FTIR vs Atomic Absorption Spectroscopy: Metals Detection
SEP 22, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Metal Detection Technology Background and Objectives
Metal detection technologies have evolved significantly over the past century, with spectroscopic methods becoming increasingly important for precise elemental analysis. The development trajectory began with rudimentary chemical tests in the early 20th century, progressing through flame tests to more sophisticated instrumental techniques by mid-century. The emergence of atomic absorption spectroscopy (AAS) in the 1950s marked a revolutionary advancement in metal detection capabilities, offering unprecedented sensitivity for trace metal analysis.
Fourier Transform Infrared Spectroscopy (FTIR), while initially developed for organic compound analysis, has increasingly found applications in metal detection through various specialized techniques. The convergence of these two methodologies represents a fascinating intersection of analytical approaches with complementary strengths and limitations for metal detection applications.
The technological evolution in this field has been driven by increasing regulatory requirements across industries including environmental monitoring, food safety, pharmaceutical manufacturing, and materials science. Particularly stringent regulations regarding heavy metal contamination have accelerated innovation in detection methodologies, pushing toward lower detection limits and higher specificity.
Current technological objectives in metal detection focus on several key areas: improving detection sensitivity to parts-per-trillion levels, enhancing specificity for complex sample matrices, developing portable and field-deployable instrumentation, reducing analysis time, and minimizing sample preparation requirements. Additionally, there is growing emphasis on developing "green" analytical methods that reduce or eliminate hazardous reagents traditionally used in metal analysis procedures.
The comparative analysis of FTIR versus AAS represents an important evaluation of complementary approaches to metal detection. While AAS has traditionally been the gold standard for quantitative metal analysis due to its excellent sensitivity and element-specific detection capabilities, FTIR offers advantages in terms of sample throughput, minimal sample preparation, and the ability to analyze multiple components simultaneously.
The integration of computational methods, including machine learning algorithms for spectral interpretation, is emerging as a significant trend that may bridge some of the traditional limitations of both technologies. These computational approaches enable more sophisticated data analysis, potentially allowing for improved detection limits and better discrimination between similar metal species in complex matrices.
As industries continue to demand more precise, rapid, and cost-effective metal detection capabilities, understanding the relative strengths and limitations of FTIR and AAS becomes increasingly important for developing optimal analytical strategies tailored to specific application requirements.
Fourier Transform Infrared Spectroscopy (FTIR), while initially developed for organic compound analysis, has increasingly found applications in metal detection through various specialized techniques. The convergence of these two methodologies represents a fascinating intersection of analytical approaches with complementary strengths and limitations for metal detection applications.
The technological evolution in this field has been driven by increasing regulatory requirements across industries including environmental monitoring, food safety, pharmaceutical manufacturing, and materials science. Particularly stringent regulations regarding heavy metal contamination have accelerated innovation in detection methodologies, pushing toward lower detection limits and higher specificity.
Current technological objectives in metal detection focus on several key areas: improving detection sensitivity to parts-per-trillion levels, enhancing specificity for complex sample matrices, developing portable and field-deployable instrumentation, reducing analysis time, and minimizing sample preparation requirements. Additionally, there is growing emphasis on developing "green" analytical methods that reduce or eliminate hazardous reagents traditionally used in metal analysis procedures.
The comparative analysis of FTIR versus AAS represents an important evaluation of complementary approaches to metal detection. While AAS has traditionally been the gold standard for quantitative metal analysis due to its excellent sensitivity and element-specific detection capabilities, FTIR offers advantages in terms of sample throughput, minimal sample preparation, and the ability to analyze multiple components simultaneously.
The integration of computational methods, including machine learning algorithms for spectral interpretation, is emerging as a significant trend that may bridge some of the traditional limitations of both technologies. These computational approaches enable more sophisticated data analysis, potentially allowing for improved detection limits and better discrimination between similar metal species in complex matrices.
As industries continue to demand more precise, rapid, and cost-effective metal detection capabilities, understanding the relative strengths and limitations of FTIR and AAS becomes increasingly important for developing optimal analytical strategies tailored to specific application requirements.
Market Applications and Demand Analysis for Spectroscopic Methods
The global market for spectroscopic methods in metal detection has experienced significant growth over the past decade, driven by increasing regulatory requirements for metal analysis in environmental monitoring, food safety, pharmaceutical quality control, and industrial applications. FTIR (Fourier Transform Infrared Spectroscopy) and Atomic Absorption Spectroscopy (AAS) represent two distinct technological approaches that serve different segments of this expanding market.
The environmental testing sector constitutes one of the largest application areas, with a strong demand for metal detection technologies to monitor water, soil, and air quality. Government regulations worldwide have become increasingly stringent regarding permissible levels of heavy metals in the environment, creating sustained demand for sensitive analytical techniques. AAS has traditionally dominated this sector due to its high sensitivity for detecting trace metals at parts-per-billion levels.
In the pharmaceutical industry, metal contamination detection represents a critical quality control parameter. Both FTIR and AAS find applications here, with FTIR being particularly valuable for identifying organic-metal complexes and AAS excelling at quantifying elemental metal content. The pharmaceutical metal testing market has been growing steadily as regulations around elemental impurities have tightened globally.
The food and beverage industry represents another significant market driver, with increasing consumer awareness regarding food safety creating demand for comprehensive metal detection solutions. AAS has established itself as the preferred technology for detecting toxic metals like lead, mercury, and arsenic in food products, while FTIR finds applications in identifying metal-containing additives and contaminants.
Industrial applications, particularly in mining, metallurgy, and manufacturing, constitute a substantial market segment where both technologies serve complementary roles. FTIR provides valuable information about metal-containing compounds and their molecular structures, while AAS delivers precise elemental analysis. The industrial sector's demand is primarily driven by quality control requirements and process optimization needs.
Geographically, North America and Europe represent the largest markets for advanced spectroscopic metal detection technologies, primarily due to stringent regulatory frameworks and well-established industrial bases. However, the Asia-Pacific region is experiencing the fastest growth rate, driven by rapid industrialization, increasing environmental concerns, and strengthening regulatory oversight in countries like China and India.
The combined market for spectroscopic metal detection technologies is projected to continue its growth trajectory, with particular expansion in portable and field-deployable systems that enable on-site analysis. This trend reflects the increasing need for real-time monitoring and rapid decision-making across various industries dealing with potential metal contamination issues.
The environmental testing sector constitutes one of the largest application areas, with a strong demand for metal detection technologies to monitor water, soil, and air quality. Government regulations worldwide have become increasingly stringent regarding permissible levels of heavy metals in the environment, creating sustained demand for sensitive analytical techniques. AAS has traditionally dominated this sector due to its high sensitivity for detecting trace metals at parts-per-billion levels.
In the pharmaceutical industry, metal contamination detection represents a critical quality control parameter. Both FTIR and AAS find applications here, with FTIR being particularly valuable for identifying organic-metal complexes and AAS excelling at quantifying elemental metal content. The pharmaceutical metal testing market has been growing steadily as regulations around elemental impurities have tightened globally.
The food and beverage industry represents another significant market driver, with increasing consumer awareness regarding food safety creating demand for comprehensive metal detection solutions. AAS has established itself as the preferred technology for detecting toxic metals like lead, mercury, and arsenic in food products, while FTIR finds applications in identifying metal-containing additives and contaminants.
Industrial applications, particularly in mining, metallurgy, and manufacturing, constitute a substantial market segment where both technologies serve complementary roles. FTIR provides valuable information about metal-containing compounds and their molecular structures, while AAS delivers precise elemental analysis. The industrial sector's demand is primarily driven by quality control requirements and process optimization needs.
Geographically, North America and Europe represent the largest markets for advanced spectroscopic metal detection technologies, primarily due to stringent regulatory frameworks and well-established industrial bases. However, the Asia-Pacific region is experiencing the fastest growth rate, driven by rapid industrialization, increasing environmental concerns, and strengthening regulatory oversight in countries like China and India.
The combined market for spectroscopic metal detection technologies is projected to continue its growth trajectory, with particular expansion in portable and field-deployable systems that enable on-site analysis. This trend reflects the increasing need for real-time monitoring and rapid decision-making across various industries dealing with potential metal contamination issues.
FTIR and AAS Current Capabilities and Technical Limitations
Fourier Transform Infrared Spectroscopy (FTIR) and Atomic Absorption Spectroscopy (AAS) represent two distinct analytical methodologies with varying capabilities for metal detection. FTIR operates by measuring the absorption of infrared radiation across a spectrum of wavelengths, identifying molecular structures based on their vibrational modes. While FTIR excels in organic compound analysis, its application in direct metal detection faces significant limitations due to metals' minimal infrared activity in their elemental state.
FTIR's current capabilities for metal detection primarily revolve around identifying metal-containing compounds rather than elemental metals. The technique can detect metal-organic frameworks, metal oxides, and metal complexes by identifying characteristic absorption bands associated with metal-ligand bonds. Modern FTIR systems offer resolution down to 0.5 cm⁻¹ and can detect compounds at concentrations as low as parts per million in optimal conditions.
Technical limitations of FTIR for metal detection include poor sensitivity for elemental metals, interference from water and atmospheric CO₂, and difficulty in quantitative analysis without extensive calibration. Additionally, sample preparation requirements can be complex, and interpretation of spectra requires significant expertise, particularly in mixed samples where overlapping bands may obscure metal-related signals.
In contrast, Atomic Absorption Spectroscopy was specifically designed for elemental metal analysis. AAS measures the absorption of optical radiation by free atoms in the gaseous state, providing highly specific detection of individual metal elements. Modern AAS systems can detect metals at concentrations as low as parts per billion, offering superior sensitivity compared to FTIR for elemental analysis.
AAS technical capabilities include excellent specificity for individual metals, high sensitivity, and well-established quantitative analysis protocols. The technique allows for the analysis of over 70 different metallic elements across various sample matrices with minimal spectral interference between elements.
However, AAS also presents several technical limitations. The technique typically analyzes only one element at a time, requiring sequential analysis for multi-element samples. Sample preparation often involves destructive digestion procedures, and the equipment requires element-specific hollow cathode lamps. AAS also faces challenges with refractory elements that form stable compounds resistant to atomization.
The detection limits vary significantly between the two techniques, with AAS generally providing 2-3 orders of magnitude better sensitivity for elemental metals. While FTIR offers simultaneous multi-component analysis capabilities, its application for direct metal detection remains limited to specific metal-containing molecular structures rather than elemental composition determination.
FTIR's current capabilities for metal detection primarily revolve around identifying metal-containing compounds rather than elemental metals. The technique can detect metal-organic frameworks, metal oxides, and metal complexes by identifying characteristic absorption bands associated with metal-ligand bonds. Modern FTIR systems offer resolution down to 0.5 cm⁻¹ and can detect compounds at concentrations as low as parts per million in optimal conditions.
Technical limitations of FTIR for metal detection include poor sensitivity for elemental metals, interference from water and atmospheric CO₂, and difficulty in quantitative analysis without extensive calibration. Additionally, sample preparation requirements can be complex, and interpretation of spectra requires significant expertise, particularly in mixed samples where overlapping bands may obscure metal-related signals.
In contrast, Atomic Absorption Spectroscopy was specifically designed for elemental metal analysis. AAS measures the absorption of optical radiation by free atoms in the gaseous state, providing highly specific detection of individual metal elements. Modern AAS systems can detect metals at concentrations as low as parts per billion, offering superior sensitivity compared to FTIR for elemental analysis.
AAS technical capabilities include excellent specificity for individual metals, high sensitivity, and well-established quantitative analysis protocols. The technique allows for the analysis of over 70 different metallic elements across various sample matrices with minimal spectral interference between elements.
However, AAS also presents several technical limitations. The technique typically analyzes only one element at a time, requiring sequential analysis for multi-element samples. Sample preparation often involves destructive digestion procedures, and the equipment requires element-specific hollow cathode lamps. AAS also faces challenges with refractory elements that form stable compounds resistant to atomization.
The detection limits vary significantly between the two techniques, with AAS generally providing 2-3 orders of magnitude better sensitivity for elemental metals. While FTIR offers simultaneous multi-component analysis capabilities, its application for direct metal detection remains limited to specific metal-containing molecular structures rather than elemental composition determination.
Comparative Analysis of FTIR and AAS Methodologies
01 FTIR spectroscopy techniques for metal detection
Fourier Transform Infrared (FTIR) spectroscopy can be used for detecting metals in various samples. This technique measures the absorption of infrared radiation by the sample and identifies the molecular composition based on the vibrational characteristics of chemical bonds. FTIR spectroscopy can be particularly useful for identifying metal complexes, metal-organic frameworks, and metal oxides in different matrices. The technique offers advantages such as rapid analysis, minimal sample preparation, and the ability to analyze both solid and liquid samples.- FTIR spectroscopy techniques for metal detection: Fourier Transform Infrared (FTIR) spectroscopy can be used for detecting metals in various samples. This technique measures the absorption of infrared radiation by the sample and provides information about the chemical composition, including metal content. FTIR spectroscopy offers advantages such as high sensitivity, rapid analysis, and minimal sample preparation. It can be applied in environmental monitoring, industrial quality control, and research applications for metal detection.
- Atomic Absorption Spectroscopy methods for metal analysis: Atomic Absorption Spectroscopy (AAS) is a technique specifically designed for the detection and quantification of metals in various samples. It works by measuring the absorption of optical radiation by free atoms in the gaseous state. The technique offers high sensitivity and selectivity for metal detection, making it suitable for trace metal analysis in environmental, industrial, and research applications. Different atomization techniques can be employed, including flame, graphite furnace, and hydride generation.
- Combined spectroscopic approaches for enhanced metal detection: Combining multiple spectroscopic techniques, such as FTIR and Atomic Absorption Spectroscopy, can provide more comprehensive and accurate metal detection capabilities. These combined approaches leverage the strengths of each technique to overcome individual limitations. For example, FTIR can provide information about chemical bonding and molecular structure, while AAS offers high sensitivity for specific metal detection. Such combined approaches are particularly useful for complex samples or when both qualitative and quantitative analyses are required.
- Sample preparation and handling for spectroscopic metal detection: Effective sample preparation and handling techniques are crucial for accurate metal detection using FTIR and Atomic Absorption Spectroscopy. These techniques may include digestion methods, extraction procedures, concentration steps, and matrix modification approaches. Proper sample preparation can enhance sensitivity, reduce interference, and improve the reliability of metal detection. Different sample types (solid, liquid, gas) require specific preparation protocols to ensure optimal results in spectroscopic analysis.
- Instrumentation and apparatus innovations for spectroscopic metal detection: Advancements in instrumentation and apparatus design have significantly improved the capabilities of FTIR and Atomic Absorption Spectroscopy for metal detection. These innovations include enhanced optical systems, improved detectors, automated sample handling, and integrated data processing. Modern instruments often feature higher sensitivity, better resolution, faster analysis times, and increased automation. Some systems also incorporate calibration standards and quality control measures to ensure reliable and reproducible metal detection results.
02 Atomic absorption spectroscopy methods for metal analysis
Atomic Absorption Spectroscopy (AAS) is a specialized technique for quantitative determination of metals in various samples. The method involves atomizing the sample and measuring the absorption of radiation by free atoms in the gaseous state. AAS offers high sensitivity and selectivity for metal detection, making it suitable for trace metal analysis in environmental, biological, and industrial samples. Different atomization techniques, including flame, graphite furnace, and hydride generation, can be employed depending on the metal of interest and required detection limits.Expand Specific Solutions03 Combined spectroscopic approaches for enhanced metal detection
Combining multiple spectroscopic techniques, particularly FTIR and AAS, can provide complementary information for comprehensive metal analysis. While AAS offers quantitative determination of metal content, FTIR provides structural information about metal-containing compounds. This combined approach allows for both identification and quantification of metals in complex matrices. Integrated systems that incorporate both techniques can offer improved sensitivity, selectivity, and reliability for metal detection in various applications including environmental monitoring, pharmaceutical analysis, and industrial quality control.Expand Specific Solutions04 Sample preparation methods for spectroscopic metal detection
Effective sample preparation is crucial for accurate metal detection using FTIR and AAS. Various preparation techniques can be employed depending on the sample matrix and the metals of interest. These include acid digestion, extraction, preconcentration, and derivatization methods. Proper sample preparation helps to eliminate matrix interferences, improve detection limits, and enhance the reliability of analytical results. Specialized sample handling devices and automated preparation systems can further improve the efficiency and reproducibility of metal analysis by spectroscopic methods.Expand Specific Solutions05 Portable and field-deployable spectroscopic systems for metal detection
Advancements in technology have led to the development of portable and field-deployable FTIR and AAS systems for on-site metal detection. These compact instruments enable real-time analysis of metals in environmental samples, industrial processes, and emergency response situations. Portable spectroscopic systems typically incorporate miniaturized components, battery operation, and simplified user interfaces while maintaining acceptable analytical performance. Field-deployable systems offer advantages such as immediate results, reduced sample transportation costs, and the ability to make rapid decisions based on analytical data.Expand Specific Solutions
Leading Manufacturers and Research Institutions in Spectroscopic Analysis
The metal detection technology landscape is currently in a mature growth phase with a global market size estimated at $5-7 billion, growing at 4-5% annually. FTIR and Atomic Absorption Spectroscopy represent complementary approaches with distinct advantages. Leading companies like Bruker Nano, Thermo Scientific, and Photothermal Spectroscopy Corp are driving innovation in FTIR technology with enhanced sensitivity and portability, while Spectra Analysis Instruments focuses on hybrid solutions. Academic institutions including MIT, EPFL, and University of Liverpool contribute significant research advancements. The technology ecosystem shows a trend toward miniaturization and integration with other analytical methods, with industrial applications expanding beyond traditional laboratory settings into field-deployable solutions for environmental monitoring and quality control.
Bruker Nano, Inc.
Technical Solution: Bruker Nano has developed advanced FTIR spectroscopy solutions specifically optimized for metals detection and analysis. Their technology integrates high-resolution FTIR systems with specialized sampling accessories designed for metal surface analysis and contamination detection. Their LUMOS II FTIR microscope enables automated chemical identification of metal contaminants with spatial resolution down to 5 μm[1]. For metals detection applications, Bruker has implemented advanced algorithms that enhance spectral discrimination between similar metal compounds and reduce interference from sample matrices. Their systems incorporate reference libraries containing over 25,000 spectra for reliable identification of metal-containing compounds[3]. Bruker's technology also features automated background correction and atmospheric compensation algorithms that significantly improve detection limits for trace metal analysis compared to conventional FTIR systems.
Strengths: Superior spatial resolution allowing precise localization of metal contaminants; extensive spectral libraries for reliable identification; advanced algorithms for matrix interference reduction. Weaknesses: Higher initial investment compared to basic AAS systems; requires more specialized operator training; limited sensitivity for certain metals compared to dedicated atomic spectroscopy techniques.
Photothermal Spectroscopy Corp.
Technical Solution: Photothermal Spectroscopy Corp. has pioneered Optical Photothermal Infrared (O-PTIR) spectroscopy, a revolutionary approach that overcomes traditional limitations of both FTIR and AAS for metals detection. Their mIRage IR microscopy platform combines the spatial resolution advantages of optical microscopy with the chemical specificity of FTIR, achieving submicron spatial resolution (approximately 500 nm) that far exceeds conventional FTIR capabilities[9]. For metals detection, their technology enables direct visualization and chemical identification of metal particles, corrosion products, and metal-organic complexes at the microscale. The company has developed specialized accessories for analyzing metal surfaces and interfaces without the sample preparation requirements of traditional techniques. Their systems incorporate advanced signal processing algorithms that enhance sensitivity for trace metal detection, with demonstrated capabilities for identifying metal species at concentrations below 0.1% in complex matrices[10]. Photothermal Spectroscopy's technology also features simultaneous Raman spectroscopy capabilities, providing complementary molecular information that aids in metal speciation analysis.
Strengths: Submicron spatial resolution enabling microscale metal analysis; non-destructive analysis with minimal sample preparation; simultaneous complementary spectroscopic information. Weaknesses: Relatively new technology with smaller installed base; higher initial investment than conventional systems; limited throughput for routine analytical applications.
Key Patents and Scientific Breakthroughs in Metal Detection
FTIR System and Method for Compositional Analysis of Matter
PatentInactiveUS20170059411A1
Innovation
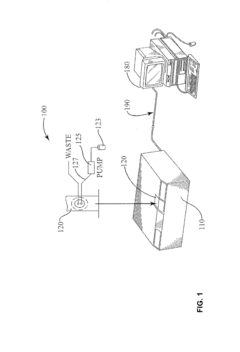
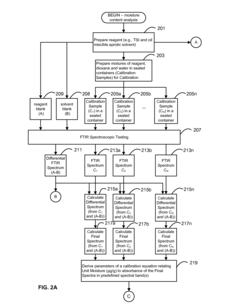
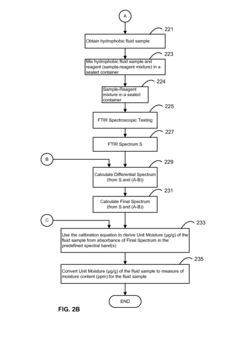
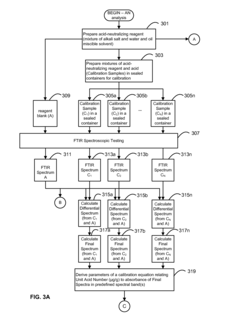
- A system and method using FTIR spectroscopy that involves preparing reagents reacting with the sample to produce carbon dioxide gas, allowing for calibration equations to be derived from standard mixtures, enabling precise measurement of moisture, acidity, or basicity by analyzing absorbance in specific spectral bands, which can be applied to various hydrophobic fluids and solid matrices.
Fourier transform infrared spectrophotometer
PatentInactiveUS7535004B2
Innovation
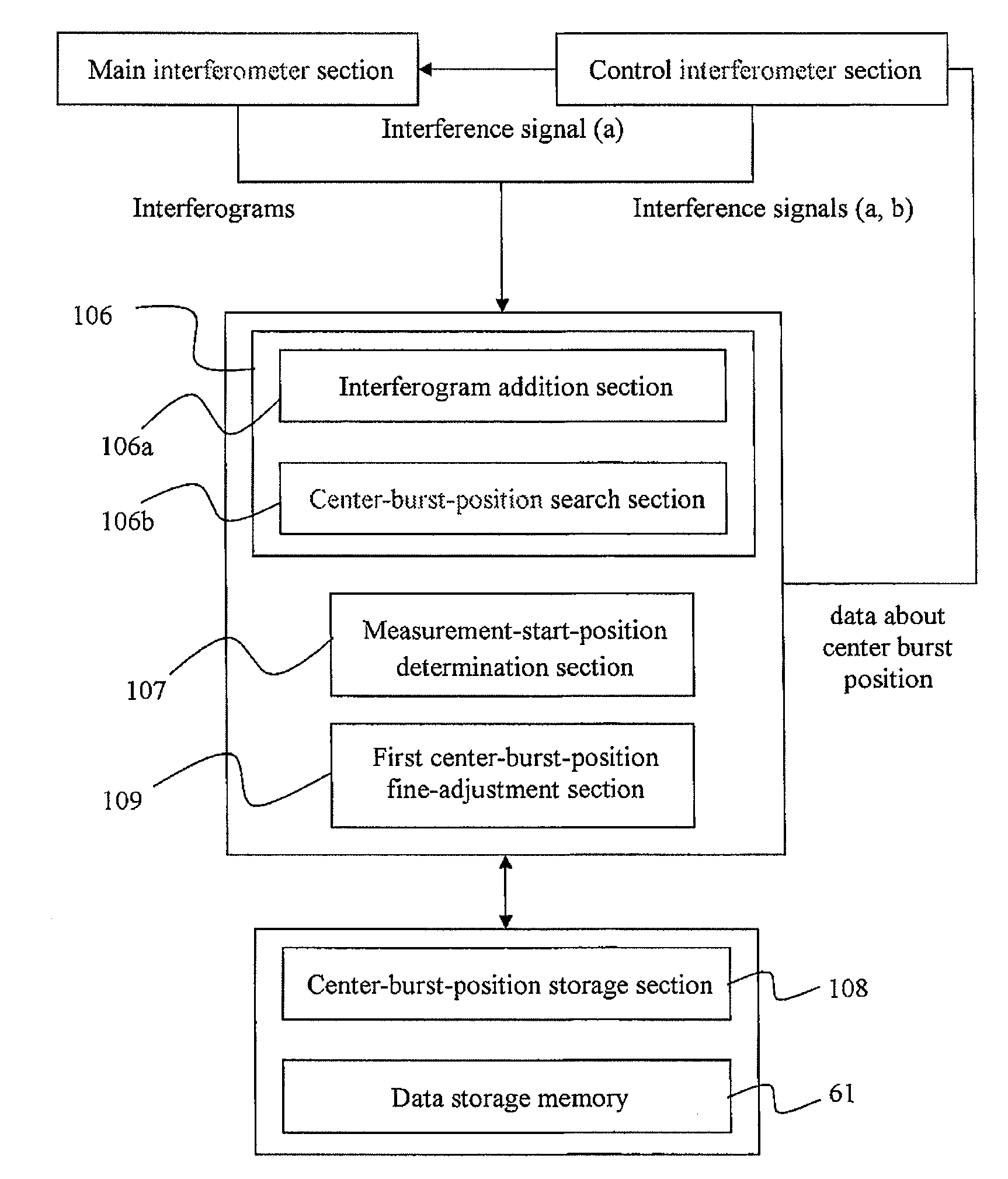
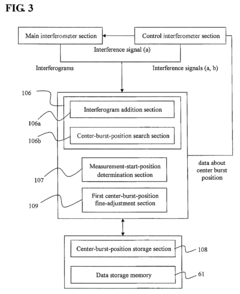
- The implementation of a center-burst-position detection section that performs addition processing on interferogram intensities to produce a cumulative interferogram, correcting positional deviations and enhancing the detection of the center burst position by increasing the intensity of true peaks while canceling out noise peaks, thereby ensuring accurate detection even under low light conditions.
Environmental Impact and Sustainability Considerations
The environmental impact of analytical techniques for metal detection is becoming increasingly important as laboratories worldwide strive for more sustainable practices. FTIR (Fourier Transform Infrared Spectroscopy) and Atomic Absorption Spectroscopy (AAS) present different environmental footprints that merit careful consideration when selecting appropriate methodologies for metals analysis.
AAS typically requires specific gases for flame or furnace operation, including acetylene, nitrous oxide, and argon, which contribute to greenhouse gas emissions during production and transportation. Additionally, the technique consumes significant electrical energy during operation, particularly for graphite furnace AAS which requires high temperatures (up to 3000°C) for atomization. The disposal of metal-containing waste solutions after analysis also presents environmental challenges, as these often contain toxic elements requiring specialized treatment.
In contrast, FTIR generally has a lower direct environmental impact during operation. It requires no carrier gases for basic functionality and consumes less electricity than AAS systems. However, FTIR sample preparation for metals analysis often necessitates chemical extraction procedures that may involve organic solvents or other reagents with their own environmental implications.
From a sustainability perspective, newer FTIR instruments incorporate energy-saving features such as standby modes and more efficient optical components, reducing their carbon footprint. Modern AAS systems have also improved in efficiency, with some manufacturers developing eco-friendly alternatives to traditional gases and implementing recycling systems for waste solutions.
The longevity of equipment represents another sustainability factor. FTIR instruments typically have fewer consumable parts and may offer longer operational lifespans than AAS systems, which require regular replacement of hollow cathode lamps, graphite tubes, and other components. This difference in maintenance requirements translates to varying levels of electronic waste generation over time.
Water consumption differs significantly between techniques. While FTIR requires minimal water for operation, AAS cooling systems can consume substantial quantities, particularly in flame systems that require continuous cooling during operation. Laboratories in water-stressed regions may find this distinction particularly relevant.
When considering the complete lifecycle assessment, both technologies require raw materials for manufacturing that involve mining and processing with associated environmental impacts. However, the operational phase typically represents the largest environmental burden for analytical instruments, making energy efficiency and consumables usage the most critical factors for sustainability evaluation.
AAS typically requires specific gases for flame or furnace operation, including acetylene, nitrous oxide, and argon, which contribute to greenhouse gas emissions during production and transportation. Additionally, the technique consumes significant electrical energy during operation, particularly for graphite furnace AAS which requires high temperatures (up to 3000°C) for atomization. The disposal of metal-containing waste solutions after analysis also presents environmental challenges, as these often contain toxic elements requiring specialized treatment.
In contrast, FTIR generally has a lower direct environmental impact during operation. It requires no carrier gases for basic functionality and consumes less electricity than AAS systems. However, FTIR sample preparation for metals analysis often necessitates chemical extraction procedures that may involve organic solvents or other reagents with their own environmental implications.
From a sustainability perspective, newer FTIR instruments incorporate energy-saving features such as standby modes and more efficient optical components, reducing their carbon footprint. Modern AAS systems have also improved in efficiency, with some manufacturers developing eco-friendly alternatives to traditional gases and implementing recycling systems for waste solutions.
The longevity of equipment represents another sustainability factor. FTIR instruments typically have fewer consumable parts and may offer longer operational lifespans than AAS systems, which require regular replacement of hollow cathode lamps, graphite tubes, and other components. This difference in maintenance requirements translates to varying levels of electronic waste generation over time.
Water consumption differs significantly between techniques. While FTIR requires minimal water for operation, AAS cooling systems can consume substantial quantities, particularly in flame systems that require continuous cooling during operation. Laboratories in water-stressed regions may find this distinction particularly relevant.
When considering the complete lifecycle assessment, both technologies require raw materials for manufacturing that involve mining and processing with associated environmental impacts. However, the operational phase typically represents the largest environmental burden for analytical instruments, making energy efficiency and consumables usage the most critical factors for sustainability evaluation.
Regulatory Standards for Metal Detection Technologies
Metal detection technologies are subject to stringent regulatory frameworks that vary across industries and geographical regions. In the United States, the Food and Drug Administration (FDA) establishes guidelines for metal detection in food products, requiring sensitivity levels capable of detecting particles as small as 0.8mm for ferrous metals and 1.2mm for non-ferrous metals. The Environmental Protection Agency (EPA) regulates metal detection methodologies for environmental monitoring, with Method 200.7 specifically addressing FTIR applications and Method 200.9 covering atomic absorption spectroscopy techniques.
The European Union enforces more comprehensive standards through the EN 1672-2 and EC 1935/2004 regulations, which mandate regular validation and calibration of metal detection equipment. These standards specify detection thresholds based on both metal type and the matrix being analyzed, with particular emphasis on heavy metals like lead, cadmium, and mercury. The International Organization for Standardization (ISO) provides globally recognized standards, including ISO 17025 for laboratory testing competence and ISO 11816 for specific metal detection protocols.
For pharmaceutical applications, the United States Pharmacopeia (USP) and European Pharmacopoeia (EP) establish strict guidelines for elemental impurity testing. USP <232> and <233> specifically outline permissible limits for metal contaminants and recommended analytical procedures, with atomic absorption spectroscopy recognized as a reference method for certain elements. Similarly, EP 2.4.20 details requirements for metal detection in pharmaceutical products.
Regulatory compliance for FTIR and atomic absorption spectroscopy differs significantly in terms of validation requirements. Atomic absorption methods typically require more extensive validation protocols due to their established status as reference methods for metal detection. FTIR methods, while increasingly accepted, often require additional verification against traditional techniques when used for regulatory compliance.
Emerging regulations are increasingly focusing on method performance rather than prescribing specific technologies. This trend allows for innovation in detection methodologies while maintaining safety standards. The International Conference on Harmonisation (ICH) Q3D guideline represents this approach, establishing permitted daily exposure limits for elemental impurities without mandating specific detection technologies.
Compliance with these regulatory standards necessitates regular performance verification, documentation of calibration procedures, and participation in proficiency testing programs. Laboratories employing either FTIR or atomic absorption spectroscopy must demonstrate method validation according to parameters including accuracy, precision, specificity, limit of detection, and robustness as outlined in respective regulatory frameworks.
The European Union enforces more comprehensive standards through the EN 1672-2 and EC 1935/2004 regulations, which mandate regular validation and calibration of metal detection equipment. These standards specify detection thresholds based on both metal type and the matrix being analyzed, with particular emphasis on heavy metals like lead, cadmium, and mercury. The International Organization for Standardization (ISO) provides globally recognized standards, including ISO 17025 for laboratory testing competence and ISO 11816 for specific metal detection protocols.
For pharmaceutical applications, the United States Pharmacopeia (USP) and European Pharmacopoeia (EP) establish strict guidelines for elemental impurity testing. USP <232> and <233> specifically outline permissible limits for metal contaminants and recommended analytical procedures, with atomic absorption spectroscopy recognized as a reference method for certain elements. Similarly, EP 2.4.20 details requirements for metal detection in pharmaceutical products.
Regulatory compliance for FTIR and atomic absorption spectroscopy differs significantly in terms of validation requirements. Atomic absorption methods typically require more extensive validation protocols due to their established status as reference methods for metal detection. FTIR methods, while increasingly accepted, often require additional verification against traditional techniques when used for regulatory compliance.
Emerging regulations are increasingly focusing on method performance rather than prescribing specific technologies. This trend allows for innovation in detection methodologies while maintaining safety standards. The International Conference on Harmonisation (ICH) Q3D guideline represents this approach, establishing permitted daily exposure limits for elemental impurities without mandating specific detection technologies.
Compliance with these regulatory standards necessitates regular performance verification, documentation of calibration procedures, and participation in proficiency testing programs. Laboratories employing either FTIR or atomic absorption spectroscopy must demonstrate method validation according to parameters including accuracy, precision, specificity, limit of detection, and robustness as outlined in respective regulatory frameworks.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!