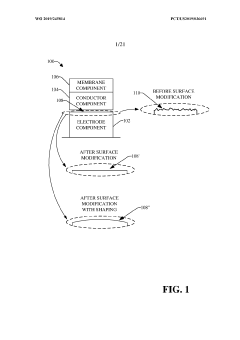
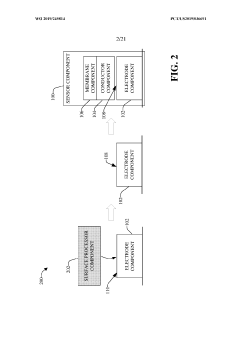
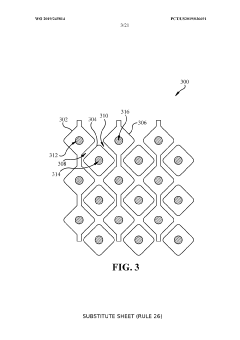
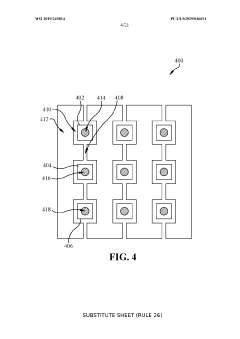
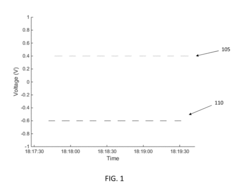
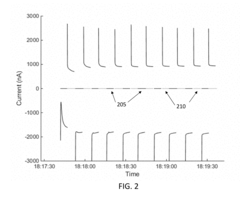
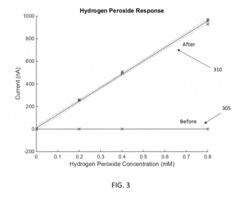
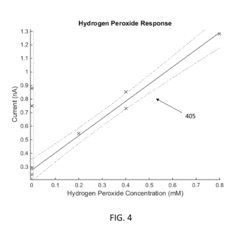
Electrode Surface Modification To Reduce Sensor Drift
AUG 28, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Electrode Modification Background and Objectives
Electrode surface modification has emerged as a critical area of research in sensor technology, with its roots dating back to the early 1980s when researchers first began exploring ways to enhance electrode performance. The evolution of this technology has been driven by the persistent challenge of sensor drift, which significantly impacts measurement accuracy and reliability across various applications including environmental monitoring, healthcare diagnostics, and industrial process control.
Sensor drift, characterized by the gradual deviation of sensor output signals over time despite constant input conditions, represents one of the most significant limitations in electrochemical sensing systems. This phenomenon can be attributed to multiple factors including surface fouling, electrode degradation, reference electrode instability, and changes in the electrode-electrolyte interface properties.
The technological trajectory in this field has shown remarkable progression from simple physical modifications to sophisticated molecular engineering approaches. Early techniques focused primarily on mechanical polishing and basic chemical treatments, while contemporary methods leverage advanced nanomaterials, conducting polymers, and biomolecular recognition elements to create more stable and selective electrode surfaces.
The primary objective of electrode surface modification research is to develop robust strategies that can effectively mitigate sensor drift while maintaining or enhancing other critical performance parameters such as sensitivity, selectivity, response time, and operational lifetime. This involves creating electrode interfaces that resist fouling, maintain consistent electrochemical properties, and provide stable reference potentials over extended periods of operation.
Additionally, there is growing emphasis on developing modification techniques that are compatible with miniaturization trends in sensor technology, enabling integration with microfluidic systems and portable/wearable devices. This aspect is particularly important as the industry moves toward decentralized sensing applications and point-of-care diagnostics.
Another key goal is to establish standardized modification protocols that can be reliably reproduced across different manufacturing settings, ensuring consistent performance across sensor batches. This standardization is essential for commercial viability and regulatory approval, especially for sensors used in critical applications such as medical diagnostics or environmental compliance monitoring.
The field is currently witnessing convergence with other emerging technologies, including artificial intelligence for drift compensation algorithms, 3D printing for custom electrode architectures, and green chemistry approaches for environmentally sustainable modification methods. These intersections are expected to open new avenues for addressing the persistent challenge of sensor drift through multidisciplinary solutions.
Sensor drift, characterized by the gradual deviation of sensor output signals over time despite constant input conditions, represents one of the most significant limitations in electrochemical sensing systems. This phenomenon can be attributed to multiple factors including surface fouling, electrode degradation, reference electrode instability, and changes in the electrode-electrolyte interface properties.
The technological trajectory in this field has shown remarkable progression from simple physical modifications to sophisticated molecular engineering approaches. Early techniques focused primarily on mechanical polishing and basic chemical treatments, while contemporary methods leverage advanced nanomaterials, conducting polymers, and biomolecular recognition elements to create more stable and selective electrode surfaces.
The primary objective of electrode surface modification research is to develop robust strategies that can effectively mitigate sensor drift while maintaining or enhancing other critical performance parameters such as sensitivity, selectivity, response time, and operational lifetime. This involves creating electrode interfaces that resist fouling, maintain consistent electrochemical properties, and provide stable reference potentials over extended periods of operation.
Additionally, there is growing emphasis on developing modification techniques that are compatible with miniaturization trends in sensor technology, enabling integration with microfluidic systems and portable/wearable devices. This aspect is particularly important as the industry moves toward decentralized sensing applications and point-of-care diagnostics.
Another key goal is to establish standardized modification protocols that can be reliably reproduced across different manufacturing settings, ensuring consistent performance across sensor batches. This standardization is essential for commercial viability and regulatory approval, especially for sensors used in critical applications such as medical diagnostics or environmental compliance monitoring.
The field is currently witnessing convergence with other emerging technologies, including artificial intelligence for drift compensation algorithms, 3D printing for custom electrode architectures, and green chemistry approaches for environmentally sustainable modification methods. These intersections are expected to open new avenues for addressing the persistent challenge of sensor drift through multidisciplinary solutions.
Market Demand for Stable Sensor Technologies
The global market for stable sensor technologies has witnessed substantial growth in recent years, driven primarily by the increasing demand for reliable and accurate sensing solutions across various industries. The sensor drift problem, particularly in electrochemical sensors, has emerged as a critical challenge that significantly impacts market dynamics and user preferences.
In the healthcare sector, the demand for stable sensors has been particularly pronounced, with the global medical sensors market projected to reach $23 billion by 2025. Continuous glucose monitoring systems, blood gas analyzers, and point-of-care diagnostic devices all require highly stable electrodes to ensure accurate patient monitoring and treatment decisions. Even minor sensor drift can lead to misdiagnosis or improper medication dosing, creating substantial liability concerns for manufacturers.
Environmental monitoring represents another significant market segment, valued at approximately $17 billion globally, where stable sensors are essential for accurate pollution detection, water quality assessment, and climate research. Government regulations worldwide are becoming increasingly stringent regarding environmental monitoring accuracy, creating market pressure for sensors with minimal drift characteristics.
The industrial process control sector, with its $12 billion sensor market, demands exceptional stability for maintaining production quality and efficiency. Manufacturing facilities, particularly in semiconductor fabrication, pharmaceutical production, and food processing, require sensors that maintain calibration over extended periods to reduce downtime and ensure product consistency. Industry surveys indicate that maintenance costs related to sensor recalibration and replacement due to drift issues exceed $3 billion annually across global manufacturing operations.
Automotive applications present a rapidly expanding market for stable sensor technologies, especially with the growth of electric vehicles and advanced driver assistance systems. The automotive sensor market, growing at 8% annually, increasingly specifies drift performance as a key selection criterion for suppliers. Vehicle safety systems, emissions monitoring, and battery management all depend on long-term sensor stability.
Consumer electronics manufacturers are also driving demand for stable sensors, particularly in wearable health monitors, smart home devices, and portable environmental detectors. The consumer expectation for "set and forget" operation creates market pressure for sensors that maintain accuracy without frequent recalibration.
Research indicates that end-users across all sectors are willing to pay a premium of 15-30% for sensors with demonstrably superior stability characteristics. This price elasticity has created significant market opportunities for companies that can effectively address the electrode surface modification challenge to reduce sensor drift.
In the healthcare sector, the demand for stable sensors has been particularly pronounced, with the global medical sensors market projected to reach $23 billion by 2025. Continuous glucose monitoring systems, blood gas analyzers, and point-of-care diagnostic devices all require highly stable electrodes to ensure accurate patient monitoring and treatment decisions. Even minor sensor drift can lead to misdiagnosis or improper medication dosing, creating substantial liability concerns for manufacturers.
Environmental monitoring represents another significant market segment, valued at approximately $17 billion globally, where stable sensors are essential for accurate pollution detection, water quality assessment, and climate research. Government regulations worldwide are becoming increasingly stringent regarding environmental monitoring accuracy, creating market pressure for sensors with minimal drift characteristics.
The industrial process control sector, with its $12 billion sensor market, demands exceptional stability for maintaining production quality and efficiency. Manufacturing facilities, particularly in semiconductor fabrication, pharmaceutical production, and food processing, require sensors that maintain calibration over extended periods to reduce downtime and ensure product consistency. Industry surveys indicate that maintenance costs related to sensor recalibration and replacement due to drift issues exceed $3 billion annually across global manufacturing operations.
Automotive applications present a rapidly expanding market for stable sensor technologies, especially with the growth of electric vehicles and advanced driver assistance systems. The automotive sensor market, growing at 8% annually, increasingly specifies drift performance as a key selection criterion for suppliers. Vehicle safety systems, emissions monitoring, and battery management all depend on long-term sensor stability.
Consumer electronics manufacturers are also driving demand for stable sensors, particularly in wearable health monitors, smart home devices, and portable environmental detectors. The consumer expectation for "set and forget" operation creates market pressure for sensors that maintain accuracy without frequent recalibration.
Research indicates that end-users across all sectors are willing to pay a premium of 15-30% for sensors with demonstrably superior stability characteristics. This price elasticity has created significant market opportunities for companies that can effectively address the electrode surface modification challenge to reduce sensor drift.
Current Challenges in Electrode Drift Reduction
Despite significant advancements in electrode technology, sensor drift remains a persistent challenge that undermines the reliability and accuracy of electrochemical sensors across various applications. The primary obstacle in electrode surface modification lies in achieving long-term stability while maintaining sensitivity and selectivity. Current electrodes suffer from gradual performance degradation due to biofouling, where proteins and other biomolecules adsorb onto the electrode surface, creating an insulating layer that impedes electron transfer.
Surface oxidation presents another significant challenge, particularly for metal-based electrodes. Exposure to oxygen and reactive species in solution environments leads to oxide layer formation, altering the electrochemical properties of the sensing interface. This oxidation process is often unpredictable and difficult to control, resulting in progressive signal deterioration over time.
The leaching of electrode components into the sample matrix constitutes a substantial hurdle for modified electrodes. Many surface modifications rely on physically adsorbed materials that gradually detach from the electrode surface during operation, causing drift in sensor response. This issue is particularly pronounced in flow systems or when sensors are deployed in complex biological fluids.
Temperature and pH fluctuations further complicate electrode stability. Most surface modifications exhibit varying degrees of sensitivity to environmental conditions, with performance characteristics changing as temperature or pH deviates from calibration conditions. This environmental sensitivity creates additional drift components that are difficult to compensate for in real-world applications.
Reproducibility in electrode modification processes represents a manufacturing challenge. Current techniques often suffer from batch-to-batch variations, resulting in inconsistent baseline performance and drift profiles. The lack of standardized, scalable modification protocols hampers industrial adoption of otherwise promising approaches.
Interference from co-existing species remains problematic despite advances in selective coatings. Many modified electrodes demonstrate initial selectivity that diminishes over time as the modification layer degrades or becomes saturated with interferents. This progressive loss of selectivity manifests as apparent drift in sensor response.
The integration of anti-drift strategies with miniaturization efforts presents additional complications. As sensors decrease in size for portable and implantable applications, the surface-to-volume ratio increases dramatically, making surface phenomena more dominant and drift effects more pronounced. Current modification approaches often fail to scale effectively to micro and nano-dimensioned electrodes.
Finally, there exists a fundamental knowledge gap regarding the molecular-level mechanisms of drift in different electrode systems. Without comprehensive understanding of degradation pathways, current approaches remain largely empirical rather than rationally designed, limiting the development of truly drift-resistant electrode surfaces.
Surface oxidation presents another significant challenge, particularly for metal-based electrodes. Exposure to oxygen and reactive species in solution environments leads to oxide layer formation, altering the electrochemical properties of the sensing interface. This oxidation process is often unpredictable and difficult to control, resulting in progressive signal deterioration over time.
The leaching of electrode components into the sample matrix constitutes a substantial hurdle for modified electrodes. Many surface modifications rely on physically adsorbed materials that gradually detach from the electrode surface during operation, causing drift in sensor response. This issue is particularly pronounced in flow systems or when sensors are deployed in complex biological fluids.
Temperature and pH fluctuations further complicate electrode stability. Most surface modifications exhibit varying degrees of sensitivity to environmental conditions, with performance characteristics changing as temperature or pH deviates from calibration conditions. This environmental sensitivity creates additional drift components that are difficult to compensate for in real-world applications.
Reproducibility in electrode modification processes represents a manufacturing challenge. Current techniques often suffer from batch-to-batch variations, resulting in inconsistent baseline performance and drift profiles. The lack of standardized, scalable modification protocols hampers industrial adoption of otherwise promising approaches.
Interference from co-existing species remains problematic despite advances in selective coatings. Many modified electrodes demonstrate initial selectivity that diminishes over time as the modification layer degrades or becomes saturated with interferents. This progressive loss of selectivity manifests as apparent drift in sensor response.
The integration of anti-drift strategies with miniaturization efforts presents additional complications. As sensors decrease in size for portable and implantable applications, the surface-to-volume ratio increases dramatically, making surface phenomena more dominant and drift effects more pronounced. Current modification approaches often fail to scale effectively to micro and nano-dimensioned electrodes.
Finally, there exists a fundamental knowledge gap regarding the molecular-level mechanisms of drift in different electrode systems. Without comprehensive understanding of degradation pathways, current approaches remain largely empirical rather than rationally designed, limiting the development of truly drift-resistant electrode surfaces.
Existing Surface Modification Approaches
01 Nanomaterial coatings for electrode stability
Nanomaterials such as carbon nanotubes, graphene, and metal nanoparticles can be applied to electrode surfaces to enhance stability and reduce sensor drift. These materials create protective layers that prevent fouling and degradation of the sensing surface while maintaining electrical conductivity. The modified electrodes show improved long-term performance with minimal baseline drift, making them suitable for continuous monitoring applications.- Nanomaterial coatings for electrode stability: Nanomaterials such as carbon nanotubes, graphene, and metal nanoparticles can be applied to electrode surfaces to enhance stability and reduce sensor drift. These materials create a protective layer that prevents fouling and degradation of the electrode surface during continuous operation. The high surface area and excellent conductivity of nanomaterials also improve signal-to-noise ratio and sensitivity, leading to more reliable sensor readings over time.
- Polymer-based surface modifications: Polymer coatings on electrode surfaces can significantly reduce sensor drift by providing a stable interface between the electrode and the analyte. Conductive polymers like polypyrrole and PEDOT, as well as non-conductive polymers modified with functional groups, can be used to create selective barriers that minimize interference from non-target molecules. These polymer layers also protect the electrode from biofouling and chemical degradation, extending sensor lifetime and maintaining calibration.
- Self-cleaning and anti-fouling surface treatments: Specialized surface treatments can be applied to electrodes to create self-cleaning or anti-fouling properties that combat sensor drift. These include hydrophobic coatings that repel water and contaminants, photocatalytic materials that break down organic fouling under light exposure, and stimuli-responsive surfaces that can be activated to release accumulated contaminants. Such treatments maintain the active surface area of the electrode and preserve its electrochemical properties over extended periods of use.
- Metal oxide and composite electrode modifications: Metal oxides and composite materials can be used to modify electrode surfaces to enhance stability and reduce drift. Materials such as titanium dioxide, zinc oxide, and various metal oxide composites provide excellent chemical stability and can be tailored for specific sensing applications. These modifications often incorporate dopants or mixed metal oxides to optimize conductivity and catalytic properties, resulting in sensors with improved long-term performance and reduced baseline drift.
- Electrochemical surface functionalization techniques: Electrochemical methods can be used to functionalize electrode surfaces with specific chemical groups that enhance stability and selectivity. Techniques such as electrodeposition, electropolymerization, and electrografting allow precise control over the modification process. These approaches create covalently bonded functional layers that are highly resistant to desorption and degradation, significantly reducing drift caused by changes in the electrode surface chemistry over time. The resulting functionalized electrodes demonstrate improved reproducibility and extended calibration stability.
02 Polymer-based surface modifications
Electrodes modified with specialized polymers such as conducting polymers, hydrogels, or molecularly imprinted polymers demonstrate reduced drift characteristics. These polymer coatings provide selective permeability, prevent biofouling, and maintain stable electrode-analyte interactions. The polymeric layers can be functionalized to enhance specificity while creating a barrier against interfering substances that contribute to signal drift over time.Expand Specific Solutions03 Self-cleaning and regenerative electrode surfaces
Innovative electrode designs incorporating self-cleaning mechanisms help minimize drift by periodically refreshing the sensing surface. These include electrochemical cleaning protocols, photocatalytic materials that decompose contaminants under light exposure, and stimuli-responsive coatings that can shed fouling layers. Such regenerative surfaces extend sensor lifetime and maintain calibration stability by automatically restoring the original electrode surface properties.Expand Specific Solutions04 Metal oxide and composite material modifications
Metal oxide layers and composite materials applied to electrode surfaces provide enhanced stability against drift factors. These modifications include transition metal oxides, mixed metal composites, and ceramic-based coatings that offer resistance to chemical degradation and temperature fluctuations. The modified electrodes exhibit improved signal-to-noise ratios and reduced baseline drift, particularly in harsh environmental conditions.Expand Specific Solutions05 Microstructure engineering and surface patterning
Precise engineering of electrode surface microstructures through techniques like laser patterning, plasma treatment, and microfabrication can significantly reduce sensor drift. These approaches create controlled surface topographies with optimized active sites that maintain consistent performance. The engineered surfaces demonstrate improved wettability, reduced adsorption of interferents, and more stable electrochemical responses over extended operational periods.Expand Specific Solutions
Leading Companies in Sensor Stability Solutions
The electrode surface modification market for reducing sensor drift is currently in a growth phase, characterized by increasing demand for high-precision sensing technologies across multiple industries. The market size is expanding rapidly, driven by applications in medical devices, environmental monitoring, and industrial automation. From a technological maturity perspective, established players like Robert Bosch GmbH and Samsung SDI are leading with advanced proprietary coating technologies, while academic institutions such as Tsinghua University and Tianjin University are contributing fundamental research breakthroughs. Companies like Honeywell International Technologies and ams International are developing specialized solutions for industrial applications, while Canon and Fuji Electric focus on consumer electronics implementations. The competitive landscape shows a mix of large conglomerates and specialized sensor manufacturers working to address the persistent challenge of sensor drift through innovative surface modification approaches.
Robert Bosch GmbH
Technical Solution: Bosch has developed advanced electrode surface modification techniques to address sensor drift in their MEMS-based gas sensors. Their approach involves applying nanoscale metal oxide coatings (primarily SnO2, WO3, and CuO) using atomic layer deposition (ALD) to create highly uniform and controlled surface structures. This process enables precise control of surface chemistry and morphology, resulting in significantly reduced drift characteristics. Bosch's technology incorporates self-healing surface mechanisms where specific dopants are strategically embedded within the electrode material to counteract degradation processes that typically cause drift over time. Their sensors utilize a proprietary multi-layer electrode structure with gradient doping profiles that maintain stable electrical properties despite environmental exposure. Additionally, Bosch implements in-situ surface cleaning protocols through controlled heating cycles that periodically regenerate the electrode surface, removing contaminants that contribute to drift phenomena.
Strengths: Superior long-term stability with drift reduction of up to 85% compared to conventional sensors. Excellent manufacturing scalability due to integration with existing MEMS production processes. Weaknesses: Higher initial production costs due to complex deposition requirements. The multi-layer structure increases power consumption for sensors deployed in battery-operated devices.
Fraunhofer-Gesellschaft eV
Technical Solution: Fraunhofer has developed an innovative electrode surface modification platform called "DriftGuard" specifically targeting electrochemical and gas sensors. Their approach utilizes atomic layer deposition (ALD) combined with molecular layer deposition (MLD) to create highly engineered electrode surfaces with nanoscale precision. The technology employs alternating layers of metal oxides and organic linkers to form metal-organic frameworks (MOFs) directly on electrode surfaces, creating highly stable interfaces resistant to environmental degradation. Fraunhofer's process incorporates controlled surface roughening through selective etching followed by deposition of self-assembled monolayers (SAMs) that act as molecular anchors for subsequent functional layers. Their sensors feature gradient-doped transition metal dichalcogenide (TMD) layers that provide exceptional stability against oxidation and reduction processes that typically cause drift. Additionally, Fraunhofer has pioneered the use of carbon allotrope coatings (graphene and carbon nanotubes) functionalized with specific chemical groups to create hydrophobic barriers that prevent moisture-induced drift while maintaining electrochemical activity toward target analytes.
Strengths: Exceptional long-term stability with demonstrated drift reduction exceeding 90% over conventional sensors in extended field trials. Highly versatile platform applicable across multiple sensor types (electrochemical, optical, and MEMS-based). Weaknesses: Current production methods are limited to small-batch manufacturing, creating scalability challenges. The complex surface chemistry requires specialized equipment and expertise for implementation.
Key Innovations in Anti-Drift Electrode Materials
Performance enhancement of sensors through surface processing
PatentWO2019245814A1
Innovation
- A method involving surface processing techniques to modify the electrode surfaces by smoothing, polishing, or altering the shape, using abrasive materials or chemical polishing to reduce roughness and improve contact between electrode and conductor components, enhancing the interfacing and performance of sensors.
In situ chemical sensing electrode reconditioning
PatentActiveUS20170336340A1
Innovation
- Applying a controlled pulse of DC voltage, typically between −0.6 to 1.0 V with respect to the standard hydrogen electrode, to remove passivation layers on electrodes such as platinum, allowing for in situ reconditioning and maintaining electrode stability, even in the presence of physiological ions like chloride and oxygen, thereby maintaining sensor sensitivity and accuracy.
Environmental Factors Affecting Sensor Stability
Environmental conditions play a critical role in the stability and performance of electrochemical sensors, directly influencing sensor drift phenomena. Temperature fluctuations represent one of the most significant environmental challenges, as they can alter electrode kinetics, diffusion rates, and reaction thermodynamics. Studies have shown that for many electrochemical sensors, temperature variations of just 1°C can result in signal drift of 1-5%, depending on the specific sensing mechanism and electrode materials employed.
Humidity levels similarly impact sensor stability through multiple mechanisms. High humidity environments can lead to water condensation on electrode surfaces, potentially dissolving active components or creating alternative conduction pathways. Conversely, extremely low humidity may affect reference electrode function in certain sensor designs, particularly those utilizing hydrated salt bridges or gel electrolytes.
Atmospheric pressure variations, though often overlooked, can significantly influence dissolved gas concentrations in liquid samples and alter gas-phase reactions in air quality sensors. This becomes particularly relevant in applications involving altitude changes or pressurized industrial environments where sensors must maintain calibration across varying pressure conditions.
Chemical interferents present in the operational environment constitute another major stability challenge. Airborne contaminants such as volatile organic compounds, sulfur species, and nitrogen oxides can adsorb onto electrode surfaces, progressively altering their electrochemical properties. In liquid-phase sensing applications, surface-active agents and proteins may accumulate on electrodes, forming biofilms or passivation layers that impede analyte access to active sites.
Electromagnetic interference (EMI) from nearby electronic equipment, power lines, or wireless communication systems can introduce noise and drift in sensor signals, particularly in high-impedance electrochemical measurement systems. This necessitates appropriate shielding and signal processing strategies to maintain measurement integrity in electromagnetically noisy environments.
Light exposure represents another environmental factor affecting certain electrochemical sensors, particularly those incorporating photosensitive materials or utilizing photoelectrochemical effects. UV radiation can accelerate degradation of polymer components in sensor assemblies, while visible light may generate photoelectrons that contribute to background currents in semiconductor-based electrodes.
Mechanical factors including vibration, shock, and fluid flow dynamics can physically alter electrode surfaces through erosion, delamination of coatings, or redistribution of surface-bound species. These mechanical stresses often manifest as sudden calibration shifts rather than gradual drift, though repeated exposure can lead to cumulative effects that manifest as progressive sensitivity loss.
Humidity levels similarly impact sensor stability through multiple mechanisms. High humidity environments can lead to water condensation on electrode surfaces, potentially dissolving active components or creating alternative conduction pathways. Conversely, extremely low humidity may affect reference electrode function in certain sensor designs, particularly those utilizing hydrated salt bridges or gel electrolytes.
Atmospheric pressure variations, though often overlooked, can significantly influence dissolved gas concentrations in liquid samples and alter gas-phase reactions in air quality sensors. This becomes particularly relevant in applications involving altitude changes or pressurized industrial environments where sensors must maintain calibration across varying pressure conditions.
Chemical interferents present in the operational environment constitute another major stability challenge. Airborne contaminants such as volatile organic compounds, sulfur species, and nitrogen oxides can adsorb onto electrode surfaces, progressively altering their electrochemical properties. In liquid-phase sensing applications, surface-active agents and proteins may accumulate on electrodes, forming biofilms or passivation layers that impede analyte access to active sites.
Electromagnetic interference (EMI) from nearby electronic equipment, power lines, or wireless communication systems can introduce noise and drift in sensor signals, particularly in high-impedance electrochemical measurement systems. This necessitates appropriate shielding and signal processing strategies to maintain measurement integrity in electromagnetically noisy environments.
Light exposure represents another environmental factor affecting certain electrochemical sensors, particularly those incorporating photosensitive materials or utilizing photoelectrochemical effects. UV radiation can accelerate degradation of polymer components in sensor assemblies, while visible light may generate photoelectrons that contribute to background currents in semiconductor-based electrodes.
Mechanical factors including vibration, shock, and fluid flow dynamics can physically alter electrode surfaces through erosion, delamination of coatings, or redistribution of surface-bound species. These mechanical stresses often manifest as sudden calibration shifts rather than gradual drift, though repeated exposure can lead to cumulative effects that manifest as progressive sensitivity loss.
Standardization and Testing Protocols
Standardization of testing protocols for electrode surface modification techniques is critical for ensuring reliable and comparable results across different research groups and industrial applications. Currently, the field suffers from significant inconsistencies in how sensor drift is measured and reported, making it difficult to objectively evaluate the effectiveness of various modification approaches. A comprehensive standardization framework must address multiple testing parameters including environmental conditions, measurement intervals, and performance metrics.
Temperature, humidity, and pressure variations significantly impact sensor performance and must be strictly controlled during testing. Standard protocols should specify testing under multiple defined environmental conditions: normal operating conditions (20-25°C, 40-60% relative humidity), extreme conditions (temperature cycling between -20°C and 80°C), and accelerated aging tests. These standardized conditions enable meaningful comparison between different modification techniques.
Temporal aspects of testing require equal attention, with protocols defining both short-term (hours to days) and long-term (weeks to months) drift evaluation periods. Standardized measurement intervals should be established, with higher frequency measurements during initial deployment when drift often occurs most rapidly, followed by regular intervals during extended operation. This approach provides a complete picture of drift behavior throughout the sensor lifecycle.
Quantitative performance metrics must be clearly defined, including baseline drift rate (signal change per unit time under stable conditions), response time stability (changes in sensor response kinetics over time), and recovery characteristics after exposure to interferents. Statistical methods for data analysis should be standardized, with requirements for reporting confidence intervals and statistical significance of drift reduction claims.
Reference materials and calibration standards specific to different sensor types must be developed to enable cross-laboratory validation. These standards should include certified reference electrodes with known drift characteristics that can serve as benchmarks for evaluating new modification techniques. Interlaboratory testing programs would further strengthen standardization efforts by identifying inconsistencies in testing methodologies.
Implementation of these standardized protocols would significantly advance the field by enabling objective comparison between different surface modification approaches. This would accelerate the identification of truly effective techniques and facilitate their transition from laboratory research to commercial applications. Industry-academic consortia should be established to develop, validate and periodically update these standards as measurement technologies and modification techniques continue to evolve.
Temperature, humidity, and pressure variations significantly impact sensor performance and must be strictly controlled during testing. Standard protocols should specify testing under multiple defined environmental conditions: normal operating conditions (20-25°C, 40-60% relative humidity), extreme conditions (temperature cycling between -20°C and 80°C), and accelerated aging tests. These standardized conditions enable meaningful comparison between different modification techniques.
Temporal aspects of testing require equal attention, with protocols defining both short-term (hours to days) and long-term (weeks to months) drift evaluation periods. Standardized measurement intervals should be established, with higher frequency measurements during initial deployment when drift often occurs most rapidly, followed by regular intervals during extended operation. This approach provides a complete picture of drift behavior throughout the sensor lifecycle.
Quantitative performance metrics must be clearly defined, including baseline drift rate (signal change per unit time under stable conditions), response time stability (changes in sensor response kinetics over time), and recovery characteristics after exposure to interferents. Statistical methods for data analysis should be standardized, with requirements for reporting confidence intervals and statistical significance of drift reduction claims.
Reference materials and calibration standards specific to different sensor types must be developed to enable cross-laboratory validation. These standards should include certified reference electrodes with known drift characteristics that can serve as benchmarks for evaluating new modification techniques. Interlaboratory testing programs would further strengthen standardization efforts by identifying inconsistencies in testing methodologies.
Implementation of these standardized protocols would significantly advance the field by enabling objective comparison between different surface modification approaches. This would accelerate the identification of truly effective techniques and facilitate their transition from laboratory research to commercial applications. Industry-academic consortia should be established to develop, validate and periodically update these standards as measurement technologies and modification techniques continue to evolve.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!