How Nanomaterials Enhance Efficiency in Electrolytic Cells
AUG 1, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Nanomaterial Electrocatalysis Background and Objectives
Nanomaterials have emerged as a revolutionary force in the field of electrocatalysis, offering unprecedented opportunities to enhance the efficiency of electrolytic cells. The evolution of nanotechnology has paved the way for significant advancements in energy conversion and storage systems, with electrolytic cells being a prime beneficiary of these innovations.
The development of nanomaterials for electrocatalysis can be traced back to the early 2000s when researchers began exploring the unique properties of materials at the nanoscale. Since then, the field has witnessed exponential growth, driven by the increasing demand for clean energy solutions and the need to optimize industrial processes.
Nanomaterials, characterized by their extremely small size and high surface-to-volume ratio, offer several advantages in electrocatalysis. They provide increased active surface area, enhanced electron transfer kinetics, and improved mass transport properties. These characteristics directly translate to higher catalytic activity, selectivity, and stability in electrolytic processes.
The primary objective of incorporating nanomaterials in electrolytic cells is to overcome the limitations of conventional catalysts. Traditional catalysts often suffer from low efficiency, poor durability, and high costs. Nanomaterials aim to address these challenges by offering superior catalytic performance while potentially reducing the overall material costs.
Key technological goals in this field include developing nanomaterials with tailored electronic structures, optimizing their morphology and composition, and enhancing their stability under harsh operating conditions. Researchers are also focused on scaling up the production of these nanomaterials to meet industrial demands while maintaining their unique properties.
The evolution of nanomaterial electrocatalysis has been marked by several milestones. These include the development of carbon nanotubes and graphene-based catalysts, the synthesis of metal nanoparticles with controlled size and shape, and the creation of hybrid nanostructures combining multiple materials for synergistic effects.
Looking ahead, the field of nanomaterial electrocatalysis is poised for further advancements. Emerging trends include the exploration of single-atom catalysts, the development of 2D materials beyond graphene, and the integration of artificial intelligence for rational design of nanomaterials. These innovations are expected to push the boundaries of efficiency in electrolytic cells, opening new avenues for sustainable energy production and chemical synthesis.
The development of nanomaterials for electrocatalysis can be traced back to the early 2000s when researchers began exploring the unique properties of materials at the nanoscale. Since then, the field has witnessed exponential growth, driven by the increasing demand for clean energy solutions and the need to optimize industrial processes.
Nanomaterials, characterized by their extremely small size and high surface-to-volume ratio, offer several advantages in electrocatalysis. They provide increased active surface area, enhanced electron transfer kinetics, and improved mass transport properties. These characteristics directly translate to higher catalytic activity, selectivity, and stability in electrolytic processes.
The primary objective of incorporating nanomaterials in electrolytic cells is to overcome the limitations of conventional catalysts. Traditional catalysts often suffer from low efficiency, poor durability, and high costs. Nanomaterials aim to address these challenges by offering superior catalytic performance while potentially reducing the overall material costs.
Key technological goals in this field include developing nanomaterials with tailored electronic structures, optimizing their morphology and composition, and enhancing their stability under harsh operating conditions. Researchers are also focused on scaling up the production of these nanomaterials to meet industrial demands while maintaining their unique properties.
The evolution of nanomaterial electrocatalysis has been marked by several milestones. These include the development of carbon nanotubes and graphene-based catalysts, the synthesis of metal nanoparticles with controlled size and shape, and the creation of hybrid nanostructures combining multiple materials for synergistic effects.
Looking ahead, the field of nanomaterial electrocatalysis is poised for further advancements. Emerging trends include the exploration of single-atom catalysts, the development of 2D materials beyond graphene, and the integration of artificial intelligence for rational design of nanomaterials. These innovations are expected to push the boundaries of efficiency in electrolytic cells, opening new avenues for sustainable energy production and chemical synthesis.
Market Demand for Efficient Electrolytic Processes
The demand for efficient electrolytic processes has been steadily increasing across various industries, driven by the need for sustainable and cost-effective production methods. Electrolytic cells play a crucial role in numerous applications, including water treatment, metal production, and energy storage systems. As global environmental concerns grow and energy costs rise, there is a pressing need for more efficient electrolytic processes that can reduce energy consumption and improve overall productivity.
In the water treatment sector, the market for electrolytic cells is expanding rapidly due to increasing water scarcity and stricter regulations on water quality. Municipalities and industrial facilities are seeking advanced electrolytic technologies to remove contaminants and purify water more effectively. This trend is particularly pronounced in developing countries where access to clean water remains a significant challenge.
The metal production industry, especially in aluminum and copper manufacturing, is another key driver of demand for efficient electrolytic processes. As the global demand for these metals continues to grow, manufacturers are under pressure to reduce energy costs and minimize environmental impact. Improved electrolytic cell efficiency can lead to substantial savings in both operational expenses and carbon emissions.
Energy storage systems, particularly those related to renewable energy sources, represent a burgeoning market for advanced electrolytic technologies. The intermittent nature of solar and wind power necessitates efficient energy storage solutions, with hydrogen production through water electrolysis emerging as a promising option. As countries worldwide commit to reducing carbon emissions, the demand for green hydrogen production is expected to soar, further driving the need for highly efficient electrolytic cells.
The chemical industry is also experiencing increased demand for efficient electrolytic processes in the production of various chemicals, including chlorine, sodium hydroxide, and hydrogen peroxide. These chemicals are essential in numerous applications, from pharmaceuticals to plastics manufacturing. Improved electrolytic efficiency can significantly reduce production costs and environmental impact in these sectors.
Furthermore, the growing focus on circular economy principles is creating new opportunities for electrolytic processes in recycling and waste management. Advanced electrolytic technologies are being explored for the recovery of valuable materials from electronic waste, industrial effluents, and other waste streams, presenting a potentially lucrative market for innovative solutions.
As industries strive to meet sustainability goals and reduce operational costs, the market demand for efficient electrolytic processes continues to expand. This trend is further reinforced by government initiatives and regulations promoting clean technologies and energy efficiency. Consequently, there is a significant opportunity for nanomaterial-enhanced electrolytic cells to address these market needs and revolutionize various industrial processes.
In the water treatment sector, the market for electrolytic cells is expanding rapidly due to increasing water scarcity and stricter regulations on water quality. Municipalities and industrial facilities are seeking advanced electrolytic technologies to remove contaminants and purify water more effectively. This trend is particularly pronounced in developing countries where access to clean water remains a significant challenge.
The metal production industry, especially in aluminum and copper manufacturing, is another key driver of demand for efficient electrolytic processes. As the global demand for these metals continues to grow, manufacturers are under pressure to reduce energy costs and minimize environmental impact. Improved electrolytic cell efficiency can lead to substantial savings in both operational expenses and carbon emissions.
Energy storage systems, particularly those related to renewable energy sources, represent a burgeoning market for advanced electrolytic technologies. The intermittent nature of solar and wind power necessitates efficient energy storage solutions, with hydrogen production through water electrolysis emerging as a promising option. As countries worldwide commit to reducing carbon emissions, the demand for green hydrogen production is expected to soar, further driving the need for highly efficient electrolytic cells.
The chemical industry is also experiencing increased demand for efficient electrolytic processes in the production of various chemicals, including chlorine, sodium hydroxide, and hydrogen peroxide. These chemicals are essential in numerous applications, from pharmaceuticals to plastics manufacturing. Improved electrolytic efficiency can significantly reduce production costs and environmental impact in these sectors.
Furthermore, the growing focus on circular economy principles is creating new opportunities for electrolytic processes in recycling and waste management. Advanced electrolytic technologies are being explored for the recovery of valuable materials from electronic waste, industrial effluents, and other waste streams, presenting a potentially lucrative market for innovative solutions.
As industries strive to meet sustainability goals and reduce operational costs, the market demand for efficient electrolytic processes continues to expand. This trend is further reinforced by government initiatives and regulations promoting clean technologies and energy efficiency. Consequently, there is a significant opportunity for nanomaterial-enhanced electrolytic cells to address these market needs and revolutionize various industrial processes.
Current Challenges in Electrolytic Cell Efficiency
Electrolytic cells play a crucial role in various industrial processes, yet they face significant challenges in terms of efficiency. One of the primary issues is the high energy consumption required for electrolysis, which often results in substantial operational costs. This inefficiency is largely attributed to the resistance encountered during ion transport and electron transfer processes within the cell.
Another major challenge is the degradation of electrode materials over time. The harsh chemical environment and continuous electrochemical reactions lead to corrosion and fouling of electrodes, reducing their performance and lifespan. This necessitates frequent maintenance and replacement, further increasing operational expenses and downtime.
The formation of gas bubbles on electrode surfaces presents an additional obstacle to efficiency. These bubbles create a barrier that impedes the contact between the electrolyte and the electrode, effectively reducing the active surface area and limiting reaction rates. This phenomenon, known as bubble overpotential, contributes significantly to overall cell inefficiency.
Concentration polarization is yet another factor hampering electrolytic cell performance. As reactions proceed, the concentration of reactants near the electrode surface becomes depleted, while products accumulate. This concentration gradient leads to increased overpotential and reduced reaction rates, ultimately diminishing overall cell efficiency.
The challenge of achieving uniform current distribution across electrode surfaces further compounds efficiency issues. Non-uniform current distribution results in localized hot spots and areas of reduced activity, leading to uneven electrode wear and suboptimal utilization of the electrode surface area.
Temperature control presents an ongoing challenge in electrolytic cells. Many electrochemical processes are sensitive to temperature fluctuations, which can affect reaction kinetics, mass transport, and even the stability of electrode materials. Maintaining optimal temperature conditions throughout the cell is often difficult, particularly in large-scale industrial applications.
Lastly, the selectivity of electrolytic processes remains a significant hurdle. Unwanted side reactions can occur, consuming energy and reducing the yield of desired products. Improving reaction selectivity while maintaining high efficiency is a complex balancing act that continues to challenge researchers and engineers in the field.
Addressing these challenges is crucial for advancing electrolytic cell technology and improving the efficiency of numerous industrial processes. The integration of nanomaterials offers promising avenues for overcoming these limitations, potentially revolutionizing the field of electrolysis.
Another major challenge is the degradation of electrode materials over time. The harsh chemical environment and continuous electrochemical reactions lead to corrosion and fouling of electrodes, reducing their performance and lifespan. This necessitates frequent maintenance and replacement, further increasing operational expenses and downtime.
The formation of gas bubbles on electrode surfaces presents an additional obstacle to efficiency. These bubbles create a barrier that impedes the contact between the electrolyte and the electrode, effectively reducing the active surface area and limiting reaction rates. This phenomenon, known as bubble overpotential, contributes significantly to overall cell inefficiency.
Concentration polarization is yet another factor hampering electrolytic cell performance. As reactions proceed, the concentration of reactants near the electrode surface becomes depleted, while products accumulate. This concentration gradient leads to increased overpotential and reduced reaction rates, ultimately diminishing overall cell efficiency.
The challenge of achieving uniform current distribution across electrode surfaces further compounds efficiency issues. Non-uniform current distribution results in localized hot spots and areas of reduced activity, leading to uneven electrode wear and suboptimal utilization of the electrode surface area.
Temperature control presents an ongoing challenge in electrolytic cells. Many electrochemical processes are sensitive to temperature fluctuations, which can affect reaction kinetics, mass transport, and even the stability of electrode materials. Maintaining optimal temperature conditions throughout the cell is often difficult, particularly in large-scale industrial applications.
Lastly, the selectivity of electrolytic processes remains a significant hurdle. Unwanted side reactions can occur, consuming energy and reducing the yield of desired products. Improving reaction selectivity while maintaining high efficiency is a complex balancing act that continues to challenge researchers and engineers in the field.
Addressing these challenges is crucial for advancing electrolytic cell technology and improving the efficiency of numerous industrial processes. The integration of nanomaterials offers promising avenues for overcoming these limitations, potentially revolutionizing the field of electrolysis.
Existing Nanomaterial Solutions for Electrolytic Cells
01 Nanomaterial synthesis and processing
Advanced techniques for synthesizing and processing nanomaterials to enhance their efficiency. This includes methods for controlling particle size, shape, and composition to optimize performance in various applications.- Nanomaterial synthesis and optimization: Advanced techniques for synthesizing and optimizing nanomaterials to enhance their efficiency in various applications. This includes methods for controlling particle size, shape, and composition to achieve desired properties and performance characteristics.
- Energy-related nanomaterial applications: Development of nanomaterials for energy-related applications, such as improved battery technologies, solar cells, and energy storage systems. These materials offer enhanced efficiency in energy conversion, storage, and utilization.
- Nanomaterials in environmental remediation: Utilization of nanomaterials for environmental remediation and pollution control. This includes the development of nanoparticles and nanostructures for water purification, air filtration, and soil decontamination, offering improved efficiency over conventional methods.
- Nanocomposites for enhanced material properties: Creation of nanocomposites by incorporating nanomaterials into bulk materials to enhance their mechanical, thermal, and electrical properties. This results in materials with improved strength, conductivity, and durability for various industrial applications.
- Nanomaterial-based catalysts: Development of highly efficient catalysts using nanomaterials, offering increased surface area and reactivity. These nanocatalysts improve the efficiency of chemical reactions in industrial processes, reducing energy consumption and waste production.
02 Energy-related nanomaterial applications
Utilization of nanomaterials in energy-related applications to improve efficiency. This covers areas such as energy storage, conversion, and harvesting, including battery technologies and solar cells.Expand Specific Solutions03 Nanomaterial-enhanced catalysis
Development of nanomaterial-based catalysts to increase reaction efficiency and selectivity. This includes the design of nanostructured catalysts for various industrial processes and environmental applications.Expand Specific Solutions04 Nanocomposites for improved material properties
Integration of nanomaterials into composite structures to enhance mechanical, thermal, and electrical properties. This approach leads to the development of high-performance materials with improved efficiency in various applications.Expand Specific Solutions05 Nanomaterial-based filtration and purification
Application of nanomaterials in filtration and purification processes to increase efficiency. This includes the development of nanostructured membranes and adsorbents for water treatment, gas separation, and other purification applications.Expand Specific Solutions
Key Players in Nanomaterial Electrocatalysis
The nanomaterials market for enhancing efficiency in electrolytic cells is in a growth phase, driven by increasing demand for energy-efficient solutions. The market size is expanding rapidly, with projections indicating significant growth in the coming years. Technologically, the field is advancing quickly, with companies like Robert Bosch GmbH, Panasonic Holdings Corp., and DENSO Corp. leading innovation. These firms are developing cutting-edge nanomaterials and integrating them into electrolytic cell designs, improving efficiency and performance. Academic institutions such as Cornell University and the Indian Institute of Science are also contributing to technological advancements through research collaborations with industry partners, further accelerating the maturation of this technology.
Indian Institute of Science
Technical Solution: The Indian Institute of Science (IISc) has made notable contributions to enhancing electrolytic cell efficiency through nanomaterial research. Their approach involves developing novel nanocomposite electrodes for various electrochemical applications. IISc researchers have successfully synthesized graphene-metal oxide nanocomposites that exhibit superior electrocatalytic properties for both the hydrogen evolution reaction (HER) and oxygen evolution reaction (OER)[8]. These nanocomposites benefit from the high conductivity of graphene and the catalytic activity of metal oxides. Additionally, the institute has explored the use of transition metal dichalcogenide (TMD) nanosheets as efficient electrocatalysts, demonstrating their potential in replacing precious metal catalysts[9]. IISc has also developed innovative methods for creating three-dimensional nanostructured electrodes, which significantly increase the active surface area and enhance mass transport in electrolytic cells[10].
Strengths: Cost-effective materials, high catalytic activity, and innovative nanostructure designs. Weaknesses: Potential challenges in long-term stability and scalability of nanocomposite electrodes.
Centre National de la Recherche Scientifique
Technical Solution: The Centre National de la Recherche Scientifique (CNRS) has developed innovative nanomaterial-based electrocatalysts to enhance the efficiency of electrolytic cells. Their approach involves using graphene-supported metal nanoparticles as electrodes, which significantly increases the active surface area and catalytic activity[1]. The CNRS team has also explored the use of metal-organic frameworks (MOFs) as precursors for highly efficient oxygen evolution reaction (OER) catalysts in water splitting[2]. These nanomaterial-based catalysts have shown remarkable improvements in current density and lower overpotentials compared to traditional catalysts[3]. Additionally, CNRS researchers have developed novel nanostructured electrodes using atomic layer deposition (ALD) techniques, allowing precise control over the catalyst composition and structure at the nanoscale[4].
Strengths: High catalytic activity, increased surface area, and precise control over nanostructures. Weaknesses: Potential high costs of nanomaterial production and scalability challenges for industrial applications.
Core Innovations in Nanomaterial Electrocatalysts
Nanomaterial compositions and methods of making the same
PatentWO2023102234A1
Innovation
- The development of an electrospun nonwoven material composition with a sacrificial polymer and dispersed ion species, which upon decomposition, forms nanoparticles with a narrow size distribution and improved crystallinity, enhancing electrochemical performance by creating a high surface area and controlled inter-fiber void volume.
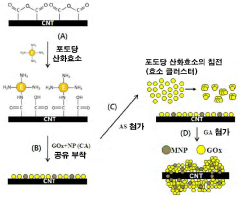
Electrolytic Material Including Minute Tube Accumulated Enzyme and Magnetic Nanoparticle and Switchable Biosensor and Biofuelcell Using the Same
PatentActiveKR1020110137756A
Innovation
- Integration of enzymes and magnetic nanoparticles into microtubes, allowing control through magnetic force for on-off switching, using methods like streptavidin-biotin binding and cross-linking with amine-linked bifunctional compounds.
Environmental Impact of Nanomaterial Electrocatalysts
The use of nanomaterial electrocatalysts in electrolytic cells has shown promising results in enhancing efficiency, but their environmental impact must be carefully considered. These materials, while offering significant performance improvements, may pose potential risks to ecosystems and human health if not properly managed throughout their lifecycle.
One of the primary environmental concerns is the potential release of nanoparticles into aquatic systems. During the operation or disposal of electrolytic cells, nanomaterials may leach into water bodies, potentially affecting aquatic organisms. Studies have shown that certain nanoparticles can accumulate in fish and other aquatic life, potentially disrupting food chains and ecosystems.
Air pollution is another consideration, as the production and handling of nanomaterials may release particles into the atmosphere. These airborne nanoparticles could have respiratory impacts on both humans and animals, particularly in areas surrounding manufacturing facilities or waste disposal sites.
The long-term persistence of nanomaterials in the environment is a subject of ongoing research. Some nanomaterials may degrade slowly, leading to potential bioaccumulation in various organisms over time. This persistence could have unforeseen consequences on biodiversity and ecosystem balance in the long run.
However, it's important to note that nanomaterial electrocatalysts also offer potential environmental benefits. Their enhanced efficiency in electrolytic processes can lead to reduced energy consumption and lower overall environmental footprint of industrial processes. This improved efficiency may result in decreased greenhouse gas emissions and reduced demand for raw materials.
Efforts are being made to develop more environmentally friendly nanomaterials and improve their lifecycle management. Green synthesis methods, using less toxic precursors and environmentally benign solvents, are being explored to minimize the environmental impact of nanomaterial production.
Proper disposal and recycling protocols for nanomaterial-containing devices are crucial to mitigate environmental risks. Research is ongoing to develop effective methods for recovering and recycling nanomaterials from spent electrolytic cells, which could significantly reduce their environmental impact and promote a circular economy approach.
Regulatory bodies worldwide are working on developing guidelines and standards for the safe use and disposal of nanomaterials. These efforts aim to balance the technological benefits of nanomaterial electrocatalysts with environmental protection, ensuring sustainable development in this field.
One of the primary environmental concerns is the potential release of nanoparticles into aquatic systems. During the operation or disposal of electrolytic cells, nanomaterials may leach into water bodies, potentially affecting aquatic organisms. Studies have shown that certain nanoparticles can accumulate in fish and other aquatic life, potentially disrupting food chains and ecosystems.
Air pollution is another consideration, as the production and handling of nanomaterials may release particles into the atmosphere. These airborne nanoparticles could have respiratory impacts on both humans and animals, particularly in areas surrounding manufacturing facilities or waste disposal sites.
The long-term persistence of nanomaterials in the environment is a subject of ongoing research. Some nanomaterials may degrade slowly, leading to potential bioaccumulation in various organisms over time. This persistence could have unforeseen consequences on biodiversity and ecosystem balance in the long run.
However, it's important to note that nanomaterial electrocatalysts also offer potential environmental benefits. Their enhanced efficiency in electrolytic processes can lead to reduced energy consumption and lower overall environmental footprint of industrial processes. This improved efficiency may result in decreased greenhouse gas emissions and reduced demand for raw materials.
Efforts are being made to develop more environmentally friendly nanomaterials and improve their lifecycle management. Green synthesis methods, using less toxic precursors and environmentally benign solvents, are being explored to minimize the environmental impact of nanomaterial production.
Proper disposal and recycling protocols for nanomaterial-containing devices are crucial to mitigate environmental risks. Research is ongoing to develop effective methods for recovering and recycling nanomaterials from spent electrolytic cells, which could significantly reduce their environmental impact and promote a circular economy approach.
Regulatory bodies worldwide are working on developing guidelines and standards for the safe use and disposal of nanomaterials. These efforts aim to balance the technological benefits of nanomaterial electrocatalysts with environmental protection, ensuring sustainable development in this field.
Scalability and Cost Analysis of Nanomaterial Solutions
The scalability and cost analysis of nanomaterial solutions for enhancing efficiency in electrolytic cells is a critical factor in determining their practical implementation and commercial viability. As the demand for more efficient and sustainable energy technologies grows, the ability to scale up nanomaterial production and integrate them into existing electrolytic systems becomes paramount.
One of the primary challenges in scaling nanomaterial solutions is the complexity of their synthesis processes. Many nanomaterials require precise control over reaction conditions, which can be difficult to maintain at larger scales. For instance, the production of carbon nanotubes or graphene often involves high-temperature processes or chemical vapor deposition techniques that are energy-intensive and may not be easily adaptable to industrial-scale production.
The cost of raw materials is another significant consideration. While some nanomaterials can be synthesized from relatively inexpensive precursors, others may require rare or expensive elements. For example, platinum-based nanoparticles, which are highly effective catalysts in electrolytic cells, face scalability issues due to the scarcity and high cost of platinum.
However, recent advancements in nanomaterial synthesis techniques offer promising solutions for scalability. Continuous flow reactors and microfluidic systems have shown potential for producing nanomaterials at larger scales while maintaining precise control over particle size and morphology. These methods could significantly reduce production costs and increase output volumes.
The integration of nanomaterials into existing electrolytic cell designs also presents challenges. While nanomaterials can dramatically enhance efficiency, their incorporation may require modifications to current manufacturing processes. This could necessitate substantial initial investments in equipment and training, which must be factored into the overall cost analysis.
Long-term stability and durability of nanomaterials in operational conditions are crucial factors affecting both scalability and cost-effectiveness. Nanomaterials that degrade quickly or lose their enhanced properties over time may require frequent replacement, potentially offsetting their initial efficiency gains. Research into protective coatings and stabilization techniques is ongoing to address these concerns.
Environmental and safety considerations also play a role in the scalability of nanomaterial solutions. As production volumes increase, the potential environmental impact and worker safety issues must be carefully evaluated. Implementing necessary safeguards and compliance measures could add to the overall cost but is essential for sustainable scaling.
Despite these challenges, the potential benefits of nanomaterial-enhanced electrolytic cells in terms of increased efficiency and reduced energy consumption could justify the initial investments required for scaling. As production techniques improve and economies of scale come into play, the cost-effectiveness of these solutions is likely to improve, making them more attractive for widespread adoption in various industrial applications.
One of the primary challenges in scaling nanomaterial solutions is the complexity of their synthesis processes. Many nanomaterials require precise control over reaction conditions, which can be difficult to maintain at larger scales. For instance, the production of carbon nanotubes or graphene often involves high-temperature processes or chemical vapor deposition techniques that are energy-intensive and may not be easily adaptable to industrial-scale production.
The cost of raw materials is another significant consideration. While some nanomaterials can be synthesized from relatively inexpensive precursors, others may require rare or expensive elements. For example, platinum-based nanoparticles, which are highly effective catalysts in electrolytic cells, face scalability issues due to the scarcity and high cost of platinum.
However, recent advancements in nanomaterial synthesis techniques offer promising solutions for scalability. Continuous flow reactors and microfluidic systems have shown potential for producing nanomaterials at larger scales while maintaining precise control over particle size and morphology. These methods could significantly reduce production costs and increase output volumes.
The integration of nanomaterials into existing electrolytic cell designs also presents challenges. While nanomaterials can dramatically enhance efficiency, their incorporation may require modifications to current manufacturing processes. This could necessitate substantial initial investments in equipment and training, which must be factored into the overall cost analysis.
Long-term stability and durability of nanomaterials in operational conditions are crucial factors affecting both scalability and cost-effectiveness. Nanomaterials that degrade quickly or lose their enhanced properties over time may require frequent replacement, potentially offsetting their initial efficiency gains. Research into protective coatings and stabilization techniques is ongoing to address these concerns.
Environmental and safety considerations also play a role in the scalability of nanomaterial solutions. As production volumes increase, the potential environmental impact and worker safety issues must be carefully evaluated. Implementing necessary safeguards and compliance measures could add to the overall cost but is essential for sustainable scaling.
Despite these challenges, the potential benefits of nanomaterial-enhanced electrolytic cells in terms of increased efficiency and reduced energy consumption could justify the initial investments required for scaling. As production techniques improve and economies of scale come into play, the cost-effectiveness of these solutions is likely to improve, making them more attractive for widespread adoption in various industrial applications.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!