulations for improved battery performance: Specialized electrolyte formulations play a crucial role in extending alkaline battery lifespan. By modifying the electrolyte composition with specific additives, stabilizers, and conductivity enhancers, ion transport efficiency can be improved while reducing corrosion and side reactions. These formulations help maintain consistent performance throughout the battery's operational life and prevent capacity loss during storage and usage.
- Separator technology and internal battery architecture: Advanced separator designs and internal battery architectures significantly impact alkaline battery lifespan. Innovative separator materials with optimized porosity, thickness, and chemical stability prevent internal short circuits while facilitating efficient ion transport. Strategic internal component arrangement and improved sealing techniques reduce electrolyte leakage and enhance the overall structural integrity of the battery, resulting in extended operational life.
- Battery management systems and discharge control: Sophisticated battery management systems and discharge control mechanisms help maximize alkaline battery lifespan. These systems monitor and regulate current flow, temperature, and voltage parameters to prevent harmful operating conditions. By implementing smart discharge algorithms and protective circuits, batteries can be protected from deep discharge, overcharging, and thermal stress, which are common factors that reduce battery life expectancy.
- Manufacturing processes and quality control techniques: Advanced manufacturing processes and quality control techniques significantly impact alkaline battery lifespan. Precision in component assembly, improved sealing methods, and contamination control during production ensure consistent battery performance. Post-production testing protocols identify potential failure points before batteries reach consumers. These manufacturing innovations result in more reliable batteries with extended shelf life and operational longevity under various usage conditions.
02 Electrolyte formulation improvements
Specialized electrolyte formulations can significantly extend alkaline battery lifespan. By incorporating corrosion inhibitors, conductivity enhancers, and stabilizing agents into the electrolyte solution, internal cell reactions that lead to self-discharge can be minimized. Modified potassium hydroxide electrolytes with specific additives help maintain optimal ionic conductivity throughout the battery's life while reducing unwanted side reactions that typically degrade performance over time.Expand Specific Solutions03 Separator design and materials
Advanced separator designs and materials play a crucial role in extending alkaline battery lifespan. Innovative separators with optimized porosity and ion-selective properties help prevent internal short circuits while facilitating efficient ion transport between electrodes. Composite separators incorporating specialized polymers and inorganic materials can reduce dendrite formation and maintain electrolyte distribution, resulting in more stable performance throughout the battery's operational life.Expand Specific Solutions04 Battery management and monitoring systems
Advanced battery management and monitoring systems can significantly extend alkaline battery lifespan. These systems incorporate sensors and control circuits that optimize discharge rates, prevent deep discharge conditions, and monitor internal cell parameters. By implementing intelligent power management algorithms and real-time diagnostics, these systems can detect early signs of battery degradation and adjust operating conditions accordingly, maximizing the useful life of electrochemical cells.Expand Specific Solutions05 Sealing and casing technologies
Improved sealing and casing technologies contribute significantly to alkaline battery lifespan. Advanced sealing methods prevent electrolyte leakage and moisture ingress, which are common causes of premature battery failure. Corrosion-resistant casings with enhanced structural integrity protect internal components from environmental factors. Specialized venting mechanisms safely release pressure buildup during operation while maintaining cell integrity, resulting in batteries with extended shelf life and operational durability.Expand Specific Solutions
Key Industry Players in Battery Manufacturing
The electrochemical cell versus alkaline battery market is in a mature growth phase, with global battery market valued at approximately $108 billion and projected to reach $280 billion by 2030. Major players like Energizer, Duracell, and Panasonic dominate the alkaline segment, while companies such as CATL, Saft Groupe, and Toyota are advancing electrochemical cell technologies. Research institutions including Nanyang Technological University, Caltech, and Wuhan University are driving innovation in both technologies. The competitive landscape is evolving with newer entrants like Altris AB developing sustainable battery solutions, while established manufacturers focus on extending lifespan metrics through improved materials and manufacturing processes to meet growing consumer and industrial demands.
Energizer Brands LLC
Technical Solution: Energizer has developed advanced alkaline battery technology with PowerSeal Technology that prevents leakage and extends shelf life up to 12 years. Their alkaline batteries utilize a zinc anode, manganese dioxide cathode, and potassium hydroxide electrolyte system optimized for long-lasting performance. Energizer's research shows their premium alkaline batteries can last up to 10 times longer than standard zinc-carbon electrochemical cells in high-drain devices. Their proprietary formula includes a unique separator system that maintains electrolyte distribution throughout discharge cycles, preventing internal short circuits and extending operational lifespan. Energizer has also implemented cathode efficiency improvements that increase the utilization of active materials by approximately 25% compared to earlier generations, resulting in higher capacity retention during storage and improved performance at temperature extremes (-20°C to 40°C).
Strengths: Superior shelf life (up to 12 years), excellent performance in high-drain devices, and advanced leak protection technology. Weaknesses: Higher cost compared to standard batteries, limited performance in extreme temperature conditions, and environmental concerns regarding disposal of alkaline batteries.
Duracell U.S. Operations, Inc.
Technical Solution: Duracell has pioneered the Duralock Power Preserve Technology for their alkaline batteries, which provides a guaranteed shelf life of up to 10 years. Their alkaline battery design incorporates a high-density manganese dioxide cathode with a zinc anode in a steel casing, utilizing a proprietary separator that enhances ion transport while preventing internal shorts. Duracell's research demonstrates their alkaline batteries deliver up to 8 times longer life in digital cameras compared to standard zinc-carbon cells. Their patented cathode formulation includes specific additives that reduce internal resistance and improve high-drain performance. Duracell has also developed a three-layer protection system that effectively prevents leakage even after complete discharge, addressing one of the primary failure modes of alkaline batteries. Their manufacturing process includes a proprietary electrolyte formulation that balances conductivity with minimal corrosion effects, extending both storage and operational lifespan significantly beyond standard electrochemical cells.
Strengths: Excellent shelf life stability, superior performance in high-drain electronic devices, and advanced leak protection technology. Weaknesses: Premium pricing compared to basic batteries, gradual voltage decline during discharge that may affect performance in voltage-sensitive devices, and limited recyclability compared to rechargeable alternatives.
Technical Deep Dive: Electrochemical vs Alkaline Chemistry
Alkaline electrochemical cell with improved lifetime
PatentInactiveUS20050287438A1
Innovation
- Incorporating barium or strontium compounds, such as BaO, Ba(OH)2, SrO, Sr(OH)2, BaSO4, or SrSO4, into the negative electrode or cell housing, in amounts ranging from 1×10−3 to 6×10−3 mol/Ah, to reduce corrosion and enhance cell performance.
Low maintenance alkaline electrochemical cell
PatentWO2014067982A1
Innovation
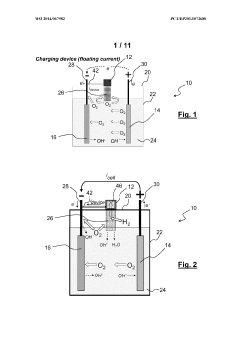
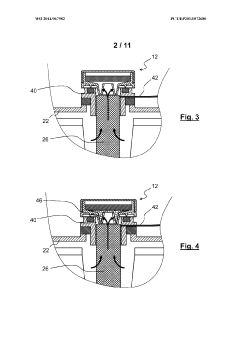
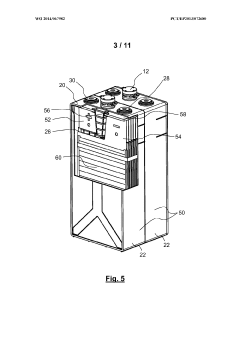
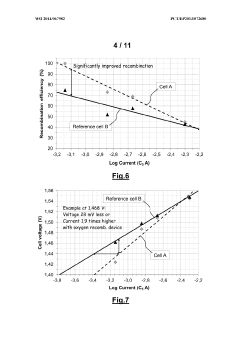
- An alkaline electrochemical cell design incorporating a catalytically active substrate for oxygen recombination, electrically connected to the negative terminal and isolated from the positive terminal, facilitates the recombination of all formed oxygen, reducing water loss and internal pressure, and enhancing volumetric energy density.
Environmental Impact and Sustainability Considerations
The environmental impact of battery technologies has become increasingly important as global sustainability concerns grow. When comparing electrochemical cells and alkaline batteries, their environmental footprints differ significantly throughout their lifecycle - from raw material extraction to disposal or recycling.
Alkaline batteries contain potentially harmful materials including zinc, manganese dioxide, and small amounts of mercury in older formulations. While modern alkaline batteries have reduced mercury content following regulatory changes, they still pose environmental challenges when improperly disposed of. These batteries typically end up in landfills where they can leach chemicals into soil and groundwater systems over time.
In contrast, many electrochemical cells, particularly rechargeable varieties like lithium-ion, nickel-metal hydride, and lead-acid cells, offer significant sustainability advantages through their extended lifespan and reusability. A single rechargeable electrochemical cell can replace hundreds of alkaline batteries over its operational life, substantially reducing waste generation and resource consumption. However, these cells often contain more toxic materials including lithium, cobalt, and heavy metals that require specialized handling at end-of-life.
The energy efficiency metrics also favor electrochemical cells. Manufacturing a single-use alkaline battery requires approximately 50 times more energy than the battery itself will deliver during use. Rechargeable electrochemical cells demonstrate superior energy return on investment, with their multiple charge cycles offsetting the initial manufacturing energy input.
Recycling infrastructure represents another critical sustainability consideration. Alkaline batteries have historically low recycling rates (below 5% globally) due to economic challenges in the recovery process. Conversely, electrochemical cells, particularly those containing valuable materials like lithium and cobalt, have more established recycling pathways with recovery rates reaching 50-95% in advanced facilities.
Carbon footprint assessments reveal that rechargeable electrochemical cells produce significantly lower greenhouse gas emissions per unit of energy delivered compared to alkaline alternatives. A lifecycle analysis shows that a rechargeable cell used for 500 cycles produces approximately 98% less waste and 90% fewer greenhouse gas emissions than the equivalent number of single-use alkaline batteries.
Water usage and pollution metrics also favor electrochemical cells when measured on a per-kilowatt-hour basis, though the manufacturing processes for both technologies require substantial improvements to reduce their environmental impact. Emerging technologies in both categories are focusing on biodegradable components, reduced toxic materials, and designs that facilitate easier recycling and material recovery.
Alkaline batteries contain potentially harmful materials including zinc, manganese dioxide, and small amounts of mercury in older formulations. While modern alkaline batteries have reduced mercury content following regulatory changes, they still pose environmental challenges when improperly disposed of. These batteries typically end up in landfills where they can leach chemicals into soil and groundwater systems over time.
In contrast, many electrochemical cells, particularly rechargeable varieties like lithium-ion, nickel-metal hydride, and lead-acid cells, offer significant sustainability advantages through their extended lifespan and reusability. A single rechargeable electrochemical cell can replace hundreds of alkaline batteries over its operational life, substantially reducing waste generation and resource consumption. However, these cells often contain more toxic materials including lithium, cobalt, and heavy metals that require specialized handling at end-of-life.
The energy efficiency metrics also favor electrochemical cells. Manufacturing a single-use alkaline battery requires approximately 50 times more energy than the battery itself will deliver during use. Rechargeable electrochemical cells demonstrate superior energy return on investment, with their multiple charge cycles offsetting the initial manufacturing energy input.
Recycling infrastructure represents another critical sustainability consideration. Alkaline batteries have historically low recycling rates (below 5% globally) due to economic challenges in the recovery process. Conversely, electrochemical cells, particularly those containing valuable materials like lithium and cobalt, have more established recycling pathways with recovery rates reaching 50-95% in advanced facilities.
Carbon footprint assessments reveal that rechargeable electrochemical cells produce significantly lower greenhouse gas emissions per unit of energy delivered compared to alkaline alternatives. A lifecycle analysis shows that a rechargeable cell used for 500 cycles produces approximately 98% less waste and 90% fewer greenhouse gas emissions than the equivalent number of single-use alkaline batteries.
Water usage and pollution metrics also favor electrochemical cells when measured on a per-kilowatt-hour basis, though the manufacturing processes for both technologies require substantial improvements to reduce their environmental impact. Emerging technologies in both categories are focusing on biodegradable components, reduced toxic materials, and designs that facilitate easier recycling and material recovery.
Cost-Performance Ratio Analysis Across Battery Types
When evaluating battery technologies from a financial perspective, the cost-performance ratio becomes a critical metric for both manufacturers and consumers. Electrochemical cells and alkaline batteries present distinctly different value propositions across various applications and usage scenarios.
Alkaline batteries typically offer a lower initial purchase price, making them attractive for budget-conscious consumers and applications where immediate cost is the primary concern. The average cost per unit ranges from $0.50 to $2.00 depending on size and brand, representing an accessible entry point for most consumers. However, when analyzed over their complete lifecycle, the cost efficiency diminishes significantly due to their relatively shorter lifespan.
In contrast, rechargeable electrochemical cells require a higher upfront investment, with prices ranging from $2.00 to $10.00 per cell plus the additional cost of charging equipment. This initial barrier is offset by their substantial reusability advantage, with modern NiMH cells capable of 500-1000 charge cycles under optimal conditions.
The cost-per-hour-of-operation metric reveals the true economic advantage of electrochemical cells. For devices with high drain requirements, rechargeable cells can achieve a cost efficiency 5-7 times greater than alkaline alternatives over their complete lifecycle. This differential becomes even more pronounced in professional and industrial applications where battery replacement logistics add significant overhead costs.
Environmental factors also influence the cost-performance equation. Many jurisdictions now impose disposal fees or taxes on non-rechargeable batteries, adding hidden costs to alkaline options. Additionally, the growing corporate emphasis on sustainability metrics means the reduced waste stream from rechargeable solutions often translates to measurable cost benefits in environmental compliance and corporate social responsibility reporting.
Market segmentation analysis indicates that consumer perception of value varies significantly across demographics. While budget-conscious segments prioritize immediate affordability, technically sophisticated consumers increasingly recognize the superior lifetime value proposition of rechargeable solutions. This perception gap represents both a marketing challenge and opportunity for manufacturers in the electrochemical cell sector.
The cost-performance landscape is also evolving with technological advancements. Recent improvements in low self-discharge NiMH formulations have extended shelf life significantly, addressing a historical disadvantage compared to alkaline batteries and further improving their lifetime value proposition.
Alkaline batteries typically offer a lower initial purchase price, making them attractive for budget-conscious consumers and applications where immediate cost is the primary concern. The average cost per unit ranges from $0.50 to $2.00 depending on size and brand, representing an accessible entry point for most consumers. However, when analyzed over their complete lifecycle, the cost efficiency diminishes significantly due to their relatively shorter lifespan.
In contrast, rechargeable electrochemical cells require a higher upfront investment, with prices ranging from $2.00 to $10.00 per cell plus the additional cost of charging equipment. This initial barrier is offset by their substantial reusability advantage, with modern NiMH cells capable of 500-1000 charge cycles under optimal conditions.
The cost-per-hour-of-operation metric reveals the true economic advantage of electrochemical cells. For devices with high drain requirements, rechargeable cells can achieve a cost efficiency 5-7 times greater than alkaline alternatives over their complete lifecycle. This differential becomes even more pronounced in professional and industrial applications where battery replacement logistics add significant overhead costs.
Environmental factors also influence the cost-performance equation. Many jurisdictions now impose disposal fees or taxes on non-rechargeable batteries, adding hidden costs to alkaline options. Additionally, the growing corporate emphasis on sustainability metrics means the reduced waste stream from rechargeable solutions often translates to measurable cost benefits in environmental compliance and corporate social responsibility reporting.
Market segmentation analysis indicates that consumer perception of value varies significantly across demographics. While budget-conscious segments prioritize immediate affordability, technically sophisticated consumers increasingly recognize the superior lifetime value proposition of rechargeable solutions. This perception gap represents both a marketing challenge and opportunity for manufacturers in the electrochemical cell sector.
The cost-performance landscape is also evolving with technological advancements. Recent improvements in low self-discharge NiMH formulations have extended shelf life significantly, addressing a historical disadvantage compared to alkaline batteries and further improving their lifetime value proposition.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!