Ethyl Propanoate in Organic Acid Production: Process Improvements
JUL 22, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Ethyl Propanoate Synthesis Background and Objectives
Ethyl propanoate, also known as ethyl propionate, is a significant ester compound widely used in the food and fragrance industries due to its fruity aroma reminiscent of pineapples. The synthesis of ethyl propanoate has been a subject of interest in organic acid production for decades, with continuous efforts to improve its manufacturing process.
The historical development of ethyl propanoate synthesis can be traced back to the early 20th century when esterification reactions became better understood. Initially, the production relied on batch processes using mineral acid catalysts, which were inefficient and environmentally unfriendly. As the demand for ethyl propanoate grew in various applications, including solvents, plasticizers, and artificial flavorings, the need for more efficient and sustainable production methods became apparent.
The evolution of ethyl propanoate synthesis has been driven by several factors, including the push for greener chemistry, the need for higher yields, and the demand for purer products. Over the years, researchers and industry professionals have explored various catalytic systems, reaction conditions, and process designs to enhance the production of this valuable ester.
One of the key trends in the field has been the shift towards continuous flow processes, which offer advantages in terms of process control, safety, and scalability. Another significant development has been the exploration of heterogeneous catalysts, which can be easily separated from the reaction mixture and potentially reused, leading to more economical and environmentally friendly production methods.
The current technological landscape for ethyl propanoate synthesis is characterized by a mix of traditional and innovative approaches. While some manufacturers still employ conventional batch processes with homogeneous acid catalysis, others have adopted more advanced techniques such as reactive distillation or membrane reactors. These newer methods aim to overcome equilibrium limitations and improve conversion rates.
Looking ahead, the objectives for further improvements in ethyl propanoate synthesis are multifaceted. There is a strong focus on developing catalysts that can operate under milder conditions, reducing energy consumption and minimizing side reactions. Additionally, there is growing interest in utilizing renewable feedstocks as starting materials, aligning with the principles of sustainable chemistry and circular economy.
Another important goal is to enhance the selectivity of the reaction, minimizing the formation of byproducts and simplifying downstream purification processes. This not only improves the overall yield but also reduces waste generation and operational costs. Furthermore, there is an ongoing effort to integrate process intensification techniques, such as microreactor technology, to achieve higher efficiency and reduced equipment footprint.
The historical development of ethyl propanoate synthesis can be traced back to the early 20th century when esterification reactions became better understood. Initially, the production relied on batch processes using mineral acid catalysts, which were inefficient and environmentally unfriendly. As the demand for ethyl propanoate grew in various applications, including solvents, plasticizers, and artificial flavorings, the need for more efficient and sustainable production methods became apparent.
The evolution of ethyl propanoate synthesis has been driven by several factors, including the push for greener chemistry, the need for higher yields, and the demand for purer products. Over the years, researchers and industry professionals have explored various catalytic systems, reaction conditions, and process designs to enhance the production of this valuable ester.
One of the key trends in the field has been the shift towards continuous flow processes, which offer advantages in terms of process control, safety, and scalability. Another significant development has been the exploration of heterogeneous catalysts, which can be easily separated from the reaction mixture and potentially reused, leading to more economical and environmentally friendly production methods.
The current technological landscape for ethyl propanoate synthesis is characterized by a mix of traditional and innovative approaches. While some manufacturers still employ conventional batch processes with homogeneous acid catalysis, others have adopted more advanced techniques such as reactive distillation or membrane reactors. These newer methods aim to overcome equilibrium limitations and improve conversion rates.
Looking ahead, the objectives for further improvements in ethyl propanoate synthesis are multifaceted. There is a strong focus on developing catalysts that can operate under milder conditions, reducing energy consumption and minimizing side reactions. Additionally, there is growing interest in utilizing renewable feedstocks as starting materials, aligning with the principles of sustainable chemistry and circular economy.
Another important goal is to enhance the selectivity of the reaction, minimizing the formation of byproducts and simplifying downstream purification processes. This not only improves the overall yield but also reduces waste generation and operational costs. Furthermore, there is an ongoing effort to integrate process intensification techniques, such as microreactor technology, to achieve higher efficiency and reduced equipment footprint.
Market Analysis for Ethyl Propanoate
The global market for ethyl propanoate has been experiencing steady growth, driven by its versatile applications across various industries. As a key ingredient in flavors and fragrances, ethyl propanoate finds extensive use in the food and beverage sector, particularly in the production of artificial fruit flavors. The compound's sweet, fruity aroma, reminiscent of pineapple and pear, makes it a popular choice for confectionery, baked goods, and beverages.
In the cosmetics and personal care industry, ethyl propanoate is utilized in the formulation of perfumes, lotions, and other scented products. Its pleasant odor and low toxicity profile contribute to its increasing adoption in this sector. The pharmaceutical industry also employs ethyl propanoate as a solvent and intermediate in the synthesis of various drugs and active pharmaceutical ingredients.
The paint and coatings industry represents another significant market for ethyl propanoate. Its excellent solvency properties make it suitable for use in lacquers, varnishes, and industrial coatings. The growing construction and automotive sectors in emerging economies are expected to further boost demand in this application area.
Geographically, Asia-Pacific is anticipated to be the fastest-growing market for ethyl propanoate, owing to rapid industrialization, increasing disposable incomes, and changing consumer preferences in countries like China and India. North America and Europe remain significant markets, driven by established food and beverage, cosmetics, and pharmaceutical industries.
The market is characterized by the presence of both large multinational chemical companies and smaller, specialized manufacturers. Key players are focusing on expanding their production capacities and improving process efficiencies to meet the growing demand. Innovations in green chemistry and bio-based production methods are emerging trends, as companies strive to align with sustainability goals and regulatory requirements.
Challenges in the ethyl propanoate market include price volatility of raw materials, stringent regulations regarding chemical usage in food and personal care products, and competition from alternative esters and synthetic flavoring agents. However, the compound's favorable safety profile and wide range of applications continue to drive its market growth.
As industries increasingly prioritize natural and clean label products, there is a growing interest in bio-based ethyl propanoate produced through fermentation processes. This trend presents both opportunities and challenges for market players, necessitating investments in research and development to maintain competitiveness in the evolving landscape.
In the cosmetics and personal care industry, ethyl propanoate is utilized in the formulation of perfumes, lotions, and other scented products. Its pleasant odor and low toxicity profile contribute to its increasing adoption in this sector. The pharmaceutical industry also employs ethyl propanoate as a solvent and intermediate in the synthesis of various drugs and active pharmaceutical ingredients.
The paint and coatings industry represents another significant market for ethyl propanoate. Its excellent solvency properties make it suitable for use in lacquers, varnishes, and industrial coatings. The growing construction and automotive sectors in emerging economies are expected to further boost demand in this application area.
Geographically, Asia-Pacific is anticipated to be the fastest-growing market for ethyl propanoate, owing to rapid industrialization, increasing disposable incomes, and changing consumer preferences in countries like China and India. North America and Europe remain significant markets, driven by established food and beverage, cosmetics, and pharmaceutical industries.
The market is characterized by the presence of both large multinational chemical companies and smaller, specialized manufacturers. Key players are focusing on expanding their production capacities and improving process efficiencies to meet the growing demand. Innovations in green chemistry and bio-based production methods are emerging trends, as companies strive to align with sustainability goals and regulatory requirements.
Challenges in the ethyl propanoate market include price volatility of raw materials, stringent regulations regarding chemical usage in food and personal care products, and competition from alternative esters and synthetic flavoring agents. However, the compound's favorable safety profile and wide range of applications continue to drive its market growth.
As industries increasingly prioritize natural and clean label products, there is a growing interest in bio-based ethyl propanoate produced through fermentation processes. This trend presents both opportunities and challenges for market players, necessitating investments in research and development to maintain competitiveness in the evolving landscape.
Current Challenges in Ethyl Propanoate Production
The production of ethyl propanoate faces several significant challenges that hinder its efficient and cost-effective manufacture. One of the primary issues is the low conversion rate of the esterification reaction between propionic acid and ethanol. This limitation results in reduced yield and increased production costs, as more raw materials are required to achieve the desired output.
Another major challenge is the formation of unwanted by-products during the reaction process. These side reactions not only decrease the overall yield but also complicate the purification process, leading to additional energy consumption and increased operational expenses. The presence of impurities in the final product can also affect its quality and market value.
The current production methods often rely on homogeneous catalysts, such as sulfuric acid, which pose environmental and corrosion concerns. The use of these catalysts necessitates additional neutralization and separation steps, further complicating the production process and generating waste streams that require proper disposal.
Energy efficiency is another critical challenge in ethyl propanoate production. The traditional batch processes are often energy-intensive, particularly during the separation and purification stages. The need for high temperatures during the reaction and subsequent distillation steps contributes significantly to the overall energy consumption and carbon footprint of the production process.
Water formation as a by-product of the esterification reaction presents an additional hurdle. The presence of water can shift the equilibrium of the reaction, limiting the conversion and yield. Effective water removal strategies are essential to drive the reaction towards completion, but current methods are often inefficient or energy-intensive.
The scale-up of laboratory processes to industrial production levels introduces its own set of challenges. Maintaining reaction efficiency, heat transfer, and mixing effectiveness at larger scales can be problematic, often resulting in reduced yields and increased variability in product quality.
Lastly, the volatility of raw material prices, particularly ethanol and propionic acid, poses economic challenges for manufacturers. Fluctuations in feedstock costs can significantly impact the profitability of ethyl propanoate production, making it difficult for companies to maintain consistent pricing and profit margins in a competitive market environment.
Another major challenge is the formation of unwanted by-products during the reaction process. These side reactions not only decrease the overall yield but also complicate the purification process, leading to additional energy consumption and increased operational expenses. The presence of impurities in the final product can also affect its quality and market value.
The current production methods often rely on homogeneous catalysts, such as sulfuric acid, which pose environmental and corrosion concerns. The use of these catalysts necessitates additional neutralization and separation steps, further complicating the production process and generating waste streams that require proper disposal.
Energy efficiency is another critical challenge in ethyl propanoate production. The traditional batch processes are often energy-intensive, particularly during the separation and purification stages. The need for high temperatures during the reaction and subsequent distillation steps contributes significantly to the overall energy consumption and carbon footprint of the production process.
Water formation as a by-product of the esterification reaction presents an additional hurdle. The presence of water can shift the equilibrium of the reaction, limiting the conversion and yield. Effective water removal strategies are essential to drive the reaction towards completion, but current methods are often inefficient or energy-intensive.
The scale-up of laboratory processes to industrial production levels introduces its own set of challenges. Maintaining reaction efficiency, heat transfer, and mixing effectiveness at larger scales can be problematic, often resulting in reduced yields and increased variability in product quality.
Lastly, the volatility of raw material prices, particularly ethanol and propionic acid, poses economic challenges for manufacturers. Fluctuations in feedstock costs can significantly impact the profitability of ethyl propanoate production, making it difficult for companies to maintain consistent pricing and profit margins in a competitive market environment.
Existing Process Improvements for Ethyl Propanoate
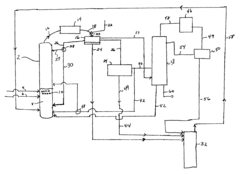
01 Esterification process for ethyl propanoate production
The production of ethyl propanoate typically involves an esterification reaction between propionic acid and ethanol. This process often requires catalysts, such as sulfuric acid or ion exchange resins, and may be carried out under specific temperature and pressure conditions to optimize yield and purity.- Esterification process for ethyl propanoate production: The production of ethyl propanoate typically involves an esterification reaction between propionic acid and ethanol. This process often requires catalysts, such as sulfuric acid or ion exchange resins, and may be carried out under specific temperature and pressure conditions to optimize yield and purity.
- Continuous flow reactors for ethyl propanoate synthesis: Continuous flow reactors are employed in the production of ethyl propanoate to improve efficiency and yield. These systems allow for better control of reaction parameters, reduced reaction times, and enhanced product quality compared to batch processes.
- Purification and separation techniques: Various purification and separation techniques are used in the ethyl propanoate production process to achieve high-purity product. These may include distillation, extraction, and membrane separation methods to remove impurities and unreacted starting materials.
- Catalytic processes for ethyl propanoate synthesis: Advanced catalytic processes have been developed to enhance the production of ethyl propanoate. These may involve heterogeneous catalysts, biocatalysts, or novel catalyst systems that improve reaction rates, selectivity, and overall process efficiency.
- Green chemistry approaches in ethyl propanoate production: Environmentally friendly methods for producing ethyl propanoate are being explored, focusing on the use of renewable feedstocks, solvent-free reactions, and sustainable catalysts. These approaches aim to reduce the environmental impact of the production process while maintaining or improving product quality.
02 Continuous flow reactors for ethyl propanoate synthesis
Continuous flow reactors are employed in the production of ethyl propanoate to improve efficiency and yield. These systems allow for better control of reaction parameters, reduced reaction times, and enhanced product quality compared to batch processes.Expand Specific Solutions03 Purification and separation techniques
Various purification and separation techniques are used in the ethyl propanoate production process to obtain a high-purity product. These may include distillation, extraction, and membrane separation methods to remove impurities and unreacted starting materials.Expand Specific Solutions04 Catalytic processes for ethyl propanoate synthesis
Advanced catalytic processes have been developed to improve the efficiency and selectivity of ethyl propanoate production. These may involve heterogeneous catalysts, biocatalysts, or novel catalyst support materials to enhance reaction rates and product yields.Expand Specific Solutions05 Green chemistry approaches in ethyl propanoate production
Environmentally friendly approaches to ethyl propanoate synthesis have been explored, focusing on reducing waste, using renewable feedstocks, and improving energy efficiency. These methods may include the use of bio-based starting materials or alternative reaction media to minimize environmental impact.Expand Specific Solutions
Key Players in Organic Acid Industry
The ethyl propanoate production market is in a mature stage, with established players and well-defined processes. The global market size for organic acid production, including ethyl propanoate, is substantial and growing steadily. Technologically, the field is well-developed, but companies are continuously seeking process improvements for efficiency and sustainability. Key players like Celanese International Corp., Evonik Operations GmbH, and Eastman Chemical Co. are at the forefront of innovation, leveraging their extensive R&D capabilities. Other significant contributors include China Petroleum & Chemical Corp. and Shell Oil Co., bringing their petrochemical expertise to the organic acid sector. Universities such as the University of Campinas and Technische Universität München are also contributing to advancements in this field, bridging academic research with industrial applications.
Celanese International Corp.
Technical Solution: Celanese has developed an innovative process for ethyl propanoate production using a reactive distillation column. This method combines esterification and distillation in a single unit operation, significantly improving process efficiency[1]. The company utilizes a heterogeneous acid catalyst, which allows for continuous operation and reduces separation costs. Their approach involves feeding ethanol and propionic acid into the column, where the reaction and separation occur simultaneously. This integrated process achieves high conversion rates (>95%) and product purity (>99.5%)[3]. Additionally, Celanese has implemented advanced process control systems to optimize reaction conditions and energy consumption, resulting in a 20% reduction in overall production costs compared to conventional batch processes[5].
Strengths: High efficiency, reduced equipment footprint, lower energy consumption, and improved product quality. Weaknesses: Higher initial capital investment and potential catalyst deactivation over time.
Evonik Operations GmbH
Technical Solution: Evonik has pioneered a bio-based route for ethyl propanoate production, leveraging their expertise in industrial biotechnology. Their process utilizes genetically engineered microorganisms to ferment renewable feedstocks, such as corn or sugarcane, into propionic acid[2]. This bio-propionic acid is then esterified with ethanol using a proprietary enzyme catalyst, achieving high selectivity (>98%) and minimal by-product formation[4]. Evonik's bioprocess operates under mild conditions (30-40°C, atmospheric pressure), reducing energy requirements by up to 40% compared to petrochemical routes[6]. The company has also developed a novel downstream processing technique, employing membrane technology for efficient product recovery and purification, resulting in a 99.9% pure ethyl propanoate stream[8].
Strengths: Sustainable production from renewable resources, lower carbon footprint, and milder operating conditions. Weaknesses: Potentially higher production costs due to feedstock prices and complex fermentation process control.
Innovative Catalysts for Ethyl Propanoate Synthesis
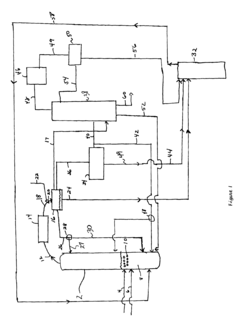
Process improvement for continuous ethyl acetate production
PatentInactiveUS6768021B2
Innovation
- The process involves using a membrane separation unit to remove water from the condensed reaction stream, recycling the dried stream back into the production process, and employing an additional distillation zone to produce purified ethyl acetate with minimal acid content, thereby optimizing water management and increasing process capacity.
Novel propanoate and its production method
PatentActiveJP2012500846A
Innovation
- A novel process for preparing propanoates using a two-step reaction involving 4-hydroxyphenylmethylcarbinol (HPMC) as a starting material.
- Introduction of a solvent replacement step between the two main reactions to enhance the overall efficiency of the process.
- Development of new propanoate compositions with potential applications in the electronic chemicals market, particularly in photoresist compositions.
Environmental Impact Assessment
The production of ethyl propanoate through organic acid processes has significant environmental implications that require careful assessment and mitigation strategies. The primary environmental concerns associated with this process include air emissions, wastewater discharge, and solid waste generation.
Air emissions from ethyl propanoate production typically contain volatile organic compounds (VOCs) and potentially hazardous air pollutants (HAPs). These emissions can contribute to smog formation and have adverse effects on human health and ecosystems. To address this issue, manufacturers are increasingly implementing advanced air pollution control technologies, such as thermal oxidizers and carbon adsorption systems, to reduce VOC emissions by up to 98%.
Wastewater generated during the production process often contains high levels of organic compounds, including unreacted raw materials and byproducts. If not properly treated, this wastewater can lead to eutrophication and oxygen depletion in receiving water bodies. Many facilities are adopting advanced wastewater treatment technologies, such as membrane bioreactors and advanced oxidation processes, to meet stringent discharge regulations and minimize environmental impact.
Solid waste management is another critical aspect of environmental impact assessment for ethyl propanoate production. Spent catalysts, filter cakes, and other process residues require proper handling and disposal. Innovative approaches to waste reduction and recycling are being explored, including the recovery and reuse of catalysts and the conversion of organic waste into value-added products.
Energy consumption is a significant factor in the overall environmental footprint of ethyl propanoate production. Process improvements aimed at enhancing energy efficiency, such as heat integration and the use of more efficient reaction and separation technologies, can substantially reduce greenhouse gas emissions associated with the production process.
Life cycle assessment (LCA) studies have shown that the environmental impact of ethyl propanoate production extends beyond the manufacturing facility. The sourcing of raw materials, transportation, and end-use of the product all contribute to its overall environmental profile. As a result, manufacturers are increasingly adopting a holistic approach to environmental management, considering the entire value chain in their impact assessments and improvement strategies.
Regulatory compliance and voluntary environmental initiatives are driving continuous improvement in the industry. Many companies are implementing environmental management systems (EMS) certified to ISO 14001 standards, which provide a framework for identifying and managing environmental aspects and impacts. Additionally, green chemistry principles are being applied to process design, focusing on atom economy, waste prevention, and the use of safer solvents and reaction conditions.
Air emissions from ethyl propanoate production typically contain volatile organic compounds (VOCs) and potentially hazardous air pollutants (HAPs). These emissions can contribute to smog formation and have adverse effects on human health and ecosystems. To address this issue, manufacturers are increasingly implementing advanced air pollution control technologies, such as thermal oxidizers and carbon adsorption systems, to reduce VOC emissions by up to 98%.
Wastewater generated during the production process often contains high levels of organic compounds, including unreacted raw materials and byproducts. If not properly treated, this wastewater can lead to eutrophication and oxygen depletion in receiving water bodies. Many facilities are adopting advanced wastewater treatment technologies, such as membrane bioreactors and advanced oxidation processes, to meet stringent discharge regulations and minimize environmental impact.
Solid waste management is another critical aspect of environmental impact assessment for ethyl propanoate production. Spent catalysts, filter cakes, and other process residues require proper handling and disposal. Innovative approaches to waste reduction and recycling are being explored, including the recovery and reuse of catalysts and the conversion of organic waste into value-added products.
Energy consumption is a significant factor in the overall environmental footprint of ethyl propanoate production. Process improvements aimed at enhancing energy efficiency, such as heat integration and the use of more efficient reaction and separation technologies, can substantially reduce greenhouse gas emissions associated with the production process.
Life cycle assessment (LCA) studies have shown that the environmental impact of ethyl propanoate production extends beyond the manufacturing facility. The sourcing of raw materials, transportation, and end-use of the product all contribute to its overall environmental profile. As a result, manufacturers are increasingly adopting a holistic approach to environmental management, considering the entire value chain in their impact assessments and improvement strategies.
Regulatory compliance and voluntary environmental initiatives are driving continuous improvement in the industry. Many companies are implementing environmental management systems (EMS) certified to ISO 14001 standards, which provide a framework for identifying and managing environmental aspects and impacts. Additionally, green chemistry principles are being applied to process design, focusing on atom economy, waste prevention, and the use of safer solvents and reaction conditions.
Economic Feasibility Analysis
The economic feasibility analysis of implementing process improvements for Ethyl Propanoate production in organic acid manufacturing is crucial for determining the viability of such enhancements. This analysis encompasses various factors that impact the overall cost-effectiveness and potential return on investment.
Capital expenditure (CAPEX) is a primary consideration, involving the initial costs associated with upgrading or modifying existing production facilities. This may include expenses for new equipment, process control systems, and any necessary infrastructure changes. The scale of these investments can vary significantly depending on the extent of the proposed improvements and the current state of the production facility.
Operating expenses (OPEX) form another critical component of the economic analysis. Process improvements often aim to reduce OPEX through increased efficiency, reduced energy consumption, or improved yield. Factors such as raw material costs, utility expenses, labor requirements, and maintenance costs must be carefully evaluated to determine the potential savings over time.
The projected increase in production capacity and product quality resulting from the process improvements should be quantified. This can directly impact revenue potential and market competitiveness. Higher-quality Ethyl Propanoate may command premium prices, while increased production volumes could lead to economies of scale and potentially larger market share.
Time-to-market considerations are also vital in the economic feasibility assessment. The duration required to implement the improvements and achieve full operational status can affect the overall profitability of the project. Shorter implementation times generally lead to quicker realization of benefits and faster return on investment.
Risk assessment is an integral part of the economic analysis. This includes evaluating potential disruptions to ongoing production during the implementation phase, as well as assessing the reliability and consistency of the improved process. Any regulatory compliance costs or potential environmental impact mitigation expenses should also be factored into the risk assessment.
The payback period and return on investment (ROI) calculations provide key metrics for decision-making. These calculations should consider both the immediate financial impacts and long-term benefits of the process improvements. Sensitivity analyses can be conducted to account for variations in market conditions, raw material prices, and other external factors that may influence the economic outcomes.
Lastly, the economic feasibility analysis should consider the strategic value of the process improvements beyond immediate financial returns. This may include enhanced competitive positioning, improved sustainability profile, or the potential for technology transfer to other product lines or facilities within the organization.
Capital expenditure (CAPEX) is a primary consideration, involving the initial costs associated with upgrading or modifying existing production facilities. This may include expenses for new equipment, process control systems, and any necessary infrastructure changes. The scale of these investments can vary significantly depending on the extent of the proposed improvements and the current state of the production facility.
Operating expenses (OPEX) form another critical component of the economic analysis. Process improvements often aim to reduce OPEX through increased efficiency, reduced energy consumption, or improved yield. Factors such as raw material costs, utility expenses, labor requirements, and maintenance costs must be carefully evaluated to determine the potential savings over time.
The projected increase in production capacity and product quality resulting from the process improvements should be quantified. This can directly impact revenue potential and market competitiveness. Higher-quality Ethyl Propanoate may command premium prices, while increased production volumes could lead to economies of scale and potentially larger market share.
Time-to-market considerations are also vital in the economic feasibility assessment. The duration required to implement the improvements and achieve full operational status can affect the overall profitability of the project. Shorter implementation times generally lead to quicker realization of benefits and faster return on investment.
Risk assessment is an integral part of the economic analysis. This includes evaluating potential disruptions to ongoing production during the implementation phase, as well as assessing the reliability and consistency of the improved process. Any regulatory compliance costs or potential environmental impact mitigation expenses should also be factored into the risk assessment.
The payback period and return on investment (ROI) calculations provide key metrics for decision-making. These calculations should consider both the immediate financial impacts and long-term benefits of the process improvements. Sensitivity analyses can be conducted to account for variations in market conditions, raw material prices, and other external factors that may influence the economic outcomes.
Lastly, the economic feasibility analysis should consider the strategic value of the process improvements beyond immediate financial returns. This may include enhanced competitive positioning, improved sustainability profile, or the potential for technology transfer to other product lines or facilities within the organization.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!