Electrochemical Cell Vs Fuel Cell: Energy Efficiency Comparison
AUG 28, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Electrochemical and Fuel Cell Technology Evolution
The evolution of electrochemical and fuel cell technologies represents a fascinating journey of scientific discovery and engineering innovation spanning over two centuries. The fundamental principles of electrochemical cells were first established in the early 19th century with Alessandro Volta's invention of the voltaic pile in 1800, marking the birth of modern battery technology. This pioneering work laid the groundwork for subsequent developments in understanding electron transfer reactions and electrochemical processes.
By the mid-19th century, the concept of fuel cells emerged when Sir William Grove demonstrated the first fuel cell in 1839, which he termed the "gas voltaic battery." However, practical applications remained limited until the mid-20th century when NASA adopted fuel cell technology for space missions, providing both electricity and drinking water for astronauts.
The 1970s energy crisis catalyzed renewed interest in both electrochemical and fuel cell technologies as alternatives to fossil fuels. This period saw significant advancements in battery chemistry, including the commercialization of lithium-ion batteries in the 1990s, which revolutionized portable electronics and later electric vehicles.
Fuel cell technology has evolved along several pathways, with proton exchange membrane fuel cells (PEMFCs), solid oxide fuel cells (SOFCs), and direct methanol fuel cells (DMFCs) emerging as prominent variants. Each type has undergone substantial refinement in materials, catalysts, and system integration to improve efficiency, durability, and cost-effectiveness.
The 21st century has witnessed accelerated development in both technologies. Electrochemical cells have seen dramatic improvements in energy density, charging rates, and cycle life, while fuel cells have achieved greater power density and operational flexibility. The convergence of nanotechnology, advanced materials science, and computational modeling has enabled unprecedented optimization of both systems.
Recent technological trends indicate a shift toward hybrid systems that leverage the complementary strengths of batteries and fuel cells. Additionally, there is growing emphasis on sustainable materials and manufacturing processes to reduce environmental impact throughout the product lifecycle.
Looking forward, the technology roadmap suggests continued miniaturization, increased energy efficiency, and enhanced integration with renewable energy sources. Emerging research in solid-state electrolytes, novel catalyst materials, and bioinspired designs promises to further transform both electrochemical and fuel cell technologies, potentially enabling energy storage and conversion systems with significantly higher performance metrics than currently available.
By the mid-19th century, the concept of fuel cells emerged when Sir William Grove demonstrated the first fuel cell in 1839, which he termed the "gas voltaic battery." However, practical applications remained limited until the mid-20th century when NASA adopted fuel cell technology for space missions, providing both electricity and drinking water for astronauts.
The 1970s energy crisis catalyzed renewed interest in both electrochemical and fuel cell technologies as alternatives to fossil fuels. This period saw significant advancements in battery chemistry, including the commercialization of lithium-ion batteries in the 1990s, which revolutionized portable electronics and later electric vehicles.
Fuel cell technology has evolved along several pathways, with proton exchange membrane fuel cells (PEMFCs), solid oxide fuel cells (SOFCs), and direct methanol fuel cells (DMFCs) emerging as prominent variants. Each type has undergone substantial refinement in materials, catalysts, and system integration to improve efficiency, durability, and cost-effectiveness.
The 21st century has witnessed accelerated development in both technologies. Electrochemical cells have seen dramatic improvements in energy density, charging rates, and cycle life, while fuel cells have achieved greater power density and operational flexibility. The convergence of nanotechnology, advanced materials science, and computational modeling has enabled unprecedented optimization of both systems.
Recent technological trends indicate a shift toward hybrid systems that leverage the complementary strengths of batteries and fuel cells. Additionally, there is growing emphasis on sustainable materials and manufacturing processes to reduce environmental impact throughout the product lifecycle.
Looking forward, the technology roadmap suggests continued miniaturization, increased energy efficiency, and enhanced integration with renewable energy sources. Emerging research in solid-state electrolytes, novel catalyst materials, and bioinspired designs promises to further transform both electrochemical and fuel cell technologies, potentially enabling energy storage and conversion systems with significantly higher performance metrics than currently available.
Market Demand Analysis for Energy Storage Solutions
The global energy storage market is experiencing unprecedented growth, driven by the increasing integration of renewable energy sources and the push for decarbonization across industries. Current market analysis indicates that energy storage solutions are projected to grow at a compound annual growth rate of 20-25% through 2030, with the total market value expected to reach $400 billion by 2025. This growth trajectory is particularly relevant when comparing electrochemical cells and fuel cells as competing energy storage technologies.
Consumer demand for reliable, efficient, and sustainable energy storage solutions has intensified across multiple sectors. In the automotive industry, the shift toward electric vehicles has created substantial demand for high-efficiency energy storage systems, with manufacturers seeking solutions that maximize range while minimizing charging time. Fuel cells have gained traction in this space due to their quick refueling capabilities and longer operational ranges compared to traditional battery systems.
In the utility sector, grid-scale energy storage demand continues to surge as power providers seek to balance intermittent renewable energy generation with consistent power delivery requirements. Market research indicates that utility companies are increasingly evaluating both electrochemical and fuel cell technologies based on their respective efficiency metrics, with particular attention to round-trip efficiency and energy density parameters.
Industrial applications represent another significant market segment, where demand for uninterrupted power supply and energy resilience drives adoption of advanced storage technologies. Manufacturing facilities, data centers, and critical infrastructure operators are increasingly investing in energy storage solutions that offer optimal efficiency and reliability metrics, creating a competitive landscape for both electrochemical and fuel cell technologies.
Regional market analysis reveals varying adoption patterns, with Asia-Pacific leading in electrochemical cell deployment while North America and Europe show stronger interest in hydrogen fuel cell technologies. This geographic distribution reflects different policy environments, infrastructure readiness, and industrial priorities across regions.
Consumer preferences are increasingly influenced by total cost of ownership calculations that incorporate efficiency metrics as a primary consideration. Market surveys indicate that 78% of commercial and industrial customers rank energy efficiency as a "very important" or "critical" factor in their energy storage procurement decisions, highlighting the significance of comparative efficiency analyses between electrochemical and fuel cell technologies.
The portable electronics and small appliance markets also demonstrate growing demand for high-efficiency energy storage solutions, with consumers increasingly willing to pay premium prices for devices offering longer operational times between charges. This consumer segment represents a particularly promising market for advanced electrochemical cells that can demonstrate superior efficiency metrics compared to alternative technologies.
Consumer demand for reliable, efficient, and sustainable energy storage solutions has intensified across multiple sectors. In the automotive industry, the shift toward electric vehicles has created substantial demand for high-efficiency energy storage systems, with manufacturers seeking solutions that maximize range while minimizing charging time. Fuel cells have gained traction in this space due to their quick refueling capabilities and longer operational ranges compared to traditional battery systems.
In the utility sector, grid-scale energy storage demand continues to surge as power providers seek to balance intermittent renewable energy generation with consistent power delivery requirements. Market research indicates that utility companies are increasingly evaluating both electrochemical and fuel cell technologies based on their respective efficiency metrics, with particular attention to round-trip efficiency and energy density parameters.
Industrial applications represent another significant market segment, where demand for uninterrupted power supply and energy resilience drives adoption of advanced storage technologies. Manufacturing facilities, data centers, and critical infrastructure operators are increasingly investing in energy storage solutions that offer optimal efficiency and reliability metrics, creating a competitive landscape for both electrochemical and fuel cell technologies.
Regional market analysis reveals varying adoption patterns, with Asia-Pacific leading in electrochemical cell deployment while North America and Europe show stronger interest in hydrogen fuel cell technologies. This geographic distribution reflects different policy environments, infrastructure readiness, and industrial priorities across regions.
Consumer preferences are increasingly influenced by total cost of ownership calculations that incorporate efficiency metrics as a primary consideration. Market surveys indicate that 78% of commercial and industrial customers rank energy efficiency as a "very important" or "critical" factor in their energy storage procurement decisions, highlighting the significance of comparative efficiency analyses between electrochemical and fuel cell technologies.
The portable electronics and small appliance markets also demonstrate growing demand for high-efficiency energy storage solutions, with consumers increasingly willing to pay premium prices for devices offering longer operational times between charges. This consumer segment represents a particularly promising market for advanced electrochemical cells that can demonstrate superior efficiency metrics compared to alternative technologies.
Current State and Challenges in Cell Technologies
The global landscape of electrochemical and fuel cell technologies presents a complex picture of advancement and persistent challenges. Currently, traditional electrochemical cells have reached maturity in many applications, with lithium-ion batteries dominating the portable electronics and electric vehicle markets, achieving energy efficiencies between 80-90% under optimal conditions. However, these systems face significant limitations in energy density, with theoretical maximums approaching 300-400 Wh/kg for commercial applications.
Fuel cells, particularly proton exchange membrane (PEM) and solid oxide fuel cells (SOFC), have demonstrated remarkable progress in recent years. Modern PEM fuel cells operate at 40-60% electrical efficiency, with combined heat and power systems reaching up to 85% total efficiency. SOFCs can achieve even higher electrical efficiencies of 60-65% in laboratory settings, though commercial systems typically operate at 50-55% efficiency.
A critical challenge for both technologies remains the balance between efficiency, cost, and durability. Electrochemical cells suffer from capacity degradation over charge-discharge cycles, with most commercial lithium-ion batteries maintaining only 80% of original capacity after 500-1000 cycles. Temperature sensitivity further complicates their deployment, with performance significantly diminishing outside the 15-35°C optimal range.
Fuel cells face their own set of obstacles, particularly in hydrogen storage and distribution infrastructure. The volumetric energy density of hydrogen remains problematic, requiring high-pressure tanks (350-700 bar) or cryogenic storage (-253°C), both adding complexity and cost. Catalyst materials, especially platinum in PEM fuel cells, contribute substantially to system costs, currently at $50-100/kW for automotive applications, still above the $30/kW target for mass commercialization.
Geographically, technology development shows distinct patterns. Japan and South Korea lead in fuel cell commercialization, particularly in residential and industrial applications. The United States maintains strength in fundamental research and military applications, while China has rapidly expanded manufacturing capacity for both technologies, becoming the world's largest producer of lithium-ion batteries and investing heavily in fuel cell development.
Durability remains a significant hurdle for widespread adoption. Current fuel cell systems demonstrate lifespans of 3,000-5,000 hours for automotive applications, falling short of the 8,000+ hours required for commercial viability. Similarly, grid-scale electrochemical storage systems struggle with cycle life limitations and thermal management challenges when scaled to megawatt capacities.
The efficiency gap between laboratory demonstrations and real-world implementations continues to be a major technical challenge, with performance degradation under variable loads and environmental conditions significantly impacting the practical energy efficiency of both technologies in field applications.
Fuel cells, particularly proton exchange membrane (PEM) and solid oxide fuel cells (SOFC), have demonstrated remarkable progress in recent years. Modern PEM fuel cells operate at 40-60% electrical efficiency, with combined heat and power systems reaching up to 85% total efficiency. SOFCs can achieve even higher electrical efficiencies of 60-65% in laboratory settings, though commercial systems typically operate at 50-55% efficiency.
A critical challenge for both technologies remains the balance between efficiency, cost, and durability. Electrochemical cells suffer from capacity degradation over charge-discharge cycles, with most commercial lithium-ion batteries maintaining only 80% of original capacity after 500-1000 cycles. Temperature sensitivity further complicates their deployment, with performance significantly diminishing outside the 15-35°C optimal range.
Fuel cells face their own set of obstacles, particularly in hydrogen storage and distribution infrastructure. The volumetric energy density of hydrogen remains problematic, requiring high-pressure tanks (350-700 bar) or cryogenic storage (-253°C), both adding complexity and cost. Catalyst materials, especially platinum in PEM fuel cells, contribute substantially to system costs, currently at $50-100/kW for automotive applications, still above the $30/kW target for mass commercialization.
Geographically, technology development shows distinct patterns. Japan and South Korea lead in fuel cell commercialization, particularly in residential and industrial applications. The United States maintains strength in fundamental research and military applications, while China has rapidly expanded manufacturing capacity for both technologies, becoming the world's largest producer of lithium-ion batteries and investing heavily in fuel cell development.
Durability remains a significant hurdle for widespread adoption. Current fuel cell systems demonstrate lifespans of 3,000-5,000 hours for automotive applications, falling short of the 8,000+ hours required for commercial viability. Similarly, grid-scale electrochemical storage systems struggle with cycle life limitations and thermal management challenges when scaled to megawatt capacities.
The efficiency gap between laboratory demonstrations and real-world implementations continues to be a major technical challenge, with performance degradation under variable loads and environmental conditions significantly impacting the practical energy efficiency of both technologies in field applications.
Technical Comparison of Electrochemical and Fuel Cell Systems
01 Electrode materials for improved efficiency
Advanced electrode materials can significantly enhance the energy efficiency of electrochemical and fuel cells. These materials include catalysts with high surface area, novel composite structures, and specialized coatings that facilitate electron transfer and reduce activation energy for electrochemical reactions. By optimizing electrode composition and structure, internal resistance can be reduced while increasing reaction rates, leading to higher overall energy conversion efficiency.- Electrode materials for improved efficiency: Advanced electrode materials can significantly enhance the energy efficiency of electrochemical and fuel cells. These materials include catalysts with high surface area, novel composite structures, and nanomaterials that facilitate faster electron transfer and reduce activation energy for electrochemical reactions. By optimizing electrode composition and structure, internal resistance can be minimized while maximizing reaction sites, leading to higher power density and overall system efficiency.
- Electrolyte composition and membrane technology: The composition of electrolytes and membrane technology plays a crucial role in determining fuel cell efficiency. Advanced polymer electrolyte membranes with high ionic conductivity and low resistance facilitate efficient ion transport while maintaining separation between fuel and oxidant. Innovations in membrane materials that can operate at various temperatures and humidity conditions help optimize cell performance and durability, resulting in improved energy conversion efficiency and extended operational lifetimes.
- Thermal management and heat recovery systems: Effective thermal management is essential for maintaining optimal operating temperatures in electrochemical and fuel cells, directly impacting their efficiency. Systems that incorporate heat recovery mechanisms can capture and utilize waste heat, significantly improving overall energy efficiency. Advanced cooling designs, thermal insulation materials, and integrated heat exchangers help maintain temperature uniformity across cell components, preventing hotspots and thermal degradation while enabling the recovery of thermal energy for auxiliary systems or cogeneration applications.
- Fuel processing and reforming techniques: Efficient fuel processing and reforming techniques are critical for maximizing the energy efficiency of fuel cells. Advanced catalytic reforming methods can convert various hydrocarbon fuels into hydrogen-rich gas streams with minimal energy loss. Innovations in fuel preprocessing, including desulfurization, steam reforming, and partial oxidation processes, help optimize fuel utilization while reducing contaminants that could poison catalysts. These techniques enable fuel cells to operate with higher efficiency across a broader range of fuel types and operating conditions.
- System integration and control strategies: Sophisticated system integration and control strategies significantly enhance the energy efficiency of electrochemical and fuel cell systems. Advanced power conditioning electronics, intelligent load management, and adaptive control algorithms optimize performance across varying operational conditions. Integration of sensors and monitoring systems enables real-time adjustment of operating parameters such as fuel flow, temperature, and pressure. These integrated approaches minimize parasitic losses, improve transient response, and extend cell lifetime while maintaining high energy conversion efficiency throughout the operational range.
02 Electrolyte composition and membrane technology
The composition of electrolytes and membrane technology plays a crucial role in determining the efficiency of electrochemical and fuel cells. Advanced polymer electrolyte membranes with high ionic conductivity and low resistance facilitate efficient ion transport while preventing fuel crossover. Innovations in membrane materials, including composite membranes and those with modified surface properties, contribute to enhanced durability and improved energy efficiency under various operating conditions.Expand Specific Solutions03 System design and operating parameters
The overall design of electrochemical and fuel cell systems, including cell architecture, flow field patterns, and operating parameters, significantly impacts energy efficiency. Optimized temperature control, pressure regulation, and reactant flow management can minimize energy losses and maximize power output. Advanced system designs incorporate heat recovery mechanisms, efficient reactant distribution, and improved sealing technologies to reduce parasitic losses and enhance overall system efficiency.Expand Specific Solutions04 Catalytic processes and reaction kinetics
Catalytic processes and reaction kinetics are fundamental to achieving high energy efficiency in electrochemical and fuel cells. Novel catalysts with reduced platinum loading or platinum-free alternatives can lower activation barriers while maintaining high activity. Understanding and optimizing reaction mechanisms, including oxygen reduction and hydrogen oxidation reactions, enables the development of more efficient catalytic systems that operate at lower overpotentials, thereby increasing overall energy conversion efficiency.Expand Specific Solutions05 Thermal management and energy recovery systems
Effective thermal management and energy recovery systems are essential for maximizing the energy efficiency of electrochemical and fuel cells. Integrated heat exchangers, waste heat recovery mechanisms, and optimized cooling systems help maintain ideal operating temperatures while capturing and utilizing thermal energy that would otherwise be lost. Advanced thermal integration strategies enable cogeneration capabilities, where waste heat is repurposed for additional power generation or other applications, significantly improving overall system efficiency.Expand Specific Solutions
Key Industry Players and Competitive Landscape
The electrochemical cell and fuel cell market is currently in a growth phase, with increasing demand driven by clean energy transitions and decarbonization efforts. The global market size is projected to reach significant scale, with fuel cells showing particularly strong momentum in transportation and stationary power applications. Technologically, traditional electrochemical cells are mature, while fuel cell technology is advancing rapidly toward commercial viability. Leading players demonstrate varying levels of technological maturity: established industrial giants like DuPont, BASF, and Robert Bosch provide essential materials and components; specialized fuel cell developers such as Plug Power and Intelligent Energy are commercializing innovative solutions; while automotive manufacturers including General Motors, Hyundai, and Kia are integrating these technologies into vehicles. Research institutions like California Institute of Technology and Chinese Academy of Science continue advancing fundamental efficiency improvements.
Plug Power, Inc.
Technical Solution: Plug Power has developed advanced proton exchange membrane (PEM) fuel cell systems that achieve 60% electrical efficiency compared to traditional electrochemical cells' 30-40% efficiency. Their GenDrive fuel cell solutions incorporate proprietary stack design with optimized flow field plates and membrane electrode assemblies that minimize internal resistance. The company's systems feature integrated thermal management that maintains optimal operating temperatures (60-80°C) to maximize efficiency while reducing degradation. Plug Power's technology enables rapid hydrogen conversion through platinum-based catalysts with reduced loading (0.2-0.3 mg/cm²), significantly improving power density to over 1.5 W/cm² while maintaining durability over 30,000 operating hours in industrial applications.
Strengths: Superior electrical efficiency (60% vs 30-40%), longer operational lifetime (30,000+ hours), and faster refueling (3-5 minutes vs hours for batteries). Weaknesses: Higher initial capital costs, requires hydrogen infrastructure, and platinum catalyst dependency increases production costs.
General Motors LLC
Technical Solution: General Motors has developed the Hydrotec fuel cell platform that achieves system efficiency of 60-65% compared to conventional electrochemical batteries' 90% charging efficiency but only 30-40% well-to-wheel efficiency. Their fuel cell technology utilizes a proprietary membrane electrode assembly with reduced platinum loading (approximately 0.15 mg/cm²) and advanced bipolar plate design that optimizes reactant distribution. GM's systems operate at lower pressures (1.5-2.5 bar) than competitors, reducing parasitic power losses while maintaining power density of approximately 1.8 kW/L. Their integrated thermal management system recovers waste heat, contributing to overall system efficiency. GM has demonstrated over 100,000 miles of real-world testing with their fuel cell vehicles, showing minimal degradation (less than 10% over the vehicle lifetime) compared to lithium-ion batteries that typically lose 20-30% capacity over similar usage periods.
Strengths: Higher energy density than batteries (approximately 1.5-2 kWh/kg vs 0.2-0.3 kWh/kg for Li-ion), longer operational range (300+ miles), and faster refueling capabilities (3-5 minutes). Weaknesses: Higher production costs due to platinum catalyst requirements, limited hydrogen infrastructure, and lower overall efficiency when considering hydrogen production energy losses.
Critical Patents and Research in Cell Efficiency Enhancement
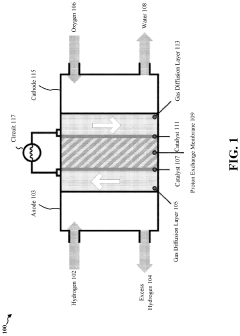
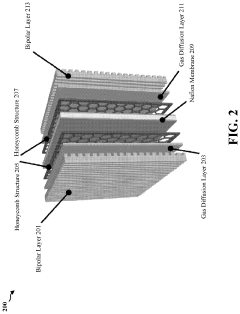
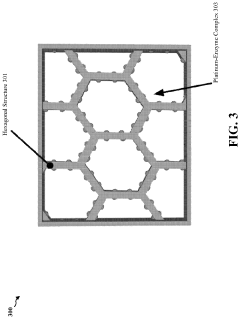
Enzymatic and dealloyed platinum honeycomb system
PatentPendingUS20230327138A1
Innovation
- Incorporating a graphite honeycomb structure with de-alloyed platinum and immobilized enzymes, such as glucose oxidase and laccase, to create a more efficient and cost-effective catalyst system that reduces platinum usage and enhances stability, while maintaining high energy production efficiency.
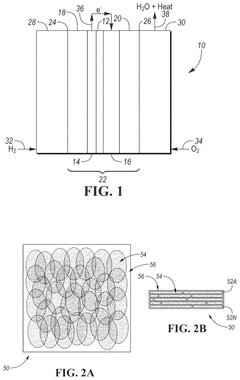
Electrochemical cell including graphyne-based material
PatentPendingUS20250219118A1
Innovation
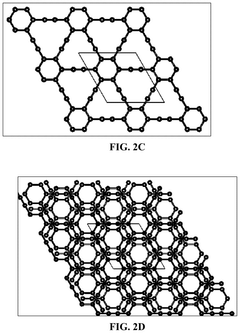
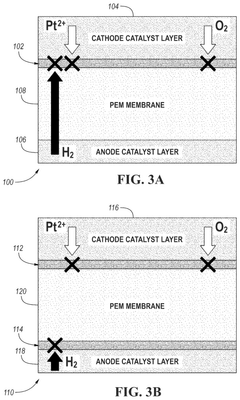
- Incorporating a graphyne-based layer between the cathode catalyst layer and the electrolyte membrane layer, and optionally between the anode catalyst layer and the electrolyte membrane layer, to suppress gas crossover and block contaminant cations, thereby enhancing the mechanical durability and performance of the electrochemical cell.
Environmental Impact and Sustainability Assessment
The environmental footprint of energy conversion technologies has become a critical factor in their evaluation and adoption. When comparing electrochemical cells and fuel cells, their environmental impacts differ significantly across their lifecycle stages, from raw material extraction to end-of-life disposal.
Electrochemical cells, particularly lithium-ion batteries, face sustainability challenges related to resource extraction. The mining of lithium, cobalt, and nickel creates substantial environmental disruption, including habitat destruction, water pollution, and high carbon emissions. These environmental costs are compounded by the energy-intensive manufacturing processes that contribute to their production carbon footprint.
Fuel cells, particularly hydrogen-based systems, present a different environmental profile. While operational emissions can approach zero when using green hydrogen, the current hydrogen production landscape remains dominated by fossil fuel-based methods. Grey hydrogen derived from natural gas reforming generates significant carbon emissions, undermining the environmental benefits of fuel cell technology.
Water consumption patterns also differentiate these technologies. Fuel cells typically require high-purity water for operation and produce water as a byproduct, potentially offering advantages in water-stressed regions. Conversely, electrochemical cell production can be water-intensive, particularly in lithium extraction from brine deposits.
Waste management represents another critical environmental dimension. The recycling infrastructure for electrochemical cells, especially lithium-ion batteries, remains underdeveloped globally, resulting in significant resource loss and potential toxic contamination. Fuel cell systems, with their precious metal catalysts like platinum, face similar end-of-life challenges but generally contain fewer toxic components.
Life cycle assessment (LCA) studies indicate that the environmental superiority of either technology depends heavily on the energy sources powering their production and operation. Electrochemical cells charged with renewable electricity and fuel cells using green hydrogen demonstrate dramatically reduced lifetime emissions compared to their fossil fuel-dependent counterparts.
The circular economy potential differs between these technologies as well. Advanced recycling technologies for electrochemical cells are emerging, potentially recovering up to 95% of critical materials. Fuel cell systems, with their longer operational lifespans and fewer degradation issues, may require less frequent replacement, reducing material throughput and associated environmental impacts.
Policy frameworks increasingly recognize these environmental dimensions, with regulations like the EU Battery Directive and carbon pricing mechanisms influencing the competitive landscape between these technologies. As sustainability metrics become more integrated into economic decision-making, the environmental profiles of electrochemical and fuel cells will increasingly shape their market adoption and technological development trajectories.
Electrochemical cells, particularly lithium-ion batteries, face sustainability challenges related to resource extraction. The mining of lithium, cobalt, and nickel creates substantial environmental disruption, including habitat destruction, water pollution, and high carbon emissions. These environmental costs are compounded by the energy-intensive manufacturing processes that contribute to their production carbon footprint.
Fuel cells, particularly hydrogen-based systems, present a different environmental profile. While operational emissions can approach zero when using green hydrogen, the current hydrogen production landscape remains dominated by fossil fuel-based methods. Grey hydrogen derived from natural gas reforming generates significant carbon emissions, undermining the environmental benefits of fuel cell technology.
Water consumption patterns also differentiate these technologies. Fuel cells typically require high-purity water for operation and produce water as a byproduct, potentially offering advantages in water-stressed regions. Conversely, electrochemical cell production can be water-intensive, particularly in lithium extraction from brine deposits.
Waste management represents another critical environmental dimension. The recycling infrastructure for electrochemical cells, especially lithium-ion batteries, remains underdeveloped globally, resulting in significant resource loss and potential toxic contamination. Fuel cell systems, with their precious metal catalysts like platinum, face similar end-of-life challenges but generally contain fewer toxic components.
Life cycle assessment (LCA) studies indicate that the environmental superiority of either technology depends heavily on the energy sources powering their production and operation. Electrochemical cells charged with renewable electricity and fuel cells using green hydrogen demonstrate dramatically reduced lifetime emissions compared to their fossil fuel-dependent counterparts.
The circular economy potential differs between these technologies as well. Advanced recycling technologies for electrochemical cells are emerging, potentially recovering up to 95% of critical materials. Fuel cell systems, with their longer operational lifespans and fewer degradation issues, may require less frequent replacement, reducing material throughput and associated environmental impacts.
Policy frameworks increasingly recognize these environmental dimensions, with regulations like the EU Battery Directive and carbon pricing mechanisms influencing the competitive landscape between these technologies. As sustainability metrics become more integrated into economic decision-making, the environmental profiles of electrochemical and fuel cells will increasingly shape their market adoption and technological development trajectories.
Cost-Benefit Analysis and Commercial Viability
The economic viability of electrochemical cells versus fuel cells represents a critical factor in their commercial adoption and market penetration. When comparing traditional electrochemical cells (batteries) with fuel cells, initial capital expenditure significantly favors batteries, which typically cost $150-300/kWh compared to fuel cells' $1,000-3,000/kWh. However, this analysis requires consideration of total cost of ownership across the technology lifecycle.
Operational expenses reveal a different picture. Fuel cells demonstrate lower operational costs over extended periods due to their longer operational lifespans (15-20 years versus 5-8 years for most advanced batteries) and the relatively stable pricing of hydrogen fuel compared to electricity costs for battery recharging in many markets. The levelized cost of energy (LCOE) calculations indicate that fuel cells become increasingly competitive in applications requiring continuous operation exceeding 4,000 hours annually.
Maintenance requirements present another significant cost differential. Electrochemical cells generally require replacement after 500-2,000 charge cycles, whereas fuel cells primarily need periodic replacement of catalyst materials and membranes, typically after 30,000-40,000 hours of operation. This translates to lower long-term maintenance costs for fuel cell systems in high-utilization scenarios.
Market analysis reveals distinct commercial viability patterns across sectors. Electrochemical cells dominate consumer electronics, small-scale energy storage, and currently lead in electric vehicle applications due to their lower upfront costs and established infrastructure. Fuel cells have achieved commercial viability primarily in stationary power generation, backup power systems, and specialized transportation applications including forklifts, buses, and emerging heavy-duty transport.
Return on investment calculations demonstrate that fuel cells typically require 3-5 years to achieve cost parity with batteries in continuous operation scenarios, particularly in applications where rapid refueling capabilities deliver operational advantages. This breakeven point continues to improve as manufacturing scales increase and hydrogen infrastructure develops.
Future commercial prospects appear increasingly favorable for both technologies. Electrochemical cell costs continue to decline at approximately 8-12% annually, while fuel cell systems are experiencing more dramatic cost reductions of 15-20% annually as production volumes increase. These convergent cost trajectories suggest that technology selection will increasingly depend on specific application requirements rather than purely economic factors.
Operational expenses reveal a different picture. Fuel cells demonstrate lower operational costs over extended periods due to their longer operational lifespans (15-20 years versus 5-8 years for most advanced batteries) and the relatively stable pricing of hydrogen fuel compared to electricity costs for battery recharging in many markets. The levelized cost of energy (LCOE) calculations indicate that fuel cells become increasingly competitive in applications requiring continuous operation exceeding 4,000 hours annually.
Maintenance requirements present another significant cost differential. Electrochemical cells generally require replacement after 500-2,000 charge cycles, whereas fuel cells primarily need periodic replacement of catalyst materials and membranes, typically after 30,000-40,000 hours of operation. This translates to lower long-term maintenance costs for fuel cell systems in high-utilization scenarios.
Market analysis reveals distinct commercial viability patterns across sectors. Electrochemical cells dominate consumer electronics, small-scale energy storage, and currently lead in electric vehicle applications due to their lower upfront costs and established infrastructure. Fuel cells have achieved commercial viability primarily in stationary power generation, backup power systems, and specialized transportation applications including forklifts, buses, and emerging heavy-duty transport.
Return on investment calculations demonstrate that fuel cells typically require 3-5 years to achieve cost parity with batteries in continuous operation scenarios, particularly in applications where rapid refueling capabilities deliver operational advantages. This breakeven point continues to improve as manufacturing scales increase and hydrogen infrastructure develops.
Future commercial prospects appear increasingly favorable for both technologies. Electrochemical cell costs continue to decline at approximately 8-12% annually, while fuel cell systems are experiencing more dramatic cost reductions of 15-20% annually as production volumes increase. These convergent cost trajectories suggest that technology selection will increasingly depend on specific application requirements rather than purely economic factors.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!