How to Adhere to Hydrofluoric Acid Safety Standards in Labs
AUG 25, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Hydrofluoric Acid Safety Background and Objectives
Hydrofluoric acid (HF) represents one of the most hazardous chemicals commonly used in laboratory settings, distinguished by its unique properties that make it particularly dangerous compared to other acids. Since its first industrial production in the 1920s, HF has become essential across multiple industries including semiconductor manufacturing, glass etching, mineral processing, and analytical chemistry. The evolution of safety standards surrounding HF has been driven largely by documented incidents, with significant advancements occurring after major industrial accidents in the 1970s and 1980s.
The primary objective of HF safety standards is to prevent both acute and chronic exposure incidents through comprehensive risk management protocols. Unlike other acids that cause immediate visible burns, HF penetrates tissue rapidly and can cause deep, progressive damage without immediate symptoms, making delayed treatment a critical concern. Furthermore, systemic toxicity from fluoride ion absorption presents risks of hypocalcemia, cardiac arrhythmias, and potentially death even from relatively small exposures.
Current global safety frameworks for HF handling vary significantly by region, with the most stringent standards established by organizations such as OSHA in the United States, the European Chemicals Agency, and similar regulatory bodies in Japan and Australia. These standards have evolved from basic personal protective equipment requirements to comprehensive management systems that address facility design, emergency response protocols, and specialized medical countermeasures.
The technological trajectory of HF safety has seen significant innovation in recent decades, including the development of specialized neutralizing agents, calcium-infused first aid products, continuous monitoring systems, and advanced containment technologies. These advancements aim to address the unique challenges posed by HF's physical and chemical properties, particularly its ability to penetrate standard laboratory glassware and many conventional protective materials.
Despite these improvements, incident data suggests that laboratory HF accidents continue to occur at concerning rates, with approximately 300-500 significant exposure incidents reported annually in research and industrial settings globally. This persistence of incidents highlights the need for continued evolution of safety standards and technologies.
The goal of this technical research report is to comprehensively evaluate current best practices for HF safety compliance, identify emerging technologies and methodologies that enhance protection, and develop strategic recommendations for laboratories to implement robust safety protocols that exceed minimum regulatory requirements. Additionally, we aim to explore potential innovations in safety equipment, training methodologies, and emergency response systems specifically tailored to the unique hazards of hydrofluoric acid.
The primary objective of HF safety standards is to prevent both acute and chronic exposure incidents through comprehensive risk management protocols. Unlike other acids that cause immediate visible burns, HF penetrates tissue rapidly and can cause deep, progressive damage without immediate symptoms, making delayed treatment a critical concern. Furthermore, systemic toxicity from fluoride ion absorption presents risks of hypocalcemia, cardiac arrhythmias, and potentially death even from relatively small exposures.
Current global safety frameworks for HF handling vary significantly by region, with the most stringent standards established by organizations such as OSHA in the United States, the European Chemicals Agency, and similar regulatory bodies in Japan and Australia. These standards have evolved from basic personal protective equipment requirements to comprehensive management systems that address facility design, emergency response protocols, and specialized medical countermeasures.
The technological trajectory of HF safety has seen significant innovation in recent decades, including the development of specialized neutralizing agents, calcium-infused first aid products, continuous monitoring systems, and advanced containment technologies. These advancements aim to address the unique challenges posed by HF's physical and chemical properties, particularly its ability to penetrate standard laboratory glassware and many conventional protective materials.
Despite these improvements, incident data suggests that laboratory HF accidents continue to occur at concerning rates, with approximately 300-500 significant exposure incidents reported annually in research and industrial settings globally. This persistence of incidents highlights the need for continued evolution of safety standards and technologies.
The goal of this technical research report is to comprehensively evaluate current best practices for HF safety compliance, identify emerging technologies and methodologies that enhance protection, and develop strategic recommendations for laboratories to implement robust safety protocols that exceed minimum regulatory requirements. Additionally, we aim to explore potential innovations in safety equipment, training methodologies, and emergency response systems specifically tailored to the unique hazards of hydrofluoric acid.
Industry Demand for HF Safety Protocols
The demand for hydrofluoric acid (HF) safety protocols has grown significantly across multiple industries as awareness of its severe hazards has increased. Chemical manufacturing, semiconductor production, glass etching, and laboratory research sectors have all experienced heightened regulatory scrutiny and internal safety requirements regarding HF handling. According to recent industry reports, over 5,000 facilities worldwide regularly use HF in their operations, creating substantial demand for comprehensive safety systems.
The semiconductor industry represents one of the largest consumers of HF, with the global market valued at approximately $450 billion. As chip manufacturing continues to expand, particularly with the push toward advanced nodes and increased production capacity, the demand for stringent HF safety protocols has grown proportionally. Industry leaders like TSMC, Samsung, and Intel have implemented extensive safety management systems specifically designed for HF handling.
Research laboratories in academic institutions and pharmaceutical companies constitute another significant segment driving demand for HF safety protocols. With thousands of research facilities worldwide using HF for various applications, there has been a marked increase in investment in specialized safety equipment, training programs, and facility modifications. The pharmaceutical research sector alone has increased spending on chemical safety protocols by 27% over the past five years.
The glass etching and metal cleaning industries have also contributed substantially to the growing demand for HF safety standards. These sectors have reported a collective 35% increase in safety-related expenditures specific to HF handling over the last decade, reflecting both regulatory pressure and internal risk management priorities.
Regulatory developments have significantly influenced market demand for HF safety solutions. OSHA in the United States, the European Chemicals Agency, and similar bodies in Asia have progressively tightened requirements for HF handling, creating a compliance-driven market for specialized safety equipment and protocols. The implementation of the Globally Harmonized System of Classification and Labeling of Chemicals (GHS) has further standardized HF hazard communication requirements across borders.
Insurance considerations represent another key driver, with many providers requiring documented HF safety protocols as a condition for coverage. Companies handling HF have reported premium reductions of up to 18% when implementing comprehensive safety programs that exceed minimum regulatory requirements.
The market for HF safety equipment, including specialized PPE, neutralization kits, monitoring systems, and emergency response equipment, has expanded at a compound annual growth rate of 8.3% since 2018. This growth trajectory is expected to continue as industries balance the necessity of using HF with the imperative to protect workers and comply with increasingly stringent regulations.
The semiconductor industry represents one of the largest consumers of HF, with the global market valued at approximately $450 billion. As chip manufacturing continues to expand, particularly with the push toward advanced nodes and increased production capacity, the demand for stringent HF safety protocols has grown proportionally. Industry leaders like TSMC, Samsung, and Intel have implemented extensive safety management systems specifically designed for HF handling.
Research laboratories in academic institutions and pharmaceutical companies constitute another significant segment driving demand for HF safety protocols. With thousands of research facilities worldwide using HF for various applications, there has been a marked increase in investment in specialized safety equipment, training programs, and facility modifications. The pharmaceutical research sector alone has increased spending on chemical safety protocols by 27% over the past five years.
The glass etching and metal cleaning industries have also contributed substantially to the growing demand for HF safety standards. These sectors have reported a collective 35% increase in safety-related expenditures specific to HF handling over the last decade, reflecting both regulatory pressure and internal risk management priorities.
Regulatory developments have significantly influenced market demand for HF safety solutions. OSHA in the United States, the European Chemicals Agency, and similar bodies in Asia have progressively tightened requirements for HF handling, creating a compliance-driven market for specialized safety equipment and protocols. The implementation of the Globally Harmonized System of Classification and Labeling of Chemicals (GHS) has further standardized HF hazard communication requirements across borders.
Insurance considerations represent another key driver, with many providers requiring documented HF safety protocols as a condition for coverage. Companies handling HF have reported premium reductions of up to 18% when implementing comprehensive safety programs that exceed minimum regulatory requirements.
The market for HF safety equipment, including specialized PPE, neutralization kits, monitoring systems, and emergency response equipment, has expanded at a compound annual growth rate of 8.3% since 2018. This growth trajectory is expected to continue as industries balance the necessity of using HF with the imperative to protect workers and comply with increasingly stringent regulations.
Current Safety Standards and Implementation Challenges
Hydrofluoric acid (HF) safety standards in laboratory environments are governed by multiple regulatory bodies worldwide, including OSHA in the United States, the Health and Safety Executive (HSE) in the UK, and similar organizations in other countries. These standards mandate specific protocols for handling, storage, disposal, and emergency response procedures related to HF. Current regulations typically require specialized training for all personnel working with HF, appropriate personal protective equipment (PPE), designated storage areas, and comprehensive emergency response plans including access to calcium gluconate antidote.
Implementation of these standards faces significant challenges across laboratory settings. One primary obstacle is the varying level of awareness regarding HF's unique hazards. Unlike other acids, HF causes deep tissue damage and systemic toxicity through calcium sequestration, yet its initial symptoms may be delayed and subtle, leading to underestimation of exposure severity. This knowledge gap creates dangerous complacency among some laboratory workers who may treat HF similarly to other less hazardous acids.
Resource constraints present another substantial challenge, particularly in academic and smaller research laboratories. The specialized equipment required for safe HF handling—including appropriate fume hoods with specific flow rates, specialized waste neutralization systems, and calcium gluconate first aid kits—represents significant financial investment. Many institutions struggle to allocate sufficient funds for these safety measures, resulting in compromised safety protocols.
Training consistency and effectiveness vary considerably across institutions. While regulations mandate training, the quality and comprehensiveness of such programs differ dramatically. Some facilities provide only basic information without practical emergency response drills, while others lack regular refresher courses. This inconsistency creates dangerous knowledge gaps, particularly in emergency response scenarios where rapid, appropriate action is critical.
Compliance monitoring presents additional challenges. Many laboratories lack robust systems for ensuring ongoing adherence to safety protocols. Regular safety audits, proper documentation of HF usage, and verification of emergency response readiness are often inadequately implemented or inconsistently enforced. This creates potential for gradual protocol drift and safety standard erosion over time.
Integration of safety standards into laboratory workflow represents perhaps the most persistent challenge. Scientists and researchers often perceive safety protocols as impediments to research efficiency rather than essential protective measures. This perception can lead to shortcuts and workarounds that compromise safety. Creating laboratory cultures where safety compliance is viewed as integral to good science rather than an administrative burden remains an ongoing challenge across research environments.
Cross-institutional standardization presents further complications. Laboratories within the same field often operate under different internal protocols despite being governed by the same regulations, creating confusion when researchers move between institutions and potentially introducing unsafe practices through habit transfer.
Implementation of these standards faces significant challenges across laboratory settings. One primary obstacle is the varying level of awareness regarding HF's unique hazards. Unlike other acids, HF causes deep tissue damage and systemic toxicity through calcium sequestration, yet its initial symptoms may be delayed and subtle, leading to underestimation of exposure severity. This knowledge gap creates dangerous complacency among some laboratory workers who may treat HF similarly to other less hazardous acids.
Resource constraints present another substantial challenge, particularly in academic and smaller research laboratories. The specialized equipment required for safe HF handling—including appropriate fume hoods with specific flow rates, specialized waste neutralization systems, and calcium gluconate first aid kits—represents significant financial investment. Many institutions struggle to allocate sufficient funds for these safety measures, resulting in compromised safety protocols.
Training consistency and effectiveness vary considerably across institutions. While regulations mandate training, the quality and comprehensiveness of such programs differ dramatically. Some facilities provide only basic information without practical emergency response drills, while others lack regular refresher courses. This inconsistency creates dangerous knowledge gaps, particularly in emergency response scenarios where rapid, appropriate action is critical.
Compliance monitoring presents additional challenges. Many laboratories lack robust systems for ensuring ongoing adherence to safety protocols. Regular safety audits, proper documentation of HF usage, and verification of emergency response readiness are often inadequately implemented or inconsistently enforced. This creates potential for gradual protocol drift and safety standard erosion over time.
Integration of safety standards into laboratory workflow represents perhaps the most persistent challenge. Scientists and researchers often perceive safety protocols as impediments to research efficiency rather than essential protective measures. This perception can lead to shortcuts and workarounds that compromise safety. Creating laboratory cultures where safety compliance is viewed as integral to good science rather than an administrative burden remains an ongoing challenge across research environments.
Cross-institutional standardization presents further complications. Laboratories within the same field often operate under different internal protocols despite being governed by the same regulations, creating confusion when researchers move between institutions and potentially introducing unsafe practices through habit transfer.
Existing HF Exposure Prevention and Response Solutions
01 Personal protective equipment for hydrofluoric acid handling
Specialized personal protective equipment (PPE) is essential when handling hydrofluoric acid to prevent exposure and injuries. This includes chemical-resistant gloves, face shields, acid-resistant clothing, and respiratory protection. The PPE must be specifically rated for hydrofluoric acid resistance, as standard chemical protection may be insufficient due to the acid's unique penetrating properties. Regular inspection and maintenance of PPE is required to ensure continued protection against this highly corrosive substance.- Personal protective equipment for hydrofluoric acid handling: Specialized personal protective equipment (PPE) is essential when handling hydrofluoric acid to prevent exposure and injury. This includes chemical-resistant gloves, face shields, acid-resistant clothing, and respiratory protection. The standards specify the materials that provide effective protection against HF penetration, such as specific types of neoprene or butyl rubber. Proper PPE selection, maintenance, and training are critical components of safety protocols for workers who may be exposed to hydrofluoric acid.
- Emergency response and first aid procedures: Specific emergency response protocols are required for hydrofluoric acid incidents due to its unique hazards. These include specialized first aid procedures involving calcium gluconate treatment for exposures, emergency shower and eyewash station requirements, and detailed decontamination procedures. Safety standards mandate immediate medical attention following any exposure, as HF injuries can worsen over time even when initial symptoms appear mild. Facilities must maintain appropriate emergency supplies and train personnel on proper response techniques.
- Facility design and engineering controls: Safety standards for facilities handling hydrofluoric acid require specific engineering controls to minimize exposure risks. These include proper ventilation systems with acid-resistant components, secondary containment structures, specialized storage facilities, and automated handling systems. Facilities must incorporate leak detection systems, emergency shutdown capabilities, and proper drainage systems. The design must account for material compatibility issues, as HF can attack glass, certain metals, and other common materials used in laboratory and industrial settings.
- Transportation and storage requirements: Stringent regulations govern the transportation and storage of hydrofluoric acid to prevent accidents and releases. These include specifications for compatible container materials, secondary containment requirements, segregation from incompatible chemicals, and proper labeling. Storage facilities must maintain specific temperature controls, ventilation standards, and access restrictions. Transportation protocols include vehicle requirements, route planning, emergency response information, and driver training specific to hydrofluoric acid hazards.
- Monitoring and detection systems: Continuous monitoring and detection systems are required in environments where hydrofluoric acid is used or stored. These include specialized HF vapor detectors, automated alarm systems, and regular air quality testing protocols. Safety standards specify the placement, sensitivity, maintenance, and calibration requirements for these systems. Monitoring programs must include regular equipment inspections, documentation procedures, and integration with emergency response systems to ensure rapid action in case of leaks or releases.
02 Containment and storage safety systems
Proper containment and storage systems are critical for hydrofluoric acid safety. These include specialized acid-resistant containers, secondary containment structures, leak detection systems, and proper labeling. Storage facilities must have adequate ventilation, temperature control, and be physically separated from incompatible materials. Automated monitoring systems can detect leaks or dangerous vapor concentrations before they reach hazardous levels, triggering alarms and emergency response protocols.Expand Specific Solutions03 Emergency response and first aid protocols
Specific emergency response and first aid protocols are essential when dealing with hydrofluoric acid incidents. This includes immediate flushing with water, application of calcium gluconate gel for skin exposure, specialized medical intervention procedures, and evacuation plans. Emergency showers and eyewash stations must be readily accessible in work areas. Personnel must be trained to recognize symptoms of exposure, which can be delayed, and know the proper immediate response actions to take before medical help arrives.Expand Specific Solutions04 Facility design and engineering controls
Specialized facility design and engineering controls are necessary for safe hydrofluoric acid handling. These include dedicated ventilation systems with acid-resistant components, automated process controls to minimize human exposure, physical barriers and containment systems, and specialized waste neutralization systems. Facilities should incorporate fail-safe mechanisms that automatically shut down processes and contain spills in the event of equipment failure or human error, reducing the risk of catastrophic releases.Expand Specific Solutions05 Training and operational safety procedures
Comprehensive training and operational safety procedures are required for all personnel working with hydrofluoric acid. This includes regular safety drills, documented standard operating procedures, certification requirements for handlers, and periodic refresher training. Workers must understand the unique hazards of hydrofluoric acid, including its ability to cause deep tissue damage without immediate pain, proper handling techniques, and the importance of never working alone when handling this chemical. Regular audits and safety reviews help ensure continued compliance with established protocols.Expand Specific Solutions
Leading Organizations in HF Safety Standards Development
Hydrofluoric acid safety standards in laboratories are evolving within a mature yet continuously developing field. The market for HF safety equipment and protocols is estimated at $1.2 billion globally, driven by stringent regulations across chemical, semiconductor, and pharmaceutical industries. Leading companies demonstrate varying levels of technical maturity: Do-Fluoride New Materials and Alkeemia SpA specialize in HF production with advanced safety protocols; Lam Research and Siemens have developed sophisticated engineering controls; while Corning and DuPont offer specialized containment materials. Emerging players like Jiangyin Runma and Fujian Tianfu are advancing high-purity electronic-grade HF handling techniques, particularly important as semiconductor manufacturing expands. The competitive landscape shows a balance between established industrial chemical companies and specialized safety equipment manufacturers focusing on innovative containment and neutralization technologies.
Siemens AG
Technical Solution: Siemens has developed the SafetyGuard HF Management Platform, an integrated safety solution for hydrofluoric acid handling in laboratory environments. The system employs a combination of engineering controls and digital monitoring technologies. At its core is a specialized ventilation system with redundant fans and pressure differential monitoring to ensure containment of HF vapors. The platform incorporates real-time air quality sensors with wireless connectivity that can detect HF concentrations as low as 0.5 ppm, triggering automated emergency protocols when threshold levels are exceeded. Siemens' solution includes specialized workstation designs with transparent safety shields made from proprietary acid-resistant polymers. Their digital management system tracks HF inventory, usage patterns, and exposure incidents through a centralized dashboard accessible to safety managers. The platform also features an emergency response system that automatically activates neutralization measures and alerts emergency personnel with precise location data in case of spills or exposures.
Strengths: Comprehensive digital integration allowing for real-time monitoring and response; excellent data collection capabilities for compliance reporting and incident investigation; scalable system suitable for facilities of various sizes. Weaknesses: Requires significant IT infrastructure investment; system complexity necessitates specialized training for maintenance personnel; potential cybersecurity vulnerabilities in networked safety systems.
Corning, Inc.
Technical Solution: Corning has developed the HF-Guard Laboratory Safety System, leveraging their expertise in glass and materials science to create specialized containment solutions for hydrofluoric acid handling. Their system features proprietary glass-ceramic composite materials that offer exceptional resistance to HF penetration while maintaining visibility for laboratory processes. Corning's approach includes modular containment workstations with integrated ventilation systems that create negative pressure environments to prevent vapor escape. The company has engineered specialized storage vessels with double-wall construction and leak detection systems that provide early warning of potential containment failures. Their safety protocols incorporate specialized waste neutralization systems that automatically treat HF-containing waste streams before disposal. Corning's solution also includes specialized first aid stations with calcium gluconate gel formulations specifically optimized for different exposure scenarios based on concentration and body area affected. The company provides comprehensive training programs that include hands-on practice with simulated emergency scenarios using non-hazardous substitutes.
Strengths: Superior containment materials specifically engineered for HF resistance; excellent visibility for laboratory processes while maintaining safety; integrated waste management solutions. Weaknesses: Higher initial cost compared to standard laboratory equipment; specialized materials may require specific cleaning and maintenance protocols; limited flexibility for custom laboratory configurations.
Critical Safety Technologies and Protocols Analysis
Process for preparing fluoroolefin compounds
PatentInactiveUS20120083632A1
Innovation
- A continuous or semicontinuous process involving a gas/solid reaction between a compound with 2-6 carbon atoms and adjacent fluorine and hydrogen atoms with calcium hydroxide, forming calcium fluoride, which allows for the efficient production of hydrofluoroolefins like 2,3,3-tetrafluoropropene, using calcium hydroxide as a solid reactant in a fluidized bed or fixed bed reactor at elevated temperatures and pressures.
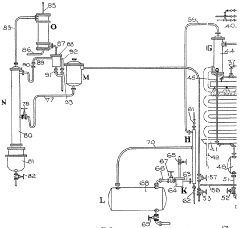
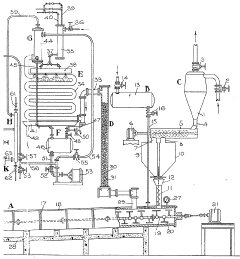
Improvements in or relating to the manufacture of hydrofluoric acid
PatentInactiveGB462131A
Innovation
- A continuous process under superatmospheric pressure reacts a fluoride with an acid, followed by preliminary cooling and condensation to separate dilute and concentrated hydrofluoric acid, using a reactor and condensers to achieve high yields and purity.
Regulatory Compliance Requirements for Laboratory HF Use
Regulatory compliance for hydrofluoric acid (HF) use in laboratories is governed by multiple international, national, and local frameworks that establish mandatory safety standards. The Occupational Safety and Health Administration (OSHA) in the United States mandates specific requirements under the Hazardous Communication Standard (29 CFR 1910.1200) and Laboratory Standard (29 CFR 1910.1450), requiring comprehensive chemical hygiene plans that specifically address HF handling protocols.
The Environmental Protection Agency (EPA) regulates HF under the Resource Conservation and Recovery Act (RCRA) and the Comprehensive Environmental Response, Compensation, and Liability Act (CERCLA), establishing strict guidelines for disposal and release reporting thresholds at 100 pounds. Laboratories must maintain detailed documentation of all HF waste management practices to demonstrate compliance with these regulations.
In the European Union, HF handling falls under the Registration, Evaluation, Authorization and Restriction of Chemicals (REACH) regulation and the Classification, Labeling and Packaging (CLP) regulation. These frameworks require extensive safety data sheets, appropriate labeling, and risk assessments before HF can be used in any laboratory setting.
Industry-specific standards provide additional compliance requirements. The American Chemical Society (ACS) and the National Institute for Occupational Safety and Health (NIOSH) have established recommended exposure limits of 3 ppm as an 8-hour time-weighted average. These standards are often incorporated into institutional policies and may become legally binding when adopted by regulatory authorities.
Documentation requirements represent a critical aspect of regulatory compliance. Laboratories must maintain records of risk assessments, standard operating procedures (SOPs), training certifications, exposure monitoring results, medical surveillance programs, and incident reports. These documents must be readily accessible during regulatory inspections and updated regularly to reflect changes in procedures or regulations.
Training certification requirements vary by jurisdiction but typically mandate initial comprehensive training followed by annual refresher courses. Training must cover HF-specific hazards, emergency response procedures, first aid protocols, and proper use of personal protective equipment. Documentation of this training must be maintained for each employee with potential HF exposure.
Facility design and engineering controls constitute another regulatory dimension. Laboratories must implement appropriate ventilation systems, including fume hoods with specific face velocities (typically 100 feet per minute), emergency eyewash stations and safety showers within 10 seconds' travel time from HF use areas, and appropriate storage facilities with secondary containment capable of holding 110% of the largest container's volume.
The Environmental Protection Agency (EPA) regulates HF under the Resource Conservation and Recovery Act (RCRA) and the Comprehensive Environmental Response, Compensation, and Liability Act (CERCLA), establishing strict guidelines for disposal and release reporting thresholds at 100 pounds. Laboratories must maintain detailed documentation of all HF waste management practices to demonstrate compliance with these regulations.
In the European Union, HF handling falls under the Registration, Evaluation, Authorization and Restriction of Chemicals (REACH) regulation and the Classification, Labeling and Packaging (CLP) regulation. These frameworks require extensive safety data sheets, appropriate labeling, and risk assessments before HF can be used in any laboratory setting.
Industry-specific standards provide additional compliance requirements. The American Chemical Society (ACS) and the National Institute for Occupational Safety and Health (NIOSH) have established recommended exposure limits of 3 ppm as an 8-hour time-weighted average. These standards are often incorporated into institutional policies and may become legally binding when adopted by regulatory authorities.
Documentation requirements represent a critical aspect of regulatory compliance. Laboratories must maintain records of risk assessments, standard operating procedures (SOPs), training certifications, exposure monitoring results, medical surveillance programs, and incident reports. These documents must be readily accessible during regulatory inspections and updated regularly to reflect changes in procedures or regulations.
Training certification requirements vary by jurisdiction but typically mandate initial comprehensive training followed by annual refresher courses. Training must cover HF-specific hazards, emergency response procedures, first aid protocols, and proper use of personal protective equipment. Documentation of this training must be maintained for each employee with potential HF exposure.
Facility design and engineering controls constitute another regulatory dimension. Laboratories must implement appropriate ventilation systems, including fume hoods with specific face velocities (typically 100 feet per minute), emergency eyewash stations and safety showers within 10 seconds' travel time from HF use areas, and appropriate storage facilities with secondary containment capable of holding 110% of the largest container's volume.
Emergency Response and Medical Treatment Protocols
Effective emergency response to hydrofluoric acid (HF) exposure requires immediate and specialized protocols due to the unique penetrating nature of this acid. The primary emergency response begins with removing the affected individual from the exposure area while ensuring responder safety through appropriate personal protective equipment. For skin contact, immediate removal of contaminated clothing and thorough rinsing with water for at least 15 minutes is critical. During this initial response, emergency services should be contacted immediately, with clear communication about the HF exposure to ensure appropriate medical resources are dispatched.
Medical treatment for HF exposure follows a distinct protocol from other acid exposures due to the fluoride ion's ability to penetrate tissue and bind with calcium and magnesium in the body. The cornerstone of medical intervention is the application of calcium gluconate, which acts as an antidote by binding with fluoride ions. For skin exposures, 2.5% calcium gluconate gel should be applied promptly and repeatedly to the affected area. In severe cases, subcutaneous or intra-arterial calcium gluconate injections may be necessary, requiring immediate medical professional intervention.
Laboratory facilities must maintain dedicated HF emergency kits containing calcium gluconate gel, eye wash solutions, and specific neutralizing agents. These kits should be strategically placed in easily accessible locations throughout areas where HF is used or stored, with clear signage and regular inventory checks to ensure readiness. Additionally, specialized eye wash stations and safety showers with extended operation capabilities are essential, as HF exposures typically require longer decontamination periods than standard acid exposures.
Training programs for laboratory personnel must include practical drills on emergency response procedures specific to HF incidents. These drills should simulate various exposure scenarios and emphasize the time-critical nature of response actions. Documentation systems must be established to record all exposure incidents, treatment administered, and outcomes, which serves both regulatory compliance purposes and continuous improvement of safety protocols.
Coordination with local emergency medical services and nearby hospitals is vital to ensure seamless transition from first aid to professional medical care. This includes pre-establishing communication channels, providing information about on-site HF quantities and concentrations, and ensuring that receiving medical facilities have appropriate treatment capabilities including calcium gluconate supplies and staff trained in HF exposure management.
Post-incident follow-up protocols are equally important, as HF injuries can develop over time with initially minimal symptoms. Medical monitoring should continue for at least 24 hours following exposure, with particular attention to potential systemic effects including hypocalcemia, hypomagnesemia, and cardiac arrhythmias in cases of significant exposure.
Medical treatment for HF exposure follows a distinct protocol from other acid exposures due to the fluoride ion's ability to penetrate tissue and bind with calcium and magnesium in the body. The cornerstone of medical intervention is the application of calcium gluconate, which acts as an antidote by binding with fluoride ions. For skin exposures, 2.5% calcium gluconate gel should be applied promptly and repeatedly to the affected area. In severe cases, subcutaneous or intra-arterial calcium gluconate injections may be necessary, requiring immediate medical professional intervention.
Laboratory facilities must maintain dedicated HF emergency kits containing calcium gluconate gel, eye wash solutions, and specific neutralizing agents. These kits should be strategically placed in easily accessible locations throughout areas where HF is used or stored, with clear signage and regular inventory checks to ensure readiness. Additionally, specialized eye wash stations and safety showers with extended operation capabilities are essential, as HF exposures typically require longer decontamination periods than standard acid exposures.
Training programs for laboratory personnel must include practical drills on emergency response procedures specific to HF incidents. These drills should simulate various exposure scenarios and emphasize the time-critical nature of response actions. Documentation systems must be established to record all exposure incidents, treatment administered, and outcomes, which serves both regulatory compliance purposes and continuous improvement of safety protocols.
Coordination with local emergency medical services and nearby hospitals is vital to ensure seamless transition from first aid to professional medical care. This includes pre-establishing communication channels, providing information about on-site HF quantities and concentrations, and ensuring that receiving medical facilities have appropriate treatment capabilities including calcium gluconate supplies and staff trained in HF exposure management.
Post-incident follow-up protocols are equally important, as HF injuries can develop over time with initially minimal symptoms. Medical monitoring should continue for at least 24 hours following exposure, with particular attention to potential systemic effects including hypocalcemia, hypomagnesemia, and cardiac arrhythmias in cases of significant exposure.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!