Exploring New Electrolyte Compounds for Electrolytic Cell Application
AUG 1, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Electrolyte Evolution
The evolution of electrolytes for electrolytic cell applications has been a journey of continuous innovation and refinement. Initially, simple aqueous solutions of acids, bases, or salts were used as electrolytes. These early electrolytes provided the necessary ionic conductivity but were limited in their electrochemical stability and operating temperature range.
As the demands for more efficient and versatile electrolytic cells grew, researchers began exploring non-aqueous electrolytes. The introduction of organic solvents in the mid-20th century marked a significant milestone. These solvents, such as propylene carbonate and dimethyl sulfoxide, allowed for a wider electrochemical window and improved the solubility of various salts.
The 1970s and 1980s saw the development of polymer electrolytes, which offered advantages in terms of mechanical stability and flexibility. Poly(ethylene oxide) (PEO) emerged as a prominent polymer host, capable of dissolving lithium salts and facilitating ion transport. This innovation paved the way for solid-state electrolytes and opened up new possibilities in battery technology.
In the 1990s, ionic liquids gained attention as potential electrolytes due to their unique properties, including negligible vapor pressure, high thermal stability, and wide electrochemical windows. These room-temperature molten salts offered new opportunities for electrochemical processes under extreme conditions.
The turn of the millennium brought increased focus on composite and hybrid electrolytes. By combining different materials, researchers aimed to synergize the benefits of various electrolyte types. For instance, ceramic-polymer composites sought to enhance the mechanical strength of polymer electrolytes while maintaining their flexibility.
Recent years have seen a surge in the development of advanced inorganic solid electrolytes, particularly for battery applications. Materials like LISICON (Lithium Super Ionic Conductor) and garnet-type structures have shown promise in terms of high ionic conductivity and stability against lithium metal anodes.
The ongoing evolution of electrolytes is now driven by the need for safer, more efficient, and environmentally friendly materials. Research is focusing on developing electrolytes with improved thermal stability, wider electrochemical windows, and enhanced compatibility with electrode materials. Additionally, there is growing interest in bio-derived and sustainable electrolyte compounds to address environmental concerns.
As we look to the future, the evolution of electrolytes is likely to continue along several paths. These include the development of multi-functional electrolytes that can serve additional roles beyond ion conduction, such as self-healing or smart responsive behaviors. The integration of nanotechnology in electrolyte design is also expected to yield novel materials with unprecedented properties and performance.
As the demands for more efficient and versatile electrolytic cells grew, researchers began exploring non-aqueous electrolytes. The introduction of organic solvents in the mid-20th century marked a significant milestone. These solvents, such as propylene carbonate and dimethyl sulfoxide, allowed for a wider electrochemical window and improved the solubility of various salts.
The 1970s and 1980s saw the development of polymer electrolytes, which offered advantages in terms of mechanical stability and flexibility. Poly(ethylene oxide) (PEO) emerged as a prominent polymer host, capable of dissolving lithium salts and facilitating ion transport. This innovation paved the way for solid-state electrolytes and opened up new possibilities in battery technology.
In the 1990s, ionic liquids gained attention as potential electrolytes due to their unique properties, including negligible vapor pressure, high thermal stability, and wide electrochemical windows. These room-temperature molten salts offered new opportunities for electrochemical processes under extreme conditions.
The turn of the millennium brought increased focus on composite and hybrid electrolytes. By combining different materials, researchers aimed to synergize the benefits of various electrolyte types. For instance, ceramic-polymer composites sought to enhance the mechanical strength of polymer electrolytes while maintaining their flexibility.
Recent years have seen a surge in the development of advanced inorganic solid electrolytes, particularly for battery applications. Materials like LISICON (Lithium Super Ionic Conductor) and garnet-type structures have shown promise in terms of high ionic conductivity and stability against lithium metal anodes.
The ongoing evolution of electrolytes is now driven by the need for safer, more efficient, and environmentally friendly materials. Research is focusing on developing electrolytes with improved thermal stability, wider electrochemical windows, and enhanced compatibility with electrode materials. Additionally, there is growing interest in bio-derived and sustainable electrolyte compounds to address environmental concerns.
As we look to the future, the evolution of electrolytes is likely to continue along several paths. These include the development of multi-functional electrolytes that can serve additional roles beyond ion conduction, such as self-healing or smart responsive behaviors. The integration of nanotechnology in electrolyte design is also expected to yield novel materials with unprecedented properties and performance.
Market Demand Analysis
The market demand for new electrolyte compounds in electrolytic cell applications has been steadily growing, driven by the increasing need for more efficient and sustainable energy storage solutions. This demand is particularly evident in sectors such as renewable energy, electric vehicles, and portable electronics, where advanced battery technologies are crucial for improved performance and longevity.
In the renewable energy sector, the push for grid-scale energy storage systems has created a significant market opportunity for innovative electrolyte compounds. These compounds are essential for developing high-capacity, long-duration storage solutions that can effectively manage the intermittent nature of renewable energy sources like solar and wind power. The global energy storage market is projected to expand rapidly, with electrolyte innovations playing a key role in this growth.
The electric vehicle (EV) industry represents another major driver for electrolyte compound development. As automakers worldwide accelerate their transition to electric powertrains, there is an urgent need for batteries with higher energy density, faster charging capabilities, and improved safety profiles. New electrolyte formulations are critical in addressing these challenges, potentially enabling EVs with longer ranges and shorter charging times.
In the consumer electronics market, the demand for longer-lasting, faster-charging devices continues to fuel research into advanced electrolyte compounds. Smartphones, laptops, and wearable devices all stand to benefit from improvements in battery technology, creating a substantial market for innovative electrolyte solutions that can enhance both performance and user experience.
The industrial sector also presents significant opportunities for new electrolyte compounds, particularly in applications such as uninterruptible power supplies, backup power systems, and large-scale industrial equipment. These applications require robust, reliable energy storage solutions that can withstand harsh operating conditions and provide consistent performance over extended periods.
Furthermore, the growing emphasis on sustainability and environmental protection has led to increased demand for electrolyte compounds that are more environmentally friendly and recyclable. This trend aligns with global efforts to reduce the carbon footprint of energy storage technologies and promote circular economy principles in battery production and disposal.
Market analysts predict that the global electrolyte market for batteries will experience substantial growth in the coming years, with particular emphasis on advanced liquid electrolytes and solid-state electrolytes. This growth is expected to be driven by ongoing research and development efforts, as well as increasing investments in battery technology across various industries.
In the renewable energy sector, the push for grid-scale energy storage systems has created a significant market opportunity for innovative electrolyte compounds. These compounds are essential for developing high-capacity, long-duration storage solutions that can effectively manage the intermittent nature of renewable energy sources like solar and wind power. The global energy storage market is projected to expand rapidly, with electrolyte innovations playing a key role in this growth.
The electric vehicle (EV) industry represents another major driver for electrolyte compound development. As automakers worldwide accelerate their transition to electric powertrains, there is an urgent need for batteries with higher energy density, faster charging capabilities, and improved safety profiles. New electrolyte formulations are critical in addressing these challenges, potentially enabling EVs with longer ranges and shorter charging times.
In the consumer electronics market, the demand for longer-lasting, faster-charging devices continues to fuel research into advanced electrolyte compounds. Smartphones, laptops, and wearable devices all stand to benefit from improvements in battery technology, creating a substantial market for innovative electrolyte solutions that can enhance both performance and user experience.
The industrial sector also presents significant opportunities for new electrolyte compounds, particularly in applications such as uninterruptible power supplies, backup power systems, and large-scale industrial equipment. These applications require robust, reliable energy storage solutions that can withstand harsh operating conditions and provide consistent performance over extended periods.
Furthermore, the growing emphasis on sustainability and environmental protection has led to increased demand for electrolyte compounds that are more environmentally friendly and recyclable. This trend aligns with global efforts to reduce the carbon footprint of energy storage technologies and promote circular economy principles in battery production and disposal.
Market analysts predict that the global electrolyte market for batteries will experience substantial growth in the coming years, with particular emphasis on advanced liquid electrolytes and solid-state electrolytes. This growth is expected to be driven by ongoing research and development efforts, as well as increasing investments in battery technology across various industries.
Current Challenges
The development of new electrolyte compounds for electrolytic cell applications faces several significant challenges. One of the primary obstacles is achieving a balance between ionic conductivity and electrochemical stability. While high ionic conductivity is crucial for efficient cell operation, it often comes at the cost of reduced electrochemical stability, leading to degradation and shortened cell lifespan.
Another major challenge lies in the compatibility of new electrolyte compounds with existing electrode materials. Many promising electrolytes exhibit excellent conductivity but react unfavorably with common electrode materials, causing capacity fade and safety issues. This necessitates a holistic approach to electrolyte development, considering the entire cell system rather than the electrolyte in isolation.
The environmental impact and safety concerns of new electrolyte compounds pose additional hurdles. Many high-performance electrolytes contain toxic or flammable components, raising concerns about large-scale production, handling, and disposal. Developing environmentally friendly alternatives that maintain performance standards remains a significant challenge for researchers and industry professionals.
Cost-effectiveness is another critical factor impeding the widespread adoption of new electrolyte compounds. Many advanced electrolytes require expensive precursors or complex synthesis processes, making them economically unfeasible for large-scale commercial applications. Striking a balance between performance and cost-effectiveness is crucial for market viability.
The scalability of new electrolyte production processes presents yet another challenge. Laboratory-scale synthesis methods often prove difficult to translate into industrial-scale production, leading to inconsistencies in quality and performance. Developing robust, scalable manufacturing processes for new electrolyte compounds is essential for their successful commercialization.
Lastly, the long-term stability and cycling performance of new electrolytes under various operating conditions remain areas of concern. Many promising compounds show initial high performance but suffer from rapid degradation or loss of efficiency over extended use. Understanding and mitigating these degradation mechanisms is crucial for developing electrolytes suitable for long-life applications.
Addressing these challenges requires interdisciplinary collaboration between chemists, materials scientists, and engineers. It also necessitates advanced characterization techniques and computational modeling to predict and optimize electrolyte performance. As research progresses, overcoming these hurdles will be key to unlocking the full potential of new electrolyte compounds in electrolytic cell applications.
Another major challenge lies in the compatibility of new electrolyte compounds with existing electrode materials. Many promising electrolytes exhibit excellent conductivity but react unfavorably with common electrode materials, causing capacity fade and safety issues. This necessitates a holistic approach to electrolyte development, considering the entire cell system rather than the electrolyte in isolation.
The environmental impact and safety concerns of new electrolyte compounds pose additional hurdles. Many high-performance electrolytes contain toxic or flammable components, raising concerns about large-scale production, handling, and disposal. Developing environmentally friendly alternatives that maintain performance standards remains a significant challenge for researchers and industry professionals.
Cost-effectiveness is another critical factor impeding the widespread adoption of new electrolyte compounds. Many advanced electrolytes require expensive precursors or complex synthesis processes, making them economically unfeasible for large-scale commercial applications. Striking a balance between performance and cost-effectiveness is crucial for market viability.
The scalability of new electrolyte production processes presents yet another challenge. Laboratory-scale synthesis methods often prove difficult to translate into industrial-scale production, leading to inconsistencies in quality and performance. Developing robust, scalable manufacturing processes for new electrolyte compounds is essential for their successful commercialization.
Lastly, the long-term stability and cycling performance of new electrolytes under various operating conditions remain areas of concern. Many promising compounds show initial high performance but suffer from rapid degradation or loss of efficiency over extended use. Understanding and mitigating these degradation mechanisms is crucial for developing electrolytes suitable for long-life applications.
Addressing these challenges requires interdisciplinary collaboration between chemists, materials scientists, and engineers. It also necessitates advanced characterization techniques and computational modeling to predict and optimize electrolyte performance. As research progresses, overcoming these hurdles will be key to unlocking the full potential of new electrolyte compounds in electrolytic cell applications.
Existing Compounds
01 Lithium-ion battery electrolyte compositions
Advanced electrolyte compositions for lithium-ion batteries, focusing on improving battery performance, safety, and longevity. These compositions may include novel solvents, additives, or salt combinations designed to enhance ionic conductivity, form stable solid electrolyte interphase (SEI) layers, and mitigate unwanted side reactions.- Lithium-based electrolyte compounds: Lithium-based electrolyte compounds are widely used in battery technologies, particularly in lithium-ion batteries. These compounds typically include lithium salts dissolved in organic solvents, providing ionic conductivity necessary for battery operation. Researchers focus on developing new lithium-based electrolytes to improve battery performance, safety, and longevity.
- Polymer electrolytes: Polymer electrolytes are solid or gel-like materials that conduct ions. They are being developed as alternatives to liquid electrolytes in batteries and other electrochemical devices. These materials can enhance safety by reducing the risk of leakage and improving the mechanical stability of the electrolyte system. Research in this area focuses on improving ionic conductivity and electrochemical stability.
- Electrolyte additives for performance enhancement: Various additives are incorporated into electrolyte formulations to enhance performance characteristics such as ionic conductivity, electrochemical stability, and interfacial properties. These additives can include organic compounds, inorganic materials, or hybrid substances that modify the electrolyte's properties or interact with electrode surfaces to improve overall device performance.
- Ionic liquid electrolytes: Ionic liquids are being explored as alternative electrolytes due to their unique properties such as low volatility, high thermal stability, and wide electrochemical window. These materials can be tailored for specific applications by modifying their cation and anion structures. Research in this area aims to overcome challenges related to viscosity and cost while leveraging the safety advantages of ionic liquids.
- Electrolyte optimization for specific battery chemistries: Researchers are developing specialized electrolyte formulations optimized for specific battery chemistries, such as lithium-sulfur, sodium-ion, or solid-state batteries. These tailored electrolytes address unique challenges associated with each battery type, including issues like polysulfide shuttling in lithium-sulfur batteries or interfacial stability in solid-state systems.
02 Solid-state electrolytes for batteries
Development of solid-state electrolytes as alternatives to liquid electrolytes in batteries. These materials aim to improve safety, energy density, and thermal stability of batteries. Research focuses on ceramic, polymer, and composite electrolytes with high ionic conductivity and mechanical strength.Expand Specific Solutions03 Electrolyte additives for performance enhancement
Incorporation of specific additives into electrolyte formulations to enhance various aspects of battery performance. These additives may improve cycling stability, rate capability, low-temperature performance, or mitigate capacity fading. The focus is on identifying synergistic combinations of additives for optimal results.Expand Specific Solutions04 Electrolytes for next-generation battery technologies
Exploration of novel electrolyte systems for emerging battery technologies such as lithium-sulfur, sodium-ion, or metal-air batteries. These electrolytes are tailored to address specific challenges associated with each battery chemistry, including polysulfide shuttling, dendrite formation, or air electrode stability.Expand Specific Solutions05 Electrolyte optimization for fast-charging applications
Development of electrolyte formulations specifically designed to enable fast charging of batteries without compromising safety or long-term performance. This includes strategies to mitigate lithium plating, reduce heat generation, and maintain stable interfaces during high-rate charging processes.Expand Specific Solutions
Key Industry Players
The exploration of new electrolyte compounds for electrolytic cell applications is currently in a dynamic phase of development, with significant market potential and ongoing technological advancements. The market size is expanding rapidly due to increasing demand for high-performance batteries in various sectors. While the technology is maturing, there is still room for innovation. Key players like Solvay SA, BASF Corp., and DuPont de Nemours, Inc. are leading research efforts, with emerging companies like Sion Power Corp. bringing fresh approaches. Universities such as Fudan University and Deakin University are contributing valuable research, indicating a collaborative ecosystem between industry and academia in advancing this technology.
Solvay SA
Technical Solution: Solvay has been actively developing advanced electrolyte solutions for various electrochemical applications. Their research focuses on high-performance fluorinated and sulfone-based electrolytes that offer improved thermal stability and wider electrochemical windows[13]. Solvay has also developed novel lithium salts, such as lithium bis(fluorosulfonyl)imide (LiFSI), which demonstrate enhanced conductivity and stability compared to traditional salts[14]. Their electrolyte formulations often incorporate additives that form stable solid electrolyte interphase (SEI) layers, improving the long-term performance and safety of electrochemical devices[15]. Solvay's approach combines their expertise in specialty chemicals with collaborative research to address specific challenges in electrolyte technology.
Strengths: Extensive chemical expertise, global research network, and strong position in specialty materials. Weaknesses: Potential for higher production costs of specialized electrolyte compounds.
BASF Corp.
Technical Solution: BASF has developed advanced electrolyte compounds for lithium-ion batteries, focusing on high-voltage electrolytes and additives. Their approach includes using fluorinated organic solvents and lithium salts to improve the electrochemical stability window of the electrolyte[1]. They have also introduced novel electrolyte formulations containing sulfone-based solvents, which demonstrate enhanced thermal stability and improved cycling performance at elevated temperatures[2]. BASF's research extends to solid electrolyte interphase (SEI) forming additives that create a more stable interface between the electrode and electrolyte, leading to improved battery life and safety[3].
Strengths: Extensive R&D capabilities, wide range of chemical expertise, and global presence. Weaknesses: High development costs and potential regulatory challenges for new chemical compounds.
Innovative Formulations
Electrolyte composition for electrochemical cell for high-temperature applications
PatentWO2019149812A1
Innovation
- An electrolyte composition for electrochemical cells, comprising a mixture of organic solvents like nitriles and lithium salts, with a boiling point above 85°C, ensuring stability at high temperatures and voltages, and optionally including cyclic carbonates for enhanced performance.
New components for electrolyte compositions
PatentWO2020025501A1
Innovation
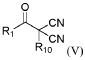
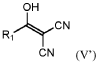
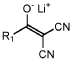
- Development of new chemical components, including specific solvents, additives, and electrolyte salts, such as compounds of formula (V) and their tautomers, salts, and solvates, which can be used in electrolyte compositions to enhance the quality of the solid electrolyte interphase (SEI), chemical stability, ionic conductivity, and thermal stability.
Environmental Impact
The environmental impact of exploring new electrolyte compounds for electrolytic cell applications is a critical consideration in the development and implementation of this technology. As the demand for more efficient and sustainable energy storage solutions grows, the potential environmental consequences of new electrolyte compounds must be carefully evaluated.
One of the primary environmental concerns associated with electrolyte compounds is their potential toxicity and persistence in the environment. Many traditional electrolytes contain harmful substances that can pose risks to ecosystems and human health if released. New electrolyte compounds must be designed with biodegradability and low toxicity in mind to minimize their environmental footprint. This includes considering the entire lifecycle of the compounds, from production to disposal.
The production process of new electrolyte compounds also warrants attention from an environmental perspective. Researchers and manufacturers must strive to develop synthesis methods that reduce energy consumption, minimize waste generation, and utilize renewable or recycled materials where possible. Green chemistry principles should be applied to ensure that the production of these compounds aligns with sustainable practices.
Water consumption and contamination are additional environmental factors to consider. Electrolytic cells often require significant amounts of water for operation and cooling. New electrolyte compounds should be designed to minimize water usage and prevent water pollution. This may involve developing closed-loop systems or exploring alternative cooling methods to reduce the overall water footprint of electrolytic cell applications.
The potential for recycling and reuse of electrolyte compounds is another crucial aspect of their environmental impact. Developing compounds that can be easily recovered and reprocessed at the end of their lifecycle can significantly reduce waste and the need for raw materials. This circular economy approach not only minimizes environmental harm but also contributes to the long-term sustainability of electrolytic cell technology.
Energy efficiency is a key factor in reducing the environmental impact of electrolytic cells. New electrolyte compounds that enable higher efficiency and lower energy consumption can indirectly contribute to reduced greenhouse gas emissions and overall environmental footprint. This is particularly important in applications such as energy storage for renewable power sources, where improved efficiency can enhance the sustainability of the entire energy system.
Lastly, the potential for unintended environmental consequences must be thoroughly investigated. This includes assessing the compounds' behavior under various environmental conditions, their potential interactions with other materials, and any long-term effects on ecosystems. Comprehensive environmental impact assessments and life cycle analyses should be conducted to identify and mitigate any unforeseen risks associated with new electrolyte compounds.
One of the primary environmental concerns associated with electrolyte compounds is their potential toxicity and persistence in the environment. Many traditional electrolytes contain harmful substances that can pose risks to ecosystems and human health if released. New electrolyte compounds must be designed with biodegradability and low toxicity in mind to minimize their environmental footprint. This includes considering the entire lifecycle of the compounds, from production to disposal.
The production process of new electrolyte compounds also warrants attention from an environmental perspective. Researchers and manufacturers must strive to develop synthesis methods that reduce energy consumption, minimize waste generation, and utilize renewable or recycled materials where possible. Green chemistry principles should be applied to ensure that the production of these compounds aligns with sustainable practices.
Water consumption and contamination are additional environmental factors to consider. Electrolytic cells often require significant amounts of water for operation and cooling. New electrolyte compounds should be designed to minimize water usage and prevent water pollution. This may involve developing closed-loop systems or exploring alternative cooling methods to reduce the overall water footprint of electrolytic cell applications.
The potential for recycling and reuse of electrolyte compounds is another crucial aspect of their environmental impact. Developing compounds that can be easily recovered and reprocessed at the end of their lifecycle can significantly reduce waste and the need for raw materials. This circular economy approach not only minimizes environmental harm but also contributes to the long-term sustainability of electrolytic cell technology.
Energy efficiency is a key factor in reducing the environmental impact of electrolytic cells. New electrolyte compounds that enable higher efficiency and lower energy consumption can indirectly contribute to reduced greenhouse gas emissions and overall environmental footprint. This is particularly important in applications such as energy storage for renewable power sources, where improved efficiency can enhance the sustainability of the entire energy system.
Lastly, the potential for unintended environmental consequences must be thoroughly investigated. This includes assessing the compounds' behavior under various environmental conditions, their potential interactions with other materials, and any long-term effects on ecosystems. Comprehensive environmental impact assessments and life cycle analyses should be conducted to identify and mitigate any unforeseen risks associated with new electrolyte compounds.
Safety Regulations
Safety regulations play a crucial role in the development and implementation of new electrolyte compounds for electrolytic cell applications. These regulations are designed to protect workers, the environment, and the general public from potential hazards associated with the use of these materials.
The primary focus of safety regulations in this field is on the handling, storage, and disposal of electrolyte compounds. Many of these substances are corrosive, toxic, or flammable, necessitating strict protocols for their management. Regulatory bodies such as the Occupational Safety and Health Administration (OSHA) in the United States and the European Chemicals Agency (ECHA) in the European Union have established comprehensive guidelines for the safe use of these materials.
One key aspect of safety regulations is the requirement for proper labeling and documentation. Manufacturers and users of new electrolyte compounds must provide detailed safety data sheets (SDS) that outline the physical and chemical properties of the substance, potential hazards, and appropriate handling procedures. This information is essential for ensuring that workers are adequately informed and protected when working with these materials.
Personal protective equipment (PPE) requirements are another critical component of safety regulations. Depending on the specific electrolyte compound, workers may need to use specialized gloves, goggles, face shields, or full-body protective suits. Ventilation systems and containment measures are also often mandated to minimize exposure risks and prevent the release of harmful vapors or spills.
Emergency response procedures form an integral part of safety regulations for electrolyte compounds. Facilities working with these materials must have well-defined protocols for addressing spills, fires, or other incidents. This includes the provision of appropriate fire suppression systems, eyewash stations, and emergency showers.
Environmental protection is a growing concern in safety regulations for electrolyte compounds. Many jurisdictions now require comprehensive waste management plans to ensure proper disposal or recycling of used electrolytes and contaminated materials. This may involve specialized treatment processes or the use of licensed hazardous waste disposal facilities.
As research into new electrolyte compounds progresses, safety regulations are continually evolving to address emerging risks and challenges. Regulatory bodies are increasingly focusing on the long-term environmental and health impacts of these materials, leading to more stringent requirements for toxicity testing and lifecycle assessments.
Compliance with safety regulations is not only a legal requirement but also a critical factor in the commercial viability of new electrolyte compounds. Companies developing these materials must consider regulatory compliance from the earliest stages of research and development to ensure that their products can be safely and legally used in electrolytic cell applications.
The primary focus of safety regulations in this field is on the handling, storage, and disposal of electrolyte compounds. Many of these substances are corrosive, toxic, or flammable, necessitating strict protocols for their management. Regulatory bodies such as the Occupational Safety and Health Administration (OSHA) in the United States and the European Chemicals Agency (ECHA) in the European Union have established comprehensive guidelines for the safe use of these materials.
One key aspect of safety regulations is the requirement for proper labeling and documentation. Manufacturers and users of new electrolyte compounds must provide detailed safety data sheets (SDS) that outline the physical and chemical properties of the substance, potential hazards, and appropriate handling procedures. This information is essential for ensuring that workers are adequately informed and protected when working with these materials.
Personal protective equipment (PPE) requirements are another critical component of safety regulations. Depending on the specific electrolyte compound, workers may need to use specialized gloves, goggles, face shields, or full-body protective suits. Ventilation systems and containment measures are also often mandated to minimize exposure risks and prevent the release of harmful vapors or spills.
Emergency response procedures form an integral part of safety regulations for electrolyte compounds. Facilities working with these materials must have well-defined protocols for addressing spills, fires, or other incidents. This includes the provision of appropriate fire suppression systems, eyewash stations, and emergency showers.
Environmental protection is a growing concern in safety regulations for electrolyte compounds. Many jurisdictions now require comprehensive waste management plans to ensure proper disposal or recycling of used electrolytes and contaminated materials. This may involve specialized treatment processes or the use of licensed hazardous waste disposal facilities.
As research into new electrolyte compounds progresses, safety regulations are continually evolving to address emerging risks and challenges. Regulatory bodies are increasingly focusing on the long-term environmental and health impacts of these materials, leading to more stringent requirements for toxicity testing and lifecycle assessments.
Compliance with safety regulations is not only a legal requirement but also a critical factor in the commercial viability of new electrolyte compounds. Companies developing these materials must consider regulatory compliance from the earliest stages of research and development to ensure that their products can be safely and legally used in electrolytic cell applications.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!