Fundamental Electrochemistry of Lithium Iron Phosphate Materials
AUG 8, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
LFP Battery Evolution
The evolution of Lithium Iron Phosphate (LFP) batteries represents a significant milestone in the development of energy storage technologies. Initially discovered in 1996 by John Goodenough and his team at the University of Texas, LFP cathode materials quickly gained attention due to their promising characteristics for battery applications.
In the early 2000s, researchers focused on overcoming the inherent limitations of LFP, such as its low electronic conductivity and slow lithium-ion diffusion. This led to the development of carbon coating techniques and nanostructuring methods, which significantly improved the material's performance. By 2005, LFP batteries began to see commercial applications, primarily in power tools and electric bicycles.
The period between 2005 and 2010 saw rapid advancements in LFP technology. Researchers explored various synthesis methods, including solid-state reactions, hydrothermal processes, and sol-gel techniques, to optimize particle size and morphology. These efforts resulted in improved capacity, rate capability, and cycle life of LFP batteries.
From 2010 to 2015, the focus shifted towards enhancing the energy density of LFP batteries. Scientists investigated doping strategies, using elements like manganese, cobalt, and nickel to modify the crystal structure and improve electrochemical properties. Additionally, research into advanced electrolytes and binders contributed to better overall battery performance.
The period from 2015 to 2020 saw increased adoption of LFP batteries in electric vehicles and grid-scale energy storage systems. This was driven by improvements in manufacturing processes, which led to reduced costs and increased production capacity. During this time, researchers also explored the potential of LFP in sodium-ion batteries as an alternative to lithium-ion systems.
In recent years, there has been a renewed interest in LFP technology, particularly for electric vehicle applications. Major automakers have announced plans to incorporate LFP batteries into their product lines, citing advantages such as lower cost, improved safety, and longer cycle life compared to other lithium-ion chemistries.
Looking ahead, the evolution of LFP batteries is expected to continue with a focus on further improving energy density, fast-charging capabilities, and low-temperature performance. Research into advanced manufacturing techniques, such as dry electrode processing and solid-state electrolyte integration, may lead to the next generation of LFP batteries with even better performance and cost-effectiveness.
In the early 2000s, researchers focused on overcoming the inherent limitations of LFP, such as its low electronic conductivity and slow lithium-ion diffusion. This led to the development of carbon coating techniques and nanostructuring methods, which significantly improved the material's performance. By 2005, LFP batteries began to see commercial applications, primarily in power tools and electric bicycles.
The period between 2005 and 2010 saw rapid advancements in LFP technology. Researchers explored various synthesis methods, including solid-state reactions, hydrothermal processes, and sol-gel techniques, to optimize particle size and morphology. These efforts resulted in improved capacity, rate capability, and cycle life of LFP batteries.
From 2010 to 2015, the focus shifted towards enhancing the energy density of LFP batteries. Scientists investigated doping strategies, using elements like manganese, cobalt, and nickel to modify the crystal structure and improve electrochemical properties. Additionally, research into advanced electrolytes and binders contributed to better overall battery performance.
The period from 2015 to 2020 saw increased adoption of LFP batteries in electric vehicles and grid-scale energy storage systems. This was driven by improvements in manufacturing processes, which led to reduced costs and increased production capacity. During this time, researchers also explored the potential of LFP in sodium-ion batteries as an alternative to lithium-ion systems.
In recent years, there has been a renewed interest in LFP technology, particularly for electric vehicle applications. Major automakers have announced plans to incorporate LFP batteries into their product lines, citing advantages such as lower cost, improved safety, and longer cycle life compared to other lithium-ion chemistries.
Looking ahead, the evolution of LFP batteries is expected to continue with a focus on further improving energy density, fast-charging capabilities, and low-temperature performance. Research into advanced manufacturing techniques, such as dry electrode processing and solid-state electrolyte integration, may lead to the next generation of LFP batteries with even better performance and cost-effectiveness.
LFP Market Analysis
The lithium iron phosphate (LFP) battery market has experienced significant growth in recent years, driven by the increasing demand for electric vehicles (EVs) and energy storage systems. LFP batteries have gained popularity due to their safety, long cycle life, and cost-effectiveness compared to other lithium-ion battery chemistries.
The global LFP battery market size was valued at over $10 billion in 2021 and is projected to grow at a compound annual growth rate (CAGR) of more than 20% from 2022 to 2030. This growth is primarily attributed to the rising adoption of EVs, particularly in China, which has been a major driver of LFP battery demand.
In the EV sector, LFP batteries have become increasingly popular, especially for mass-market vehicles and commercial applications. Major automakers, including Tesla, Volkswagen, and Ford, have announced plans to incorporate LFP batteries into their vehicle lineups, further boosting market demand.
The energy storage sector is another key market for LFP batteries, with applications ranging from residential to utility-scale installations. The growing focus on renewable energy integration and grid stability has led to increased demand for LFP-based energy storage solutions.
Geographically, China dominates the LFP battery market, accounting for over 70% of global production capacity. However, other regions, including Europe and North America, are ramping up their LFP battery manufacturing capabilities to reduce dependence on Chinese suppliers and meet local demand.
The market landscape is characterized by intense competition among established players and new entrants. Key manufacturers include CATL, BYD, Gotion High-Tech, and A123 Systems. These companies are investing heavily in research and development to improve LFP battery performance and reduce production costs.
Despite the positive market outlook, challenges remain. The ongoing global supply chain disruptions and raw material price fluctuations have impacted LFP battery production and pricing. Additionally, the development of next-generation battery technologies, such as solid-state batteries, may pose a long-term challenge to LFP market growth.
In conclusion, the LFP battery market is poised for substantial growth, driven by the EV revolution and the increasing need for energy storage solutions. As technology advances and production scales up, LFP batteries are expected to play a crucial role in the transition to a sustainable energy future.
The global LFP battery market size was valued at over $10 billion in 2021 and is projected to grow at a compound annual growth rate (CAGR) of more than 20% from 2022 to 2030. This growth is primarily attributed to the rising adoption of EVs, particularly in China, which has been a major driver of LFP battery demand.
In the EV sector, LFP batteries have become increasingly popular, especially for mass-market vehicles and commercial applications. Major automakers, including Tesla, Volkswagen, and Ford, have announced plans to incorporate LFP batteries into their vehicle lineups, further boosting market demand.
The energy storage sector is another key market for LFP batteries, with applications ranging from residential to utility-scale installations. The growing focus on renewable energy integration and grid stability has led to increased demand for LFP-based energy storage solutions.
Geographically, China dominates the LFP battery market, accounting for over 70% of global production capacity. However, other regions, including Europe and North America, are ramping up their LFP battery manufacturing capabilities to reduce dependence on Chinese suppliers and meet local demand.
The market landscape is characterized by intense competition among established players and new entrants. Key manufacturers include CATL, BYD, Gotion High-Tech, and A123 Systems. These companies are investing heavily in research and development to improve LFP battery performance and reduce production costs.
Despite the positive market outlook, challenges remain. The ongoing global supply chain disruptions and raw material price fluctuations have impacted LFP battery production and pricing. Additionally, the development of next-generation battery technologies, such as solid-state batteries, may pose a long-term challenge to LFP market growth.
In conclusion, the LFP battery market is poised for substantial growth, driven by the EV revolution and the increasing need for energy storage solutions. As technology advances and production scales up, LFP batteries are expected to play a crucial role in the transition to a sustainable energy future.
LFP Challenges
Lithium Iron Phosphate (LFP) materials have emerged as a promising cathode technology for lithium-ion batteries, offering advantages in safety, cost, and environmental impact. However, several challenges persist in the fundamental electrochemistry of LFP materials, hindering their widespread adoption and optimal performance.
One of the primary challenges is the inherently low electronic conductivity of LFP. This limitation restricts the material's ability to efficiently transport electrons during charge and discharge cycles, potentially leading to reduced power density and rate capability. Researchers have explored various strategies to address this issue, including carbon coating and doping with conductive elements, but further improvements are still needed to fully overcome this intrinsic limitation.
Another significant challenge lies in the complex phase transition mechanism during lithium insertion and extraction. The olivine structure of LFP undergoes a two-phase reaction, which can lead to structural stress and potential capacity loss over extended cycling. Understanding and controlling this phase transition process is crucial for enhancing the long-term stability and performance of LFP-based batteries.
The relatively low theoretical capacity of LFP compared to other cathode materials poses another challenge. While LFP offers excellent stability, its energy density is lower than some competing technologies. This limitation necessitates the development of strategies to increase the capacity without compromising the material's inherent advantages.
Surface reactivity and electrolyte compatibility present additional challenges in LFP electrochemistry. The formation of surface films and potential side reactions with the electrolyte can impact the electrode-electrolyte interface, affecting battery performance and longevity. Optimizing this interface is critical for maximizing the electrochemical performance of LFP materials.
Furthermore, the lithium diffusion kinetics within the LFP structure can be a limiting factor in high-rate applications. The one-dimensional lithium diffusion channels in the olivine structure can lead to diffusion limitations, particularly at high charge/discharge rates. Addressing this challenge requires innovative approaches to enhance lithium mobility within the material.
Lastly, the synthesis and processing of LFP materials present their own set of challenges. Achieving uniform particle size distribution, optimal morphology, and consistent composition are crucial for ensuring reproducible and high-performance LFP cathodes. Developing scalable and cost-effective manufacturing processes that maintain precise control over these parameters remains an ongoing challenge in the commercialization of LFP technology.
One of the primary challenges is the inherently low electronic conductivity of LFP. This limitation restricts the material's ability to efficiently transport electrons during charge and discharge cycles, potentially leading to reduced power density and rate capability. Researchers have explored various strategies to address this issue, including carbon coating and doping with conductive elements, but further improvements are still needed to fully overcome this intrinsic limitation.
Another significant challenge lies in the complex phase transition mechanism during lithium insertion and extraction. The olivine structure of LFP undergoes a two-phase reaction, which can lead to structural stress and potential capacity loss over extended cycling. Understanding and controlling this phase transition process is crucial for enhancing the long-term stability and performance of LFP-based batteries.
The relatively low theoretical capacity of LFP compared to other cathode materials poses another challenge. While LFP offers excellent stability, its energy density is lower than some competing technologies. This limitation necessitates the development of strategies to increase the capacity without compromising the material's inherent advantages.
Surface reactivity and electrolyte compatibility present additional challenges in LFP electrochemistry. The formation of surface films and potential side reactions with the electrolyte can impact the electrode-electrolyte interface, affecting battery performance and longevity. Optimizing this interface is critical for maximizing the electrochemical performance of LFP materials.
Furthermore, the lithium diffusion kinetics within the LFP structure can be a limiting factor in high-rate applications. The one-dimensional lithium diffusion channels in the olivine structure can lead to diffusion limitations, particularly at high charge/discharge rates. Addressing this challenge requires innovative approaches to enhance lithium mobility within the material.
Lastly, the synthesis and processing of LFP materials present their own set of challenges. Achieving uniform particle size distribution, optimal morphology, and consistent composition are crucial for ensuring reproducible and high-performance LFP cathodes. Developing scalable and cost-effective manufacturing processes that maintain precise control over these parameters remains an ongoing challenge in the commercialization of LFP technology.
LFP Cathode Design
01 Synthesis methods for lithium iron phosphate materials
Various synthesis methods are employed to produce lithium iron phosphate materials, including solid-state reactions, hydrothermal processes, and sol-gel techniques. These methods aim to optimize particle size, morphology, and crystallinity to enhance the electrochemical performance of the resulting materials for use in lithium-ion batteries.- Synthesis methods for lithium iron phosphate materials: Various synthesis methods are employed to produce lithium iron phosphate materials, including hydrothermal, solid-state, and sol-gel processes. These methods aim to optimize particle size, morphology, and crystallinity to enhance the electrochemical performance of the resulting materials for use in lithium-ion batteries.
- Doping and surface modification of lithium iron phosphate: Doping with various elements and surface modification techniques are used to improve the conductivity and stability of lithium iron phosphate materials. These enhancements can lead to better rate capability, cycling performance, and overall battery efficiency.
- Nanostructured lithium iron phosphate materials: Development of nanostructured lithium iron phosphate materials, including nanoparticles, nanowires, and nanocomposites, to increase the surface area and improve lithium-ion diffusion. These nanostructures can significantly enhance the power density and rate performance of lithium-ion batteries.
- Carbon coating and conductive additives: Application of carbon coatings and incorporation of conductive additives to lithium iron phosphate materials to enhance their electronic conductivity. These approaches can mitigate the inherent low conductivity of lithium iron phosphate and improve its electrochemical performance in battery applications.
- Scalable production and industrial applications: Development of scalable production methods and industrial applications for lithium iron phosphate materials, focusing on cost-effective manufacturing processes, quality control, and large-scale implementation in electric vehicles and energy storage systems.
02 Doping and surface modification of lithium iron phosphate
Doping with various elements and surface modification techniques are used to improve the conductivity and stability of lithium iron phosphate materials. These enhancements can lead to better rate capability, cycling performance, and overall battery efficiency.Expand Specific Solutions03 Nanostructured lithium iron phosphate materials
Development of nanostructured lithium iron phosphate materials, including nanoparticles, nanowires, and nanocomposites, aims to increase the surface area and shorten lithium diffusion paths. This approach can significantly improve the material's electrochemical properties and battery performance.Expand Specific Solutions04 Carbon coating and conductive additives
Carbon coating and the incorporation of conductive additives are widely used techniques to enhance the electronic conductivity of lithium iron phosphate materials. These methods can effectively improve the rate performance and capacity utilization of the cathode material in lithium-ion batteries.Expand Specific Solutions05 Scalable production and industrial applications
Research focuses on developing scalable production methods for lithium iron phosphate materials to meet industrial demands. This includes optimizing manufacturing processes, reducing costs, and ensuring consistent quality for large-scale battery production applications.Expand Specific Solutions
LFP Industry Leaders
The fundamental electrochemistry of lithium iron phosphate materials is a mature field within the broader lithium-ion battery industry, which is experiencing rapid growth. The market for these materials is expanding, driven by increasing demand for electric vehicles and energy storage systems. Key players like BYD, LG Energy Solution, and CATL are leading the commercialization efforts, while research institutions such as Tsinghua University and Centre National de la Recherche Scientifique continue to advance the technology. Smaller specialized companies like Hubei Wanrun New Energy Technology and Saft Groupe SA are also contributing to innovation in this space, indicating a competitive and diverse landscape.
BYD Co., Ltd.
Technical Solution: BYD has pioneered the Blade Battery, an innovative LFP battery design that significantly improves energy density and safety. The Blade Battery utilizes a novel cell-to-pack integration method, arranging long, thin LFP cells in a blade-like format[4]. This design increases volumetric energy density by up to 50% compared to traditional LFP batteries[5]. BYD's approach includes optimized electrode materials with controlled particle size distribution and morphology to enhance lithium-ion diffusion. They have also developed proprietary electrolyte additives to form a stable solid electrolyte interphase (SEI), improving the battery's long-term performance and safety[6]. BYD's research extends to advanced manufacturing processes, including precise control of synthesis conditions to achieve uniform LFP crystal structures.
Strengths: Exceptional safety performance, high energy density for LFP, and cost-effectiveness. Weaknesses: Limited to prismatic cell format, potentially higher manufacturing complexity.
LG Energy Solution Ltd.
Technical Solution: LG Energy Solution has made significant strides in LFP battery technology, focusing on overcoming traditional limitations. Their approach includes developing nano-structured LFP cathode materials with enhanced surface area and conductivity. LG has implemented advanced carbon coating techniques to improve electron transfer within LFP particles[7]. They have also explored the use of conductive additives and binders to optimize electrode structure and performance. LG's research extends to the development of high-voltage LFP formulations, aiming to increase energy density while maintaining the inherent safety advantages of LFP chemistry[8]. Additionally, they have invested in advanced manufacturing processes, including precise control of synthesis parameters to achieve uniform particle size distribution and optimal crystal structure.
Strengths: High-quality manufacturing capabilities, potential for high-voltage LFP formulations. Weaknesses: Relatively late entry into LFP market, potential catch-up required in terms of energy density.
LFP Innovations
High-compaction lithium iron phosphate positive electrode material, preparation method thereof, positive electrode and battery including the same
PatentActiveIN202224069334A
Innovation
- A high-compaction lithium iron phosphate positive electrode material is developed by doping with vanadium, titanium, and boron, and coating with carbon, using a specific particle size distribution and sintering process to achieve a compacted density of 2.5-3.0 g/mL and a specific capacity of 100-200 mAh/g.
Lithium iron phosphate having olivine structure and method for analyzing the same
PatentActiveUS20100261060A1
Innovation
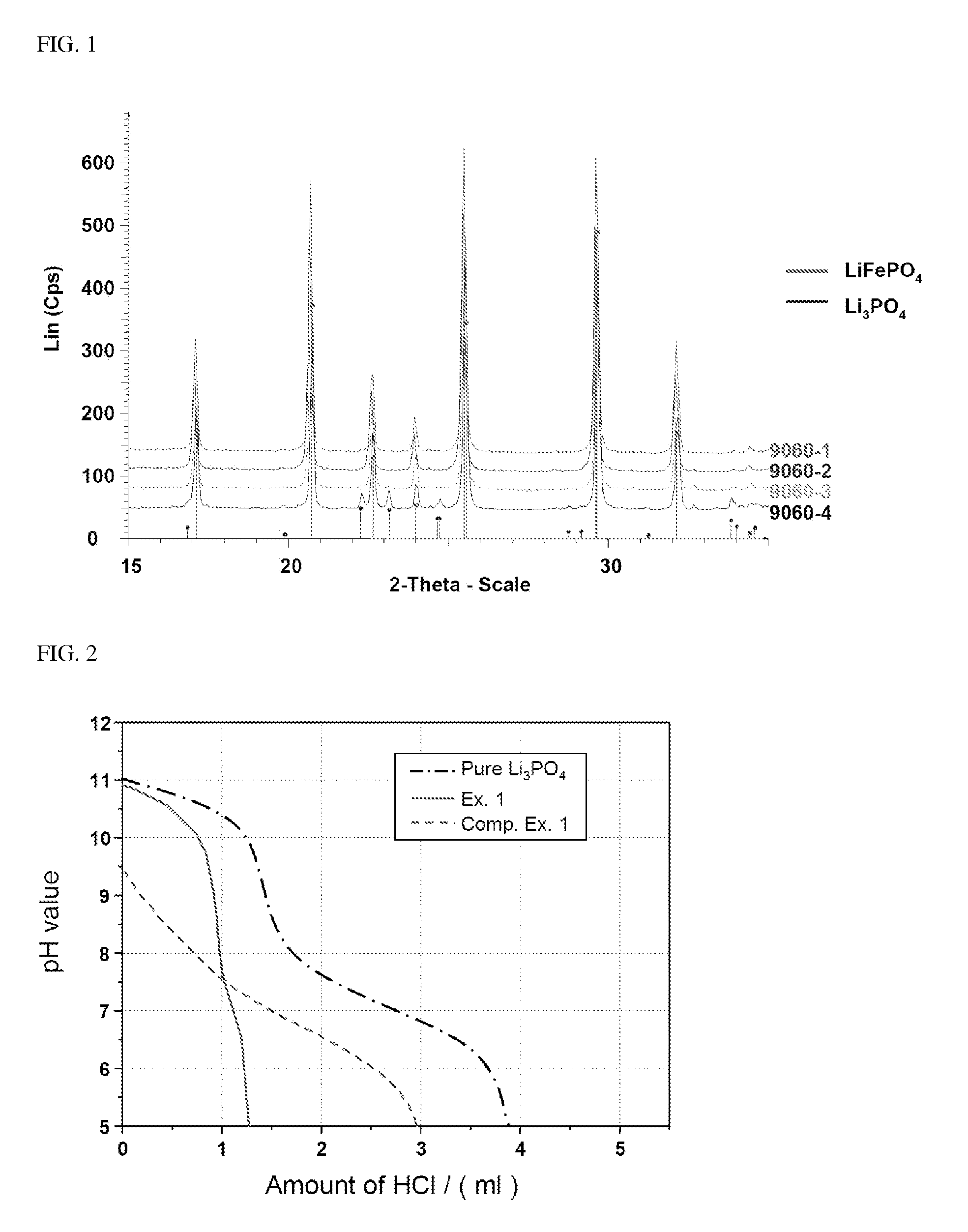
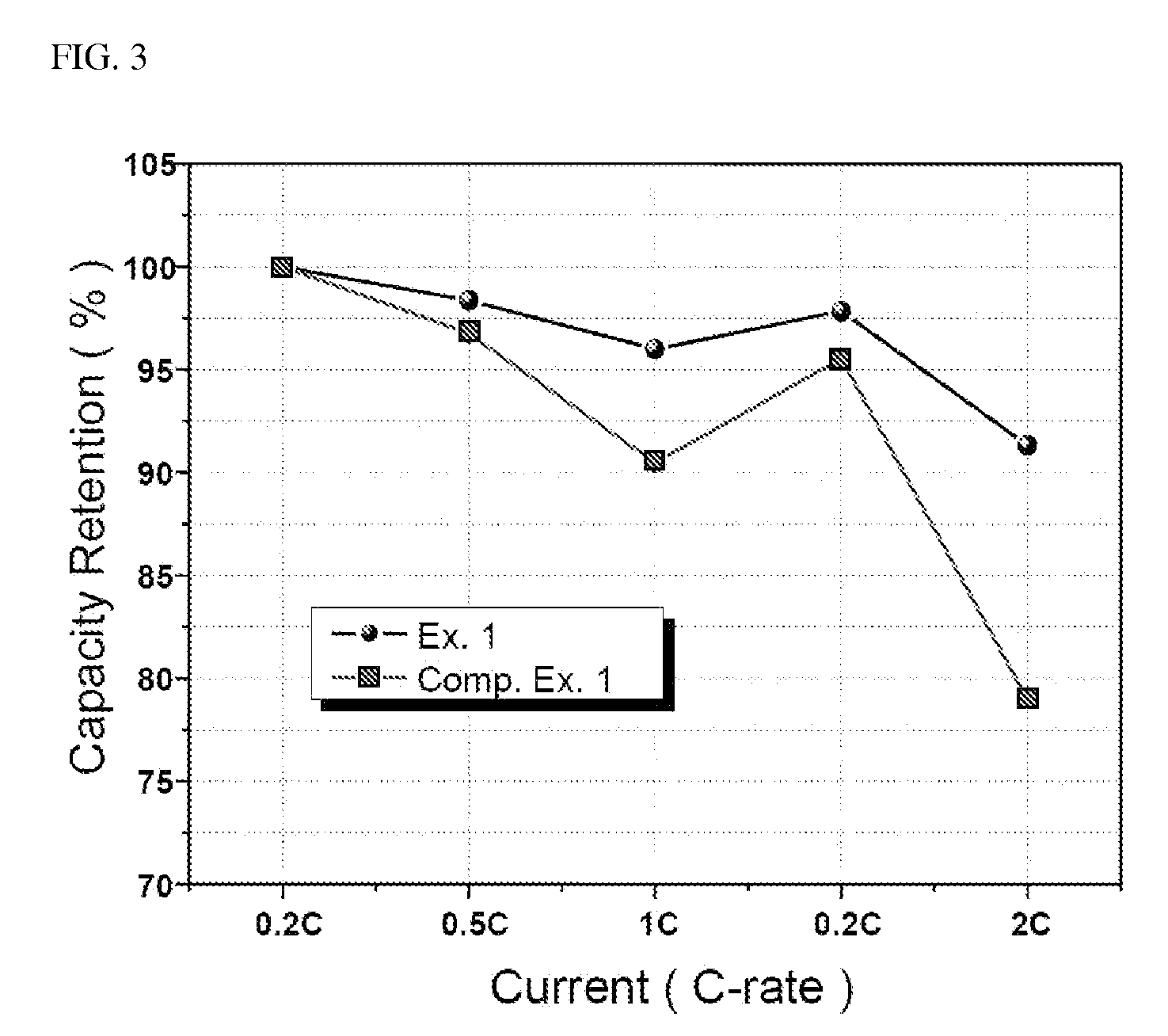
- Incorporating a minimal amount of Li3PO4 into the lithium iron phosphate, specifically 0.1 to 5% by weight, to enhance ionic conductivity and reduce Li2CO3 content to less than 0.25% by weight, thereby improving high-temperature stability and rate properties without inducing side reactions or capacity deterioration.
LFP Safety Standards
Safety standards for Lithium Iron Phosphate (LFP) batteries are crucial in ensuring the safe operation and widespread adoption of this technology. These standards encompass various aspects of battery design, manufacturing, testing, and usage. The International Electrotechnical Commission (IEC) has established several standards specifically for LFP batteries, including IEC 62619 for industrial applications and IEC 62660 for electric vehicle applications.
One of the key safety requirements for LFP batteries is thermal stability. LFP cells must undergo rigorous thermal runaway tests to ensure they can withstand high temperatures without catastrophic failure. These tests typically involve subjecting the cells to temperatures exceeding 130°C and monitoring their behavior. The cells should not ignite, explode, or release hazardous materials during these tests.
Mechanical integrity is another critical aspect of LFP safety standards. Batteries must withstand various mechanical stresses, including vibration, shock, and crush tests. These tests simulate real-world conditions that batteries might encounter during transportation or in the event of an accident. The standards specify the minimum force and duration that the batteries must endure without compromising their safety features.
Electrical safety is also a primary concern in LFP battery standards. Overcharge and over-discharge protection mechanisms are mandatory to prevent dangerous situations that could lead to battery failure or fire. The standards specify the maximum allowable voltage and current limits, as well as the response time for safety circuits to activate in case of abnormal conditions.
Environmental testing is an integral part of LFP safety standards. Batteries must demonstrate their ability to function safely across a wide range of temperatures and humidity levels. This includes both storage and operational conditions, ensuring that the batteries remain stable and perform reliably in various climates and environments.
Quality control and traceability requirements are embedded within LFP safety standards. Manufacturers must implement rigorous quality management systems and maintain detailed records of battery production, including material sourcing, manufacturing processes, and test results. This traceability is essential for identifying and addressing potential safety issues throughout the battery's lifecycle.
Transportation safety is a critical component of LFP battery standards, given the potential risks associated with shipping large quantities of batteries. The United Nations has established specific regulations for the transport of lithium batteries, including LFP, which are incorporated into various international and national transportation codes. These regulations cover aspects such as packaging, labeling, and documentation requirements.
As the technology evolves, safety standards for LFP batteries continue to be updated and refined. Regulatory bodies and industry stakeholders collaborate to address emerging safety concerns and incorporate new testing methodologies. This ongoing process ensures that LFP batteries maintain their reputation as one of the safest lithium-ion chemistries available, supporting their widespread adoption in various applications.
One of the key safety requirements for LFP batteries is thermal stability. LFP cells must undergo rigorous thermal runaway tests to ensure they can withstand high temperatures without catastrophic failure. These tests typically involve subjecting the cells to temperatures exceeding 130°C and monitoring their behavior. The cells should not ignite, explode, or release hazardous materials during these tests.
Mechanical integrity is another critical aspect of LFP safety standards. Batteries must withstand various mechanical stresses, including vibration, shock, and crush tests. These tests simulate real-world conditions that batteries might encounter during transportation or in the event of an accident. The standards specify the minimum force and duration that the batteries must endure without compromising their safety features.
Electrical safety is also a primary concern in LFP battery standards. Overcharge and over-discharge protection mechanisms are mandatory to prevent dangerous situations that could lead to battery failure or fire. The standards specify the maximum allowable voltage and current limits, as well as the response time for safety circuits to activate in case of abnormal conditions.
Environmental testing is an integral part of LFP safety standards. Batteries must demonstrate their ability to function safely across a wide range of temperatures and humidity levels. This includes both storage and operational conditions, ensuring that the batteries remain stable and perform reliably in various climates and environments.
Quality control and traceability requirements are embedded within LFP safety standards. Manufacturers must implement rigorous quality management systems and maintain detailed records of battery production, including material sourcing, manufacturing processes, and test results. This traceability is essential for identifying and addressing potential safety issues throughout the battery's lifecycle.
Transportation safety is a critical component of LFP battery standards, given the potential risks associated with shipping large quantities of batteries. The United Nations has established specific regulations for the transport of lithium batteries, including LFP, which are incorporated into various international and national transportation codes. These regulations cover aspects such as packaging, labeling, and documentation requirements.
As the technology evolves, safety standards for LFP batteries continue to be updated and refined. Regulatory bodies and industry stakeholders collaborate to address emerging safety concerns and incorporate new testing methodologies. This ongoing process ensures that LFP batteries maintain their reputation as one of the safest lithium-ion chemistries available, supporting their widespread adoption in various applications.
LFP Sustainability
Lithium iron phosphate (LFP) batteries have gained significant attention in recent years due to their inherent sustainability advantages over other lithium-ion battery chemistries. The sustainability of LFP materials is a crucial factor in their widespread adoption and long-term viability in the energy storage sector.
One of the primary sustainability benefits of LFP batteries is their use of abundant and relatively low-cost materials. Iron and phosphate are widely available resources, reducing the reliance on scarce or geopolitically sensitive materials such as cobalt or nickel. This abundance contributes to a more stable and sustainable supply chain for LFP battery production.
The production process of LFP materials also offers environmental advantages. Compared to other cathode materials, LFP synthesis typically requires lower temperatures and less energy-intensive processes. This results in a reduced carbon footprint during manufacturing, aligning with global efforts to minimize industrial emissions and combat climate change.
LFP batteries exhibit excellent thermal stability and safety characteristics, reducing the risk of thermal runaway and fire incidents. This enhanced safety profile not only protects users and infrastructure but also contributes to sustainability by minimizing the potential for environmental contamination from battery failures or accidents.
The long cycle life of LFP batteries is another key sustainability feature. With the ability to withstand thousands of charge-discharge cycles without significant degradation, LFP batteries can remain in service for extended periods. This longevity reduces the need for frequent replacements, conserving resources and minimizing waste generation over time.
End-of-life considerations also favor LFP batteries from a sustainability perspective. The materials used in LFP cathodes are generally easier to recycle compared to other lithium-ion chemistries. The recycling process can recover a high percentage of the original materials, supporting a circular economy approach and reducing the demand for virgin resources.
The sustainability of LFP extends beyond the battery itself to its applications. LFP batteries are well-suited for renewable energy storage systems, electric vehicles, and grid stabilization. By enabling these clean energy technologies, LFP contributes to the broader transition towards a more sustainable energy landscape.
However, challenges remain in fully realizing the sustainability potential of LFP materials. Ongoing research is focused on further improving the energy density of LFP batteries to enhance their competitiveness in high-energy applications. Additionally, efforts are being made to optimize recycling processes and establish robust recycling infrastructure to maximize material recovery at the end of battery life.
One of the primary sustainability benefits of LFP batteries is their use of abundant and relatively low-cost materials. Iron and phosphate are widely available resources, reducing the reliance on scarce or geopolitically sensitive materials such as cobalt or nickel. This abundance contributes to a more stable and sustainable supply chain for LFP battery production.
The production process of LFP materials also offers environmental advantages. Compared to other cathode materials, LFP synthesis typically requires lower temperatures and less energy-intensive processes. This results in a reduced carbon footprint during manufacturing, aligning with global efforts to minimize industrial emissions and combat climate change.
LFP batteries exhibit excellent thermal stability and safety characteristics, reducing the risk of thermal runaway and fire incidents. This enhanced safety profile not only protects users and infrastructure but also contributes to sustainability by minimizing the potential for environmental contamination from battery failures or accidents.
The long cycle life of LFP batteries is another key sustainability feature. With the ability to withstand thousands of charge-discharge cycles without significant degradation, LFP batteries can remain in service for extended periods. This longevity reduces the need for frequent replacements, conserving resources and minimizing waste generation over time.
End-of-life considerations also favor LFP batteries from a sustainability perspective. The materials used in LFP cathodes are generally easier to recycle compared to other lithium-ion chemistries. The recycling process can recover a high percentage of the original materials, supporting a circular economy approach and reducing the demand for virgin resources.
The sustainability of LFP extends beyond the battery itself to its applications. LFP batteries are well-suited for renewable energy storage systems, electric vehicles, and grid stabilization. By enabling these clean energy technologies, LFP contributes to the broader transition towards a more sustainable energy landscape.
However, challenges remain in fully realizing the sustainability potential of LFP materials. Ongoing research is focused on further improving the energy density of LFP batteries to enhance their competitiveness in high-energy applications. Additionally, efforts are being made to optimize recycling processes and establish robust recycling infrastructure to maximize material recovery at the end of battery life.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!