How to Achieve Consistency in Hydrochloric Acid Production?
JUL 1, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
HCl Production Background and Objectives
Hydrochloric acid (HCl) production has been a cornerstone of the chemical industry for over a century, playing a crucial role in various industrial processes and applications. The journey of HCl production began in the early 19th century with the Leblanc process, which produced HCl as a byproduct of soda ash manufacturing. As industrial needs evolved, so did the methods of HCl production, leading to the development of more efficient and environmentally friendly processes.
The primary objective in modern HCl production is to achieve consistency in both quality and quantity. This goal is driven by the increasing demand for high-purity HCl in semiconductor manufacturing, pharmaceutical production, and water treatment industries. Consistency in production ensures that end-users receive a reliable product that meets their specific requirements, which is critical for maintaining product quality and process efficiency in various applications.
The evolution of HCl production technology has been marked by several key milestones. The introduction of the chlor-alkali process in the early 20th century revolutionized HCl production by providing a more direct and efficient method. This was followed by the development of the burner process, which allowed for the direct synthesis of HCl from hydrogen and chlorine gases. More recently, advancements in process control and automation have further enhanced the ability to maintain consistent production parameters.
Current technological trends in HCl production focus on improving process efficiency, reducing environmental impact, and enhancing product purity. These trends include the development of more efficient catalysts for the burner process, implementation of advanced process control systems, and the integration of real-time monitoring technologies. Additionally, there is a growing emphasis on recycling and recovering HCl from waste streams, aligning with broader sustainability goals in the chemical industry.
The quest for consistency in HCl production faces several challenges. These include fluctuations in raw material quality, variations in process conditions, and the need for precise control over reaction parameters. Overcoming these challenges requires a multifaceted approach, combining advanced process control technologies, robust quality management systems, and continuous improvement initiatives.
Looking ahead, the future of HCl production is likely to be shaped by emerging technologies such as artificial intelligence and machine learning for process optimization, as well as the development of novel production methods that offer greater control over product consistency. The industry is also exploring ways to integrate HCl production with other chemical processes to create more efficient and sustainable manufacturing ecosystems.
The primary objective in modern HCl production is to achieve consistency in both quality and quantity. This goal is driven by the increasing demand for high-purity HCl in semiconductor manufacturing, pharmaceutical production, and water treatment industries. Consistency in production ensures that end-users receive a reliable product that meets their specific requirements, which is critical for maintaining product quality and process efficiency in various applications.
The evolution of HCl production technology has been marked by several key milestones. The introduction of the chlor-alkali process in the early 20th century revolutionized HCl production by providing a more direct and efficient method. This was followed by the development of the burner process, which allowed for the direct synthesis of HCl from hydrogen and chlorine gases. More recently, advancements in process control and automation have further enhanced the ability to maintain consistent production parameters.
Current technological trends in HCl production focus on improving process efficiency, reducing environmental impact, and enhancing product purity. These trends include the development of more efficient catalysts for the burner process, implementation of advanced process control systems, and the integration of real-time monitoring technologies. Additionally, there is a growing emphasis on recycling and recovering HCl from waste streams, aligning with broader sustainability goals in the chemical industry.
The quest for consistency in HCl production faces several challenges. These include fluctuations in raw material quality, variations in process conditions, and the need for precise control over reaction parameters. Overcoming these challenges requires a multifaceted approach, combining advanced process control technologies, robust quality management systems, and continuous improvement initiatives.
Looking ahead, the future of HCl production is likely to be shaped by emerging technologies such as artificial intelligence and machine learning for process optimization, as well as the development of novel production methods that offer greater control over product consistency. The industry is also exploring ways to integrate HCl production with other chemical processes to create more efficient and sustainable manufacturing ecosystems.
Market Analysis for HCl Demand
The global hydrochloric acid (HCl) market has shown steady growth in recent years, driven by increasing demand across various industries. The market size was valued at approximately 39.3 million metric tons in 2020 and is projected to reach 46.2 million metric tons by 2027, growing at a compound annual growth rate (CAGR) of 2.5% during the forecast period.
The demand for HCl is primarily fueled by its widespread applications in diverse sectors. The chemical industry remains the largest consumer, utilizing HCl in the production of various chemicals, including vinyl chloride, chlorinated solvents, and other organic compounds. The steel industry also contributes significantly to HCl demand, employing it in steel pickling processes to remove rust and scale from metal surfaces.
In the oil and gas sector, HCl plays a crucial role in well acidizing and hydraulic fracturing operations, enhancing oil and gas recovery rates. The growing exploration and production activities in unconventional oil and gas reserves are expected to drive HCl demand further in this sector.
The pharmaceutical industry represents another key market for HCl, where it is used in drug manufacturing processes and as a reagent in various chemical reactions. With the expansion of the global pharmaceutical market, particularly in emerging economies, the demand for HCl in this sector is anticipated to increase.
Geographically, Asia-Pacific dominates the HCl market, accounting for over 40% of global consumption. China, in particular, is the largest producer and consumer of HCl, driven by its robust chemical and manufacturing industries. North America and Europe follow as significant markets, with steady demand from established industrial sectors.
Environmental regulations and sustainability concerns are influencing market dynamics. There is a growing emphasis on recycling and recovering HCl from industrial processes, which may impact the overall demand for virgin HCl. However, this trend also presents opportunities for innovative production methods that align with circular economy principles.
The market is characterized by the presence of both large multinational corporations and regional players. Key market participants are focusing on capacity expansion, technological advancements, and strategic partnerships to strengthen their market position and meet the growing demand for high-purity HCl in specialized applications.
The demand for HCl is primarily fueled by its widespread applications in diverse sectors. The chemical industry remains the largest consumer, utilizing HCl in the production of various chemicals, including vinyl chloride, chlorinated solvents, and other organic compounds. The steel industry also contributes significantly to HCl demand, employing it in steel pickling processes to remove rust and scale from metal surfaces.
In the oil and gas sector, HCl plays a crucial role in well acidizing and hydraulic fracturing operations, enhancing oil and gas recovery rates. The growing exploration and production activities in unconventional oil and gas reserves are expected to drive HCl demand further in this sector.
The pharmaceutical industry represents another key market for HCl, where it is used in drug manufacturing processes and as a reagent in various chemical reactions. With the expansion of the global pharmaceutical market, particularly in emerging economies, the demand for HCl in this sector is anticipated to increase.
Geographically, Asia-Pacific dominates the HCl market, accounting for over 40% of global consumption. China, in particular, is the largest producer and consumer of HCl, driven by its robust chemical and manufacturing industries. North America and Europe follow as significant markets, with steady demand from established industrial sectors.
Environmental regulations and sustainability concerns are influencing market dynamics. There is a growing emphasis on recycling and recovering HCl from industrial processes, which may impact the overall demand for virgin HCl. However, this trend also presents opportunities for innovative production methods that align with circular economy principles.
The market is characterized by the presence of both large multinational corporations and regional players. Key market participants are focusing on capacity expansion, technological advancements, and strategic partnerships to strengthen their market position and meet the growing demand for high-purity HCl in specialized applications.
Current Challenges in HCl Production Consistency
Achieving consistency in hydrochloric acid (HCl) production remains a significant challenge in the chemical industry. One of the primary issues is the variability in raw material quality, particularly in the chlorine gas and hydrogen used as feedstocks. Fluctuations in the purity and composition of these inputs can lead to inconsistencies in the final HCl product, affecting its concentration and overall quality.
Process control presents another major hurdle. Maintaining precise temperature and pressure conditions throughout the synthesis reaction is crucial for consistent HCl production. However, industrial-scale reactors often face difficulties in achieving uniform heat distribution and pressure regulation, leading to variations in reaction rates and product characteristics across different batches.
Equipment wear and corrosion pose ongoing challenges to production consistency. The highly corrosive nature of HCl necessitates the use of specialized materials and equipment. Over time, degradation of reactor linings, piping, and storage tanks can introduce contaminants into the product and alter reaction conditions, compromising consistency.
Absorption efficiency in the production process is another critical factor affecting consistency. The effectiveness of dissolving hydrogen chloride gas in water to form aqueous HCl can vary due to factors such as water quality, gas flow rates, and absorption tower design. Inconsistencies in this stage can result in fluctuations in acid concentration and purity.
Environmental factors, including ambient temperature and humidity, can impact production consistency, especially in facilities without adequate climate control. These external variables can affect reaction kinetics and absorption rates, leading to variations in product quality across different production runs.
Analytical and quality control methods present their own set of challenges. Ensuring accurate and consistent measurement of HCl concentration and impurities is essential for maintaining product consistency. However, variations in sampling techniques, analytical equipment calibration, and human error can introduce discrepancies in quality assessment.
Lastly, the demand for increased production rates to meet market needs often conflicts with the goal of maintaining consistency. Accelerated production schedules may lead to compromises in process control and quality assurance measures, potentially resulting in greater variability in the final product.
Addressing these challenges requires a multifaceted approach, combining advanced process control technologies, improved equipment design, enhanced analytical methods, and rigorous quality management systems. Overcoming these obstacles is crucial for meeting the stringent quality requirements of various industries that rely on consistent, high-purity hydrochloric acid.
Process control presents another major hurdle. Maintaining precise temperature and pressure conditions throughout the synthesis reaction is crucial for consistent HCl production. However, industrial-scale reactors often face difficulties in achieving uniform heat distribution and pressure regulation, leading to variations in reaction rates and product characteristics across different batches.
Equipment wear and corrosion pose ongoing challenges to production consistency. The highly corrosive nature of HCl necessitates the use of specialized materials and equipment. Over time, degradation of reactor linings, piping, and storage tanks can introduce contaminants into the product and alter reaction conditions, compromising consistency.
Absorption efficiency in the production process is another critical factor affecting consistency. The effectiveness of dissolving hydrogen chloride gas in water to form aqueous HCl can vary due to factors such as water quality, gas flow rates, and absorption tower design. Inconsistencies in this stage can result in fluctuations in acid concentration and purity.
Environmental factors, including ambient temperature and humidity, can impact production consistency, especially in facilities without adequate climate control. These external variables can affect reaction kinetics and absorption rates, leading to variations in product quality across different production runs.
Analytical and quality control methods present their own set of challenges. Ensuring accurate and consistent measurement of HCl concentration and impurities is essential for maintaining product consistency. However, variations in sampling techniques, analytical equipment calibration, and human error can introduce discrepancies in quality assessment.
Lastly, the demand for increased production rates to meet market needs often conflicts with the goal of maintaining consistency. Accelerated production schedules may lead to compromises in process control and quality assurance measures, potentially resulting in greater variability in the final product.
Addressing these challenges requires a multifaceted approach, combining advanced process control technologies, improved equipment design, enhanced analytical methods, and rigorous quality management systems. Overcoming these obstacles is crucial for meeting the stringent quality requirements of various industries that rely on consistent, high-purity hydrochloric acid.
Existing Methods for HCl Consistency Control
01 Hydrochloric acid production methods
Various methods are employed to produce hydrochloric acid with consistent quality. These methods include the reaction of hydrogen and chlorine gases, the chlorination of organic compounds, and the treatment of metal chlorides with sulfuric acid. The production processes are optimized to ensure uniform acid concentration and purity.- Hydrochloric acid production methods: Various methods are employed to produce hydrochloric acid with consistent quality. These methods may include the reaction of hydrogen and chlorine gases, the chlorination of organic compounds, or the treatment of metal chlorides with sulfuric acid. The production processes are optimized to ensure uniform concentration and purity of the resulting hydrochloric acid.
- Concentration control techniques: Maintaining consistent hydrochloric acid concentration is crucial for many industrial applications. Techniques such as continuous monitoring, automated dilution systems, and precise mixing processes are employed to control and adjust the acid concentration. These methods ensure that the hydrochloric acid maintains its desired strength and properties throughout its production and use.
- Storage and handling solutions: Proper storage and handling of hydrochloric acid are essential for maintaining its consistency. Specialized containers, storage tanks, and transportation systems are designed to prevent contamination, evaporation, or degradation of the acid. These solutions may include corrosion-resistant materials, temperature-controlled environments, and safety features to ensure the acid's stability and quality over time.
- Quality control and testing methods: To ensure hydrochloric acid consistency, various quality control and testing methods are implemented. These may include spectroscopic analysis, titration techniques, and automated monitoring systems. Regular testing and documentation of acid properties such as concentration, purity, and contaminant levels help maintain consistent quality across batches and production runs.
- Stabilization and additive technologies: Additives and stabilization technologies are used to enhance the consistency of hydrochloric acid. These may include inhibitors to prevent corrosion, stabilizers to maintain concentration, or additives to improve specific properties. Such technologies help in maintaining the acid's characteristics during storage, transportation, and application, ensuring consistent performance across various industrial processes.
02 Concentration control techniques
Maintaining consistent hydrochloric acid concentration is crucial for many industrial applications. Techniques such as continuous monitoring, automated dilution systems, and precise mixing processes are used to control and adjust the acid concentration. These methods help achieve the desired consistency in acid strength for various manufacturing processes.Expand Specific Solutions03 Storage and handling solutions
Proper storage and handling of hydrochloric acid are essential to maintain its consistency. Specialized containers, corrosion-resistant materials, and temperature-controlled environments are used to prevent degradation and ensure stability. Advanced storage systems may include agitation mechanisms to prevent stratification and maintain uniform concentration.Expand Specific Solutions04 Quality control and testing methods
To ensure hydrochloric acid consistency, various quality control and testing methods are employed. These include titration, spectrophotometry, and conductivity measurements. Automated inline analysis systems are also used for real-time monitoring of acid properties, allowing for immediate adjustments to maintain consistency.Expand Specific Solutions05 Purification and stabilization techniques
Purification and stabilization techniques are crucial for maintaining hydrochloric acid consistency. These may include distillation, membrane separation, and the use of stabilizing additives. Advanced purification methods help remove impurities and ensure uniform acid composition, while stabilizers prevent degradation and maintain consistent properties over time.Expand Specific Solutions
Key Players in HCl Industry
The hydrochloric acid production market is in a mature stage, with a global market size estimated to be in the billions of dollars. The technology for producing hydrochloric acid is well-established, but achieving consistency remains a challenge due to variations in raw materials and process conditions. Key players in this field include BASF Corp., Covestro Deutschland AG, and Shin-Etsu Chemical Co., Ltd., who are focusing on improving process control and automation to enhance consistency. Emerging technologies, such as advanced sensors and AI-driven process optimization, are being explored by companies like Taiwan Semiconductor Manufacturing Co., Ltd. to further refine production methods and ensure consistent quality across batches.
BASF Corp.
Technical Solution: BASF has developed an innovative approach to achieve consistency in hydrochloric acid production through advanced process control and automation. Their system utilizes real-time monitoring of key parameters such as temperature, pressure, and reactant concentrations to maintain optimal reaction conditions[1]. The company employs a closed-loop control system that automatically adjusts process variables to compensate for fluctuations, ensuring a stable and consistent output. Additionally, BASF has implemented predictive modeling algorithms that anticipate potential deviations and proactively adjust the production process[3]. This technology allows for precise control of acid concentration and purity, meeting stringent quality standards across various industrial applications.
Strengths: High precision control, adaptability to varying input conditions, and consistent product quality. Weaknesses: High initial investment costs and complexity in implementation and maintenance.
Tokuyama Corp.
Technical Solution: Tokuyama Corporation has developed a proprietary membrane electrolysis technology for hydrochloric acid production, which ensures high consistency and purity. Their process utilizes ion-exchange membranes to separate chlorine and hydrogen gases produced during electrolysis, allowing for precise control of the reaction[2]. The company's system incorporates advanced electrode materials that enhance efficiency and reduce energy consumption. Tokuyama's technology also features a unique gas absorption system that optimizes the conversion of chlorine gas to hydrochloric acid, maintaining consistent concentration levels[5]. The process is further enhanced by a sophisticated control system that monitors and adjusts electrolysis parameters in real-time, ensuring stable and reproducible acid production.
Strengths: High purity output, energy efficiency, and precise concentration control. Weaknesses: Specialized equipment requirements and potential membrane degradation over time.
Innovative Technologies in HCl Production
Method for producing high-purity hydrochloric acid
PatentWO2001025144A1
Innovation
- A process involving heating hydrochloric acid with a hydrogen chloride content above 21% to pass through a retention column and demister made of fluorinated polyolefin, followed by absorption in ultrapure water, with the option to recycle the hydrogen chloride solution and adjust concentration using ultrapure water, while maintaining low flow resistance and constant pressure, utilizing components and storage tanks made of fluorinated polyolefin materials.
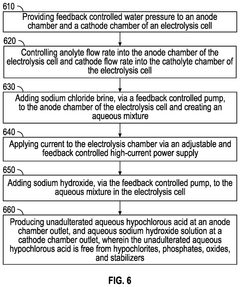
Smart tank predictive production feedback system and method
PatentPendingUS20250101603A1
Innovation
- A system for remote, predictive feedback-controlled production of pure HOCl using electrolysis, which continuously monitors and adjusts parameters like pH, ORP, and chlorine concentration, ensuring the production of HOCl free from hypochlorites and stabilizers, using feedback-controlled water pressure, electric current, and sodium chloride and hydroxide levels.
Environmental Impact of HCl Production
The production of hydrochloric acid (HCl) has significant environmental implications that must be carefully considered and managed. The primary environmental concerns associated with HCl production stem from potential air and water pollution, as well as the generation of hazardous waste. Air emissions from HCl production facilities often contain chlorine gas, hydrogen chloride, and other volatile compounds, which can contribute to air quality degradation and pose health risks to nearby communities if not properly controlled.
Water pollution is another critical environmental issue in HCl production. Wastewater from the manufacturing process may contain residual acid, dissolved metals, and other contaminants. If not adequately treated before discharge, these effluents can harm aquatic ecosystems and potentially contaminate groundwater resources. Additionally, the transportation and storage of HCl present risks of accidental spills or leaks, which can have severe environmental consequences, including soil contamination and damage to vegetation.
The energy-intensive nature of HCl production also contributes to its environmental footprint. The process typically requires substantial amounts of electricity and thermal energy, often derived from fossil fuels, leading to greenhouse gas emissions and exacerbating climate change concerns. Furthermore, the production of chlorine, a key raw material in HCl synthesis, involves energy-intensive electrolysis processes that further compound the environmental impact.
Waste management is another crucial aspect of HCl production's environmental impact. The process generates various waste streams, including spent catalysts, contaminated packaging materials, and by-products that may require special handling and disposal. Improper management of these wastes can lead to soil and water contamination, as well as potential long-term environmental liabilities.
To mitigate these environmental impacts, the HCl production industry has implemented various measures. These include advanced air pollution control technologies, such as scrubbers and absorption systems, to reduce emissions of chlorine and hydrogen chloride. Wastewater treatment facilities have been improved to ensure effluents meet stringent environmental standards before discharge. Many producers have also adopted closed-loop systems and recycling processes to minimize waste generation and resource consumption.
Efforts to improve energy efficiency in HCl production have led to the development of more sustainable manufacturing processes. Some facilities have implemented heat recovery systems and optimized reaction conditions to reduce energy consumption. Additionally, there is a growing trend towards using renewable energy sources to power HCl production plants, further reducing their carbon footprint.
As environmental regulations become increasingly stringent, HCl producers are investing in research and development to find more environmentally friendly production methods. This includes exploring alternative raw materials, developing catalysts that enable more efficient reactions, and investigating novel synthesis routes that minimize waste generation and energy consumption. These ongoing efforts aim to balance the industrial demand for HCl with the imperative of environmental protection, working towards a more sustainable future for the chemical industry.
Water pollution is another critical environmental issue in HCl production. Wastewater from the manufacturing process may contain residual acid, dissolved metals, and other contaminants. If not adequately treated before discharge, these effluents can harm aquatic ecosystems and potentially contaminate groundwater resources. Additionally, the transportation and storage of HCl present risks of accidental spills or leaks, which can have severe environmental consequences, including soil contamination and damage to vegetation.
The energy-intensive nature of HCl production also contributes to its environmental footprint. The process typically requires substantial amounts of electricity and thermal energy, often derived from fossil fuels, leading to greenhouse gas emissions and exacerbating climate change concerns. Furthermore, the production of chlorine, a key raw material in HCl synthesis, involves energy-intensive electrolysis processes that further compound the environmental impact.
Waste management is another crucial aspect of HCl production's environmental impact. The process generates various waste streams, including spent catalysts, contaminated packaging materials, and by-products that may require special handling and disposal. Improper management of these wastes can lead to soil and water contamination, as well as potential long-term environmental liabilities.
To mitigate these environmental impacts, the HCl production industry has implemented various measures. These include advanced air pollution control technologies, such as scrubbers and absorption systems, to reduce emissions of chlorine and hydrogen chloride. Wastewater treatment facilities have been improved to ensure effluents meet stringent environmental standards before discharge. Many producers have also adopted closed-loop systems and recycling processes to minimize waste generation and resource consumption.
Efforts to improve energy efficiency in HCl production have led to the development of more sustainable manufacturing processes. Some facilities have implemented heat recovery systems and optimized reaction conditions to reduce energy consumption. Additionally, there is a growing trend towards using renewable energy sources to power HCl production plants, further reducing their carbon footprint.
As environmental regulations become increasingly stringent, HCl producers are investing in research and development to find more environmentally friendly production methods. This includes exploring alternative raw materials, developing catalysts that enable more efficient reactions, and investigating novel synthesis routes that minimize waste generation and energy consumption. These ongoing efforts aim to balance the industrial demand for HCl with the imperative of environmental protection, working towards a more sustainable future for the chemical industry.
Quality Control and Safety Measures
Quality control and safety measures are paramount in achieving consistency in hydrochloric acid production. Implementing robust quality management systems is essential to ensure the uniformity and reliability of the final product. This involves establishing stringent specifications for raw materials, process parameters, and finished products. Regular testing and analysis of samples at various stages of production help maintain quality standards and detect any deviations promptly.
Statistical process control (SPC) techniques play a crucial role in monitoring and controlling the production process. By utilizing control charts and other statistical tools, manufacturers can identify trends, detect anomalies, and make data-driven decisions to optimize production consistency. Implementing automated process control systems further enhances the ability to maintain precise control over critical parameters such as temperature, pressure, and flow rates.
Proper equipment maintenance and calibration are vital for consistent production. Regular inspections, preventive maintenance schedules, and calibration of instruments ensure that all equipment operates within specified tolerances. This minimizes the risk of unexpected breakdowns or inaccuracies that could compromise product quality.
Safety measures are equally important in hydrochloric acid production due to the corrosive nature of the substance. Implementing comprehensive safety protocols, including proper personal protective equipment (PPE), emergency response procedures, and containment systems, is essential. Regular safety training for personnel and conducting risk assessments help mitigate potential hazards and ensure a safe working environment.
Environmental considerations are also crucial in maintaining consistency and safety. Implementing effective emission control systems and waste management practices helps minimize environmental impact and ensures compliance with regulatory requirements. This includes proper handling and disposal of by-products and waste materials generated during the production process.
Continuous improvement initiatives, such as Six Sigma methodologies, can be employed to identify and eliminate sources of variability in the production process. By systematically analyzing and optimizing each step of the production chain, manufacturers can achieve higher levels of consistency and quality in their hydrochloric acid output.
Documentation and traceability are key components of quality control. Maintaining detailed records of production parameters, test results, and any deviations or corrective actions taken provides valuable data for analysis and continuous improvement. Implementing a robust batch tracking system ensures that any quality issues can be quickly traced to their source and addressed effectively.
Human Resource Management plays a significant role in maintaining consistency and safety. Ensuring that operators and technicians are well-trained, competent, and motivated contributes to the overall quality and safety of the production process. Regular skill assessments, training programs, and clear standard operating procedures (SOPs) help maintain a high level of expertise among the workforce.
Statistical process control (SPC) techniques play a crucial role in monitoring and controlling the production process. By utilizing control charts and other statistical tools, manufacturers can identify trends, detect anomalies, and make data-driven decisions to optimize production consistency. Implementing automated process control systems further enhances the ability to maintain precise control over critical parameters such as temperature, pressure, and flow rates.
Proper equipment maintenance and calibration are vital for consistent production. Regular inspections, preventive maintenance schedules, and calibration of instruments ensure that all equipment operates within specified tolerances. This minimizes the risk of unexpected breakdowns or inaccuracies that could compromise product quality.
Safety measures are equally important in hydrochloric acid production due to the corrosive nature of the substance. Implementing comprehensive safety protocols, including proper personal protective equipment (PPE), emergency response procedures, and containment systems, is essential. Regular safety training for personnel and conducting risk assessments help mitigate potential hazards and ensure a safe working environment.
Environmental considerations are also crucial in maintaining consistency and safety. Implementing effective emission control systems and waste management practices helps minimize environmental impact and ensures compliance with regulatory requirements. This includes proper handling and disposal of by-products and waste materials generated during the production process.
Continuous improvement initiatives, such as Six Sigma methodologies, can be employed to identify and eliminate sources of variability in the production process. By systematically analyzing and optimizing each step of the production chain, manufacturers can achieve higher levels of consistency and quality in their hydrochloric acid output.
Documentation and traceability are key components of quality control. Maintaining detailed records of production parameters, test results, and any deviations or corrective actions taken provides valuable data for analysis and continuous improvement. Implementing a robust batch tracking system ensures that any quality issues can be quickly traced to their source and addressed effectively.
Human Resource Management plays a significant role in maintaining consistency and safety. Ensuring that operators and technicians are well-trained, competent, and motivated contributes to the overall quality and safety of the production process. Regular skill assessments, training programs, and clear standard operating procedures (SOPs) help maintain a high level of expertise among the workforce.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!