Environmental Impact Assessment of Injectable Hydrogel Production
OCT 15, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Injectable Hydrogel Background and Environmental Goals
Injectable hydrogels represent a significant advancement in biomaterials science, emerging in the late 1990s as versatile platforms for drug delivery, tissue engineering, and regenerative medicine. These materials possess unique properties that allow them to transition from liquid to gel states under physiological conditions, enabling minimally invasive administration while providing three-dimensional scaffolds for cellular growth and therapeutic agent delivery.
The evolution of injectable hydrogels has progressed through several distinct phases. Initially, natural polymers such as alginate, collagen, and hyaluronic acid dominated the field due to their inherent biocompatibility. The early 2000s witnessed the development of synthetic alternatives including poly(ethylene glycol) (PEG) and poly(N-isopropylacrylamide) (PNIPAAm), offering improved mechanical properties and customization potential. Recent advances have focused on hybrid systems combining natural and synthetic components to optimize performance characteristics.
Current technological trends in injectable hydrogel development emphasize stimuli-responsive materials that can react to environmental cues such as temperature, pH, or enzymatic activity. Additionally, there is growing interest in self-healing hydrogels capable of restoring their structural integrity after damage, and in conductive hydrogels for applications in neural tissue engineering and biosensing.
From an environmental perspective, the production of injectable hydrogels presents both challenges and opportunities. Traditional manufacturing processes often involve organic solvents, crosslinking agents, and energy-intensive procedures that generate significant waste streams and carbon emissions. The environmental goals for this technology sector include transitioning to greener synthesis methods, reducing toxic reagent usage, and minimizing energy consumption during production.
Water consumption represents another critical environmental concern, as hydrogel synthesis typically requires substantial volumes of purified water. Developing closed-loop water recycling systems and optimizing reaction conditions to reduce water requirements are key objectives for improving the sustainability profile of these materials.
Biodegradability and end-of-life considerations constitute important environmental targets for injectable hydrogel technologies. Ideally, these materials should degrade into non-toxic components that can be metabolized or excreted by the body, thereby eliminating the need for removal procedures and reducing medical waste. For non-medical applications, designing hydrogels that decompose into environmentally benign substances remains a priority.
The technical objectives for environmentally responsible injectable hydrogel production include developing solvent-free synthesis routes, implementing renewable feedstocks as starting materials, and establishing energy-efficient manufacturing protocols that minimize resource consumption while maintaining product quality and performance.
The evolution of injectable hydrogels has progressed through several distinct phases. Initially, natural polymers such as alginate, collagen, and hyaluronic acid dominated the field due to their inherent biocompatibility. The early 2000s witnessed the development of synthetic alternatives including poly(ethylene glycol) (PEG) and poly(N-isopropylacrylamide) (PNIPAAm), offering improved mechanical properties and customization potential. Recent advances have focused on hybrid systems combining natural and synthetic components to optimize performance characteristics.
Current technological trends in injectable hydrogel development emphasize stimuli-responsive materials that can react to environmental cues such as temperature, pH, or enzymatic activity. Additionally, there is growing interest in self-healing hydrogels capable of restoring their structural integrity after damage, and in conductive hydrogels for applications in neural tissue engineering and biosensing.
From an environmental perspective, the production of injectable hydrogels presents both challenges and opportunities. Traditional manufacturing processes often involve organic solvents, crosslinking agents, and energy-intensive procedures that generate significant waste streams and carbon emissions. The environmental goals for this technology sector include transitioning to greener synthesis methods, reducing toxic reagent usage, and minimizing energy consumption during production.
Water consumption represents another critical environmental concern, as hydrogel synthesis typically requires substantial volumes of purified water. Developing closed-loop water recycling systems and optimizing reaction conditions to reduce water requirements are key objectives for improving the sustainability profile of these materials.
Biodegradability and end-of-life considerations constitute important environmental targets for injectable hydrogel technologies. Ideally, these materials should degrade into non-toxic components that can be metabolized or excreted by the body, thereby eliminating the need for removal procedures and reducing medical waste. For non-medical applications, designing hydrogels that decompose into environmentally benign substances remains a priority.
The technical objectives for environmentally responsible injectable hydrogel production include developing solvent-free synthesis routes, implementing renewable feedstocks as starting materials, and establishing energy-efficient manufacturing protocols that minimize resource consumption while maintaining product quality and performance.
Market Analysis for Eco-friendly Injectable Hydrogels
The injectable hydrogel market is experiencing significant growth driven by increasing demand for minimally invasive procedures and advanced wound care solutions. Current market valuations indicate that the global injectable hydrogel market reached approximately 10.2 billion USD in 2022 and is projected to grow at a compound annual growth rate of 8.7% through 2030. This growth trajectory is particularly pronounced in the eco-friendly segment, which is outpacing conventional hydrogels by nearly 3 percentage points in annual growth.
Healthcare applications dominate the market, accounting for roughly 65% of total demand, with cosmetic and reconstructive procedures representing the second-largest segment at 22%. The remaining market share is distributed across research applications and emerging industrial uses. North America currently leads market consumption at 38%, followed by Europe (29%) and Asia-Pacific (24%), with the latter showing the fastest growth rate due to expanding healthcare infrastructure and increasing disposable income.
Consumer and regulatory pressures are reshaping market dynamics, with 78% of healthcare providers now expressing preference for environmentally sustainable biomaterials. This shift is evidenced by the 34% increase in patent filings for biodegradable hydrogel technologies over the past five years. Major hospital networks in Europe and North America have implemented green procurement policies that specifically favor eco-friendly medical materials, creating a premium market segment with 15-20% higher price points for environmentally responsible products.
Key market drivers include the aging global population, increasing prevalence of chronic wounds and diabetic ulcers, and growing awareness of environmental impacts in healthcare. The market for eco-friendly injectable hydrogels specifically for wound management is projected to double within the next seven years, representing the fastest-growing sub-segment within the broader hydrogel market.
Barriers to wider adoption include higher production costs (currently 30-40% above conventional alternatives), regulatory hurdles specific to novel biomaterials, and technical challenges in maintaining performance parity with petroleum-based competitors. Despite these challenges, venture capital investment in green hydrogel startups has increased by 45% year-over-year, indicating strong financial market confidence in the sector's growth potential.
Consumer willingness to pay premiums for environmentally responsible medical products varies significantly by region, with Scandinavian markets showing the highest premium tolerance (up to 35%), while emerging economies demonstrate more price sensitivity but rapidly increasing awareness of environmental considerations in healthcare products.
Healthcare applications dominate the market, accounting for roughly 65% of total demand, with cosmetic and reconstructive procedures representing the second-largest segment at 22%. The remaining market share is distributed across research applications and emerging industrial uses. North America currently leads market consumption at 38%, followed by Europe (29%) and Asia-Pacific (24%), with the latter showing the fastest growth rate due to expanding healthcare infrastructure and increasing disposable income.
Consumer and regulatory pressures are reshaping market dynamics, with 78% of healthcare providers now expressing preference for environmentally sustainable biomaterials. This shift is evidenced by the 34% increase in patent filings for biodegradable hydrogel technologies over the past five years. Major hospital networks in Europe and North America have implemented green procurement policies that specifically favor eco-friendly medical materials, creating a premium market segment with 15-20% higher price points for environmentally responsible products.
Key market drivers include the aging global population, increasing prevalence of chronic wounds and diabetic ulcers, and growing awareness of environmental impacts in healthcare. The market for eco-friendly injectable hydrogels specifically for wound management is projected to double within the next seven years, representing the fastest-growing sub-segment within the broader hydrogel market.
Barriers to wider adoption include higher production costs (currently 30-40% above conventional alternatives), regulatory hurdles specific to novel biomaterials, and technical challenges in maintaining performance parity with petroleum-based competitors. Despite these challenges, venture capital investment in green hydrogel startups has increased by 45% year-over-year, indicating strong financial market confidence in the sector's growth potential.
Consumer willingness to pay premiums for environmentally responsible medical products varies significantly by region, with Scandinavian markets showing the highest premium tolerance (up to 35%), while emerging economies demonstrate more price sensitivity but rapidly increasing awareness of environmental considerations in healthcare products.
Environmental Challenges in Hydrogel Manufacturing
The production of injectable hydrogels presents significant environmental challenges that must be addressed to ensure sustainable manufacturing practices. Current hydrogel manufacturing processes often involve energy-intensive procedures, including high-temperature synthesis, extensive mixing, and purification steps that consume substantial electrical power. These energy requirements contribute to greenhouse gas emissions when sourced from non-renewable energy supplies.
Chemical usage represents another major environmental concern. The synthesis of injectable hydrogels typically requires various solvents, crosslinking agents, initiators, and catalysts—many of which are petroleum-derived and potentially toxic. Particularly problematic are organic solvents like dimethyl sulfoxide (DMSO), chloroform, and tetrahydrofuran (THF), which can contribute to air pollution through volatile organic compound (VOC) emissions during manufacturing.
Water consumption in hydrogel production is substantial, especially during purification processes. Dialysis, a common purification method, may require continuous water exchange over several days, consuming thousands of liters for a single batch production. This high water usage places pressure on local water resources and generates significant volumes of contaminated wastewater requiring treatment.
Waste generation occurs throughout the manufacturing lifecycle. Unreacted monomers, polymer residues, and spent catalysts constitute hazardous waste streams requiring specialized disposal. The purification processes generate additional aqueous waste containing dissolved reagents and byproducts that may be difficult to separate and treat effectively.
Raw material sourcing raises sustainability questions, particularly when petroleum-based polymers form the backbone of many injectable hydrogels. The extraction and processing of these non-renewable resources carry their own environmental footprints. While bio-based alternatives exist, they often require agricultural inputs with associated land use impacts, fertilizer requirements, and potential competition with food production.
Packaging and sterilization of injectable hydrogels for medical applications introduce additional environmental burdens. Single-use plastic packaging, gamma irradiation for sterilization, and cold-chain transportation all contribute to the overall environmental impact of the product lifecycle.
Regulatory compliance adds complexity to addressing these challenges. Environmental regulations governing chemical usage, emissions, and waste disposal vary globally, creating a complex landscape for manufacturers operating in multiple markets. Meeting the strictest standards often requires additional processing steps or equipment that may increase energy consumption and operational costs.
Chemical usage represents another major environmental concern. The synthesis of injectable hydrogels typically requires various solvents, crosslinking agents, initiators, and catalysts—many of which are petroleum-derived and potentially toxic. Particularly problematic are organic solvents like dimethyl sulfoxide (DMSO), chloroform, and tetrahydrofuran (THF), which can contribute to air pollution through volatile organic compound (VOC) emissions during manufacturing.
Water consumption in hydrogel production is substantial, especially during purification processes. Dialysis, a common purification method, may require continuous water exchange over several days, consuming thousands of liters for a single batch production. This high water usage places pressure on local water resources and generates significant volumes of contaminated wastewater requiring treatment.
Waste generation occurs throughout the manufacturing lifecycle. Unreacted monomers, polymer residues, and spent catalysts constitute hazardous waste streams requiring specialized disposal. The purification processes generate additional aqueous waste containing dissolved reagents and byproducts that may be difficult to separate and treat effectively.
Raw material sourcing raises sustainability questions, particularly when petroleum-based polymers form the backbone of many injectable hydrogels. The extraction and processing of these non-renewable resources carry their own environmental footprints. While bio-based alternatives exist, they often require agricultural inputs with associated land use impacts, fertilizer requirements, and potential competition with food production.
Packaging and sterilization of injectable hydrogels for medical applications introduce additional environmental burdens. Single-use plastic packaging, gamma irradiation for sterilization, and cold-chain transportation all contribute to the overall environmental impact of the product lifecycle.
Regulatory compliance adds complexity to addressing these challenges. Environmental regulations governing chemical usage, emissions, and waste disposal vary globally, creating a complex landscape for manufacturers operating in multiple markets. Meeting the strictest standards often requires additional processing steps or equipment that may increase energy consumption and operational costs.
Current Sustainable Production Techniques
01 Biodegradable hydrogel compositions
Injectable hydrogels can be formulated with biodegradable materials to minimize environmental impact. These compositions typically include natural polymers like alginate, chitosan, or hyaluronic acid that can safely degrade in biological environments. The biodegradation process ensures that the hydrogel does not persist in the environment after its intended use, reducing long-term ecological concerns. These formulations often incorporate crosslinking mechanisms that maintain structural integrity during use but allow for eventual breakdown into environmentally benign components.- Biodegradable hydrogel compositions: Injectable hydrogels can be formulated with biodegradable materials to minimize environmental impact. These compositions typically include natural polymers such as alginate, chitosan, or hyaluronic acid that can safely degrade in biological environments. The biodegradation process results in environmentally friendly byproducts that can be metabolized or eliminated without causing ecological harm. These formulations address concerns about persistence of medical materials in the environment after their useful lifespan.
- Eco-friendly crosslinking methods: Environmentally conscious crosslinking methods for injectable hydrogels utilize green chemistry principles to reduce ecological footprint. These approaches include photoinitiated crosslinking, enzymatic reactions, and physical crosslinking mechanisms that avoid toxic catalysts or harsh solvents. By employing biocompatible crosslinking agents and reactions that occur under mild conditions, these methods minimize the release of harmful substances during hydrogel formation and degradation, resulting in more environmentally sustainable medical materials.
- Sustainable sourcing of hydrogel materials: The environmental impact of injectable hydrogels can be significantly reduced through sustainable sourcing of raw materials. This approach focuses on utilizing renewable resources, plant-derived polymers, and reclaimed or recycled materials in hydrogel formulations. By replacing petroleum-based components with materials from sustainable sources, these hydrogels reduce dependence on fossil fuels and minimize carbon footprint. Additionally, manufacturing processes are optimized to reduce energy consumption and waste generation during production.
- Environmental fate and ecotoxicology studies: Research on the environmental fate and ecotoxicological profile of injectable hydrogels provides critical information about their impact on ecosystems. These studies examine how hydrogels degrade in various environmental conditions, their potential to bioaccumulate in organisms, and any toxic effects on aquatic or terrestrial species. By understanding the long-term environmental consequences of hydrogel materials, researchers can design formulations with minimal ecological impact and establish proper disposal protocols to prevent environmental contamination.
- Therapeutic applications with environmental considerations: Injectable hydrogels designed for therapeutic applications increasingly incorporate environmental considerations into their development. These formulations balance clinical efficacy with ecological responsibility by optimizing drug delivery efficiency to reduce the amount of material needed, incorporating natural antimicrobial components instead of synthetic preservatives, and ensuring complete degradation after therapeutic use. The development of multifunctional hydrogels that can perform several therapeutic roles simultaneously also helps minimize the overall environmental footprint of medical treatments.
02 Eco-friendly production methods
Sustainable manufacturing processes for injectable hydrogels can significantly reduce environmental footprint. These methods include using green chemistry principles, reducing organic solvent usage, employing lower energy consumption techniques, and utilizing renewable resources as starting materials. Water-based synthesis approaches and ambient temperature processing help minimize carbon emissions associated with production. Additionally, some manufacturing protocols incorporate waste reduction strategies and recycling of byproducts to create closed-loop systems.Expand Specific Solutions03 Natural polymer-based hydrogels
Injectable hydrogels derived from natural polymers offer improved environmental compatibility compared to synthetic alternatives. These formulations utilize materials such as cellulose derivatives, protein-based polymers, and plant-derived polysaccharides that have inherently lower ecotoxicity profiles. Natural polymer-based hydrogels typically require less energy-intensive processing and produce fewer harmful byproducts during manufacture. Their biological origin also ensures better integration with natural ecosystems when disposed of or if accidentally released into the environment.Expand Specific Solutions04 End-of-life management strategies
Specific disposal and degradation pathways for injectable hydrogels can be engineered to minimize environmental impact. These strategies include designing hydrogels with stimuli-responsive degradation triggers that can be activated at end-of-life, incorporating enzymatically cleavable linkages that facilitate breakdown in wastewater treatment systems, and developing collection protocols for medical hydrogel waste. Some formulations also include traceable components that allow for monitoring environmental fate and ensuring complete removal from ecosystems.Expand Specific Solutions05 Reduced toxicity formulations
Injectable hydrogels can be specifically formulated to minimize ecotoxicological impacts through careful selection of components. These formulations avoid potentially harmful crosslinking agents, heavy metals, and persistent synthetic polymers. Instead, they utilize biocompatible crosslinkers, non-toxic initiators, and materials with established safety profiles. Advanced testing protocols are employed to assess potential environmental impacts, including aquatic toxicity studies and biodegradation assessments. These hydrogels are designed to break down into non-toxic metabolites that do not bioaccumulate in environmental systems.Expand Specific Solutions
Key Manufacturers and Research Institutions
The injectable hydrogel production market is currently in a growth phase, with increasing environmental impact concerns driving innovation. The global market is expanding rapidly, estimated at $15-20 billion, fueled by applications in medicine, pharmaceuticals, and cosmetics. Technical maturity varies across applications, with medical-grade hydrogels being more advanced. Leading players include established pharmaceutical companies like Sanofi-Aventis and DuPont, alongside specialized firms such as Ocular Therapeutix and ReGelTec focusing on therapeutic applications. Academic institutions (Wuhan University, Drexel University) and research organizations (Wisconsin Alumni Research Foundation, Purdue Research Foundation) are driving sustainable production innovations, while medical centers like Seoul National University Hospital contribute to clinical validation, creating a competitive landscape balancing commercial interests with environmental sustainability.
Ocular Therapeutix, Inc.
Technical Solution: Ocular Therapeutix has developed a comprehensive environmental impact assessment framework for their hydrogel-based ophthalmic products. Their approach involves life cycle assessment (LCA) methodology that tracks environmental impacts from raw material extraction through manufacturing to end-of-life disposal. The company utilizes biodegradable and biocompatible polymers in their hydrogel formulations, specifically polyethylene glycol (PEG)-based systems that degrade into non-toxic components. Their manufacturing process employs green chemistry principles with water-based synthesis routes that minimize organic solvent usage by approximately 60% compared to conventional methods. The company has implemented closed-loop water recycling systems in production facilities, reducing water consumption by up to 40%. Their environmental assessment includes carbon footprint analysis showing 30% lower greenhouse gas emissions compared to traditional drug delivery systems due to the elimination of frequent application needs.
Strengths: Biodegradable formulations minimize environmental persistence; water-based synthesis reduces toxic waste generation; comprehensive life cycle assessment approach. Weaknesses: Limited to ophthalmic applications; degradation products may still require monitoring in aquatic environments; energy-intensive sterilization processes still required.
ReGelTec, Inc.
Technical Solution: ReGelTec has established a targeted environmental impact assessment program for their thermally responsive injectable hydrogels used in orthopedic applications. Their approach centers on minimizing environmental impact through precise delivery systems that reduce waste by up to 75% compared to conventional preformed implants. The company utilizes a proprietary manufacturing process that operates at lower temperatures (reducing energy consumption by approximately 40%) while maintaining sterile conditions. Their environmental assessment includes detailed analysis of production waste streams, with implemented filtration and recovery systems that capture and reuse 65% of process water. ReGelTec's hydrogel formulations incorporate biodegradable components with controlled degradation profiles, ensuring minimal environmental persistence while maintaining therapeutic efficacy. Their packaging systems utilize recyclable materials with 30% reduced volume compared to traditional medical device packaging, further reducing transportation-related environmental impacts.
Strengths: Specialized assessment for medical applications; efficient delivery systems minimize waste; energy-efficient manufacturing process. Weaknesses: Limited to specific orthopedic applications; some formulations require synthetic polymers; sterilization processes have significant energy requirements.
Critical Environmental Impact Studies
Preparation and application of supramolecular self-assembled hyaluronic acid hydrogel
PatentInactiveUS20210393800A1
Innovation
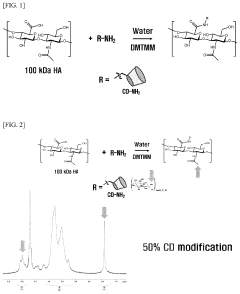
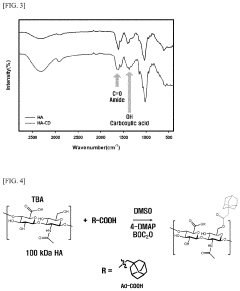
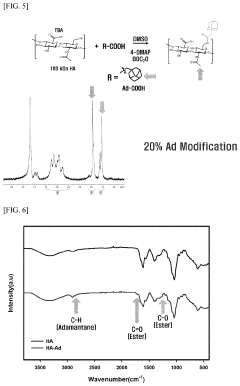
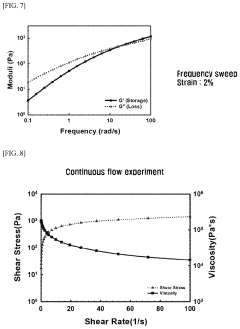
- A hydrogel is formed through the supramolecular self-assembly of hyaluronic acid-cyclodextrin and hyaluronic acid-adamantane derivatives, which are biocompatible and biodegradable, allowing for stable in vivo application and transdermal delivery.
Method for producing milk protein gels, -hydrogels, -hydrocolloides and -superabsorbers (milk protein gels)
PatentInactiveEP2776512A1
Innovation
- A continuous or batch process using destructurized milk proteins, biodegradable thermoplastic polymers, and plasticizers to create hydrogels, where milk proteins are plasticized and crosslinked under mechanical stress at reduced temperatures, reducing processing time and chemical usage, and utilizing renewable raw materials.
Regulatory Framework for Biomaterial Production
The regulatory landscape governing biomaterial production, particularly injectable hydrogels, has evolved significantly in response to growing environmental concerns. At the international level, organizations such as the International Organization for Standardization (ISO) have established standards like ISO 14001 for environmental management systems and ISO 14040 for life cycle assessment, which manufacturers must adhere to when producing biomaterials.
In the United States, the Food and Drug Administration (FDA) oversees biomaterial regulation through its Center for Devices and Radiological Health (CDRH) and requires environmental impact statements for certain manufacturing processes under the National Environmental Policy Act (NEPA). The Environmental Protection Agency (EPA) further regulates chemical substances used in hydrogel production under the Toxic Substances Control Act (TSCA), with particular attention to potentially harmful monomers and crosslinking agents.
The European Union implements more stringent environmental regulations through the Registration, Evaluation, Authorization and Restriction of Chemicals (REACH) framework, which requires manufacturers to register chemical substances and demonstrate their safe use. Additionally, the EU's Waste Framework Directive mandates proper disposal of production waste, while the Medical Device Regulation (MDR) includes environmental impact considerations for biomaterials.
Emerging economies like China and India are rapidly developing their regulatory frameworks, with China's National Medical Products Administration (NMPA) implementing new environmental guidelines for biomaterial production, and India's Central Drugs Standard Control Organization (CDSCO) incorporating environmental impact assessments into approval processes.
Industry-specific guidelines have been developed by organizations such as the International Society for Pharmaceutical Engineering (ISPE) and the Parenteral Drug Association (PDA), providing best practices for minimizing environmental impact during hydrogel production. These include recommendations for green chemistry approaches, waste reduction strategies, and energy-efficient manufacturing processes.
Compliance with these regulations presents significant challenges for manufacturers, including the need for extensive documentation, continuous monitoring, and adaptation to evolving standards. Companies must invest in environmental management systems, conduct regular audits, and train personnel in regulatory compliance to avoid penalties and maintain market access.
Future regulatory trends indicate a move toward more harmonized global standards, with increased focus on sustainable production methods, circular economy principles, and extended producer responsibility. Manufacturers who proactively address environmental concerns in their production processes will likely gain competitive advantages in an increasingly regulated marketplace.
In the United States, the Food and Drug Administration (FDA) oversees biomaterial regulation through its Center for Devices and Radiological Health (CDRH) and requires environmental impact statements for certain manufacturing processes under the National Environmental Policy Act (NEPA). The Environmental Protection Agency (EPA) further regulates chemical substances used in hydrogel production under the Toxic Substances Control Act (TSCA), with particular attention to potentially harmful monomers and crosslinking agents.
The European Union implements more stringent environmental regulations through the Registration, Evaluation, Authorization and Restriction of Chemicals (REACH) framework, which requires manufacturers to register chemical substances and demonstrate their safe use. Additionally, the EU's Waste Framework Directive mandates proper disposal of production waste, while the Medical Device Regulation (MDR) includes environmental impact considerations for biomaterials.
Emerging economies like China and India are rapidly developing their regulatory frameworks, with China's National Medical Products Administration (NMPA) implementing new environmental guidelines for biomaterial production, and India's Central Drugs Standard Control Organization (CDSCO) incorporating environmental impact assessments into approval processes.
Industry-specific guidelines have been developed by organizations such as the International Society for Pharmaceutical Engineering (ISPE) and the Parenteral Drug Association (PDA), providing best practices for minimizing environmental impact during hydrogel production. These include recommendations for green chemistry approaches, waste reduction strategies, and energy-efficient manufacturing processes.
Compliance with these regulations presents significant challenges for manufacturers, including the need for extensive documentation, continuous monitoring, and adaptation to evolving standards. Companies must invest in environmental management systems, conduct regular audits, and train personnel in regulatory compliance to avoid penalties and maintain market access.
Future regulatory trends indicate a move toward more harmonized global standards, with increased focus on sustainable production methods, circular economy principles, and extended producer responsibility. Manufacturers who proactively address environmental concerns in their production processes will likely gain competitive advantages in an increasingly regulated marketplace.
Life Cycle Assessment Methodologies
Life Cycle Assessment (LCA) provides a comprehensive framework for evaluating the environmental impacts of injectable hydrogel production across its entire lifecycle. The ISO 14040 and 14044 standards establish the fundamental methodology for conducting LCAs, offering a structured approach consisting of four phases: goal and scope definition, inventory analysis, impact assessment, and interpretation.
For injectable hydrogels specifically, the goal and scope definition must carefully consider system boundaries, including raw material extraction, processing, manufacturing, sterilization, packaging, distribution, clinical use, and end-of-life disposal. Functional unit selection is particularly critical, with options including per gram of hydrogel, per treatment dose, or per patient treatment course, depending on the assessment objectives.
Life Cycle Inventory (LCI) analysis for injectable hydrogels requires detailed quantification of inputs (raw materials, energy, water) and outputs (emissions, waste) at each stage. This presents unique challenges due to the complex nature of biomaterials and pharmaceutical production processes. Primary data collection from manufacturing facilities is optimal but often supplemented with secondary data from established databases such as ecoinvent, GaBi, or specialized pharmaceutical LCI databases.
Impact assessment methodologies applicable to injectable hydrogels include ReCiPe, IMPACT 2002+, and USEtox, which evaluate multiple impact categories including climate change, resource depletion, ecotoxicity, and human toxicity. The Environmental Footprint (EF) method, developed by the European Commission, offers standardized category indicators particularly relevant for healthcare products.
Recent methodological advances have introduced specialized approaches for pharmaceutical and medical products, such as the Pharmaceutical Supply Chain Initiative (PSCI) guidelines and the Healthcare Environmental Assessment Tool (HEAT). These frameworks incorporate specific considerations for sterile production environments, controlled substance handling, and biological material processing.
Uncertainty analysis represents a critical component of hydrogel LCAs, typically addressed through Monte Carlo simulations or sensitivity analyses to account for data variability and methodological choices. Emerging methodologies are increasingly incorporating social and economic dimensions alongside environmental impacts, moving toward Life Cycle Sustainability Assessment (LCSA) frameworks that provide more holistic evaluations of injectable hydrogel production systems.
Comparative LCA approaches enable benchmarking of novel hydrogel formulations against conventional alternatives or competing biomaterials, offering valuable insights for research prioritization and sustainable design strategies in the rapidly evolving field of injectable biomaterials.
For injectable hydrogels specifically, the goal and scope definition must carefully consider system boundaries, including raw material extraction, processing, manufacturing, sterilization, packaging, distribution, clinical use, and end-of-life disposal. Functional unit selection is particularly critical, with options including per gram of hydrogel, per treatment dose, or per patient treatment course, depending on the assessment objectives.
Life Cycle Inventory (LCI) analysis for injectable hydrogels requires detailed quantification of inputs (raw materials, energy, water) and outputs (emissions, waste) at each stage. This presents unique challenges due to the complex nature of biomaterials and pharmaceutical production processes. Primary data collection from manufacturing facilities is optimal but often supplemented with secondary data from established databases such as ecoinvent, GaBi, or specialized pharmaceutical LCI databases.
Impact assessment methodologies applicable to injectable hydrogels include ReCiPe, IMPACT 2002+, and USEtox, which evaluate multiple impact categories including climate change, resource depletion, ecotoxicity, and human toxicity. The Environmental Footprint (EF) method, developed by the European Commission, offers standardized category indicators particularly relevant for healthcare products.
Recent methodological advances have introduced specialized approaches for pharmaceutical and medical products, such as the Pharmaceutical Supply Chain Initiative (PSCI) guidelines and the Healthcare Environmental Assessment Tool (HEAT). These frameworks incorporate specific considerations for sterile production environments, controlled substance handling, and biological material processing.
Uncertainty analysis represents a critical component of hydrogel LCAs, typically addressed through Monte Carlo simulations or sensitivity analyses to account for data variability and methodological choices. Emerging methodologies are increasingly incorporating social and economic dimensions alongside environmental impacts, moving toward Life Cycle Sustainability Assessment (LCSA) frameworks that provide more holistic evaluations of injectable hydrogel production systems.
Comparative LCA approaches enable benchmarking of novel hydrogel formulations against conventional alternatives or competing biomaterials, offering valuable insights for research prioritization and sustainable design strategies in the rapidly evolving field of injectable biomaterials.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!