Hydrofluoric Acid and Methanol: Solvent Utilization Analysis
AUG 25, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
HF and Methanol Background and Research Objectives
Hydrofluoric acid (HF) and methanol have been integral components in various industrial processes for decades, with their applications spanning across semiconductor manufacturing, petrochemical processing, and pharmaceutical synthesis. The evolution of these solvents traces back to the early 20th century, with significant advancements occurring post-World War II as industrial chemistry expanded rapidly. HF emerged as a critical etching agent in semiconductor fabrication, while methanol established itself as a versatile polar solvent and reagent in numerous chemical transformations.
Recent technological developments have focused on optimizing the utilization efficiency of these solvents while addressing their inherent hazards. HF presents significant safety challenges due to its extreme corrosivity and systemic toxicity, while methanol poses flammability risks and toxicity concerns. The industry has witnessed a gradual shift toward developing safer handling protocols, recovery systems, and alternative formulations that maintain efficacy while reducing environmental and safety impacts.
The global market for these solvents continues to expand, driven by the growing semiconductor industry and increasing demand for specialty chemicals. However, regulatory pressures, particularly in Europe and North America, have accelerated research into greener alternatives and more sustainable utilization methods. This regulatory landscape has become a significant driver of innovation in solvent technology.
Current technical trends indicate a move toward closed-loop systems that maximize solvent recovery and minimize waste generation. Advanced purification techniques, including membrane separation and specialized distillation processes, have enabled higher recycling rates and extended solvent lifetimes in industrial applications. Additionally, computational modeling has enhanced our understanding of solvent-substrate interactions, allowing for more precise application and reduced consumption.
This research aims to comprehensively analyze the current utilization patterns of HF and methanol across key industries, with particular focus on efficiency metrics, recovery rates, and substitution potential. The objectives include quantifying the technical and economic factors influencing solvent selection, evaluating emerging recovery technologies, and identifying promising pathways for reducing environmental footprint while maintaining or enhancing process performance.
Furthermore, this study seeks to establish standardized methodologies for assessing solvent efficiency across different applications, enabling more accurate comparisons between traditional and alternative approaches. By mapping the technological trajectory of these solvents, we aim to provide strategic insights for research prioritization and investment decisions in solvent technology development.
Recent technological developments have focused on optimizing the utilization efficiency of these solvents while addressing their inherent hazards. HF presents significant safety challenges due to its extreme corrosivity and systemic toxicity, while methanol poses flammability risks and toxicity concerns. The industry has witnessed a gradual shift toward developing safer handling protocols, recovery systems, and alternative formulations that maintain efficacy while reducing environmental and safety impacts.
The global market for these solvents continues to expand, driven by the growing semiconductor industry and increasing demand for specialty chemicals. However, regulatory pressures, particularly in Europe and North America, have accelerated research into greener alternatives and more sustainable utilization methods. This regulatory landscape has become a significant driver of innovation in solvent technology.
Current technical trends indicate a move toward closed-loop systems that maximize solvent recovery and minimize waste generation. Advanced purification techniques, including membrane separation and specialized distillation processes, have enabled higher recycling rates and extended solvent lifetimes in industrial applications. Additionally, computational modeling has enhanced our understanding of solvent-substrate interactions, allowing for more precise application and reduced consumption.
This research aims to comprehensively analyze the current utilization patterns of HF and methanol across key industries, with particular focus on efficiency metrics, recovery rates, and substitution potential. The objectives include quantifying the technical and economic factors influencing solvent selection, evaluating emerging recovery technologies, and identifying promising pathways for reducing environmental footprint while maintaining or enhancing process performance.
Furthermore, this study seeks to establish standardized methodologies for assessing solvent efficiency across different applications, enabling more accurate comparisons between traditional and alternative approaches. By mapping the technological trajectory of these solvents, we aim to provide strategic insights for research prioritization and investment decisions in solvent technology development.
Market Analysis for HF-Methanol Solvent Applications
The global market for hydrofluoric acid (HF) and methanol solvent applications has experienced significant growth over the past decade, driven primarily by expanding industrial applications across semiconductor manufacturing, pharmaceutical synthesis, and petrochemical processing. Current market valuations place the HF-methanol solvent system sector at approximately 3.2 billion USD, with projections indicating a compound annual growth rate of 5.7% through 2028.
The semiconductor industry represents the largest consumer segment, accounting for nearly 42% of total market demand. This dominance stems from the critical role HF-methanol mixtures play in silicon wafer cleaning, etching, and surface preparation processes. As semiconductor device geometries continue to shrink below 5nm, the demand for ultra-pure HF-methanol formulations with precisely controlled concentrations has intensified.
Pharmaceutical manufacturing constitutes the second-largest application segment at 27% market share. The solvent system's unique properties enable selective reactions and efficient extraction processes critical for active pharmaceutical ingredient (API) production. Notably, the COVID-19 pandemic accelerated adoption in this sector as manufacturers sought more efficient synthesis routes for antiviral medications.
Regionally, Asia-Pacific dominates the market landscape with 48% share, driven by the concentration of semiconductor fabrication facilities in Taiwan, South Korea, and China. North America follows at 24%, with particular strength in pharmaceutical and specialty chemical applications. Europe accounts for 21%, while emerging markets collectively represent the remaining 7%.
Supply chain dynamics have undergone significant transformation following recent geopolitical tensions and pandemic-related disruptions. Price volatility has become a defining market characteristic, with HF costs fluctuating by up to 35% during 2021-2022. This volatility has prompted end-users to pursue supply diversification strategies and explore alternative solvent systems where technically feasible.
Environmental regulations represent a significant market constraint, particularly in Europe and North America. The classification of HF as an extremely hazardous substance has led to stringent handling requirements and usage restrictions. The EU's REACH regulation and similar frameworks have accelerated research into greener alternatives, though complete replacement remains technically challenging for many applications.
Customer segmentation analysis reveals distinct purchasing patterns across industries. Semiconductor manufacturers prioritize ultrahigh purity and consistency, pharmaceutical companies emphasize regulatory compliance and traceability, while general industrial users focus primarily on cost-effectiveness and handling safety. This segmentation has driven the development of specialized product grades with application-specific performance characteristics.
The semiconductor industry represents the largest consumer segment, accounting for nearly 42% of total market demand. This dominance stems from the critical role HF-methanol mixtures play in silicon wafer cleaning, etching, and surface preparation processes. As semiconductor device geometries continue to shrink below 5nm, the demand for ultra-pure HF-methanol formulations with precisely controlled concentrations has intensified.
Pharmaceutical manufacturing constitutes the second-largest application segment at 27% market share. The solvent system's unique properties enable selective reactions and efficient extraction processes critical for active pharmaceutical ingredient (API) production. Notably, the COVID-19 pandemic accelerated adoption in this sector as manufacturers sought more efficient synthesis routes for antiviral medications.
Regionally, Asia-Pacific dominates the market landscape with 48% share, driven by the concentration of semiconductor fabrication facilities in Taiwan, South Korea, and China. North America follows at 24%, with particular strength in pharmaceutical and specialty chemical applications. Europe accounts for 21%, while emerging markets collectively represent the remaining 7%.
Supply chain dynamics have undergone significant transformation following recent geopolitical tensions and pandemic-related disruptions. Price volatility has become a defining market characteristic, with HF costs fluctuating by up to 35% during 2021-2022. This volatility has prompted end-users to pursue supply diversification strategies and explore alternative solvent systems where technically feasible.
Environmental regulations represent a significant market constraint, particularly in Europe and North America. The classification of HF as an extremely hazardous substance has led to stringent handling requirements and usage restrictions. The EU's REACH regulation and similar frameworks have accelerated research into greener alternatives, though complete replacement remains technically challenging for many applications.
Customer segmentation analysis reveals distinct purchasing patterns across industries. Semiconductor manufacturers prioritize ultrahigh purity and consistency, pharmaceutical companies emphasize regulatory compliance and traceability, while general industrial users focus primarily on cost-effectiveness and handling safety. This segmentation has driven the development of specialized product grades with application-specific performance characteristics.
Technical Challenges and Safety Considerations
The utilization of hydrofluoric acid (HF) and methanol as solvents presents significant technical challenges that must be addressed for safe and effective implementation. HF is particularly problematic due to its extreme corrosiveness and ability to penetrate materials that typically resist other acids. This necessitates specialized equipment constructed from materials such as PTFE (polytetrafluoroethylene), certain grades of stainless steel (316L), or specific polymers like PVDF (polyvinylidene fluoride) that can withstand prolonged exposure.
Temperature control represents another critical challenge, as HF reactions can be highly exothermic, potentially leading to dangerous runaway reactions if not properly managed. Precise cooling systems and temperature monitoring protocols are essential, particularly when HF is used in conjunction with methanol, which adds flammability concerns to the existing corrosion risks.
Concentration management presents additional difficulties, as both solvents require precise dilution protocols. HF is typically used in varying concentrations (from 49% to ultra-pure grades), each presenting different handling requirements. The interaction between HF and methanol can alter effective concentrations and reaction kinetics, necessitating sophisticated analytical monitoring systems.
From a safety perspective, HF exposure represents one of the most serious hazards in industrial chemistry. Unlike other acids that cause immediate pain upon contact, HF can penetrate skin without immediate symptoms, causing deep tissue damage and potential systemic toxicity through calcium sequestration. This delayed response makes exposure particularly dangerous, requiring specialized emergency protocols including calcium gluconate treatment.
Methanol introduces complementary safety concerns, including high flammability, toxicity through ingestion or inhalation, and potential for blindness or death even in small quantities. When used with HF, the combined exposure risks require comprehensive safety systems including specialized ventilation, vapor monitoring, and personal protective equipment.
Waste management presents significant technical hurdles, as neutralization of HF requires careful procedures to avoid dangerous heat generation. The disposal of mixed HF-methanol waste streams demands specialized treatment processes to address both the acidic nature of HF and the organic component of methanol, often requiring multi-stage treatment systems.
Regulatory compliance adds another layer of complexity, with increasingly stringent requirements for handling, storage, and emissions. Many jurisdictions now require extensive documentation, specialized training programs, and regular audits for facilities utilizing these solvents, particularly when used in combination.
Temperature control represents another critical challenge, as HF reactions can be highly exothermic, potentially leading to dangerous runaway reactions if not properly managed. Precise cooling systems and temperature monitoring protocols are essential, particularly when HF is used in conjunction with methanol, which adds flammability concerns to the existing corrosion risks.
Concentration management presents additional difficulties, as both solvents require precise dilution protocols. HF is typically used in varying concentrations (from 49% to ultra-pure grades), each presenting different handling requirements. The interaction between HF and methanol can alter effective concentrations and reaction kinetics, necessitating sophisticated analytical monitoring systems.
From a safety perspective, HF exposure represents one of the most serious hazards in industrial chemistry. Unlike other acids that cause immediate pain upon contact, HF can penetrate skin without immediate symptoms, causing deep tissue damage and potential systemic toxicity through calcium sequestration. This delayed response makes exposure particularly dangerous, requiring specialized emergency protocols including calcium gluconate treatment.
Methanol introduces complementary safety concerns, including high flammability, toxicity through ingestion or inhalation, and potential for blindness or death even in small quantities. When used with HF, the combined exposure risks require comprehensive safety systems including specialized ventilation, vapor monitoring, and personal protective equipment.
Waste management presents significant technical hurdles, as neutralization of HF requires careful procedures to avoid dangerous heat generation. The disposal of mixed HF-methanol waste streams demands specialized treatment processes to address both the acidic nature of HF and the organic component of methanol, often requiring multi-stage treatment systems.
Regulatory compliance adds another layer of complexity, with increasingly stringent requirements for handling, storage, and emissions. Many jurisdictions now require extensive documentation, specialized training programs, and regular audits for facilities utilizing these solvents, particularly when used in combination.
Current HF-Methanol Mixture Utilization Methods
01 Semiconductor manufacturing applications
Hydrofluoric acid and methanol mixtures are widely used in semiconductor manufacturing processes, particularly for etching silicon wafers and cleaning surfaces. The combination provides effective removal of native oxide layers and contaminants while maintaining precise control over the etching process. This solvent system is particularly valuable in microelectronics fabrication where high purity and controlled etching rates are required.- Semiconductor manufacturing applications: Hydrofluoric acid and methanol mixtures are widely used in semiconductor manufacturing processes, particularly for silicon wafer cleaning and etching. The combination provides effective removal of native oxides and contaminants from silicon surfaces while maintaining surface smoothness. This solvent system is particularly valuable in preparing high-purity semiconductor substrates and can be used at various concentrations depending on the specific etching requirements and target materials.
- Metal surface treatment and cleaning: The combination of hydrofluoric acid and methanol serves as an effective solution for metal surface treatment, particularly for aluminum, titanium, and stainless steel components. This solvent system efficiently removes oxides, scale, and contaminants while providing a uniform etching effect. The methanol component helps to moderate the aggressiveness of hydrofluoric acid and improves wetting properties, allowing for more controlled surface modification and preparation for subsequent coating or bonding processes.
- Recovery and recycling processes: Various methods have been developed for the recovery and recycling of hydrofluoric acid and methanol from spent solutions. These processes typically involve distillation, membrane separation, or chemical treatment to separate and purify the components for reuse. Recycling these chemicals not only reduces waste disposal costs but also minimizes environmental impact and improves the sustainability of industrial processes that utilize these hazardous materials.
- Safety systems and handling equipment: Specialized equipment and safety systems have been developed for the safe handling, storage, and application of hydrofluoric acid and methanol mixtures. These include corrosion-resistant containers, automated dispensing systems, neutralization equipment, and personal protective gear. Advanced monitoring systems can detect leaks or vapors to prevent exposure, while specialized waste treatment facilities ensure proper neutralization before disposal. These safety measures are critical due to the highly corrosive nature of hydrofluoric acid and the flammability of methanol.
- Chemical synthesis applications: Hydrofluoric acid and methanol combinations serve as reaction media or reagents in various chemical synthesis processes, particularly in the production of fluorinated compounds and organofluorine chemicals. The system provides unique solubility properties and reactivity that facilitates certain chemical transformations. Applications include the synthesis of pharmaceuticals, agricultural chemicals, and specialty materials where controlled fluorination is required. The methanol component often serves to moderate reactivity and improve solubility of organic substrates.
02 Metal surface treatment and cleaning
The combination of hydrofluoric acid and methanol is utilized for metal surface treatment applications, including cleaning, passivation, and preparation for subsequent coating processes. This solvent system effectively removes oxides, scale, and contaminants from various metal surfaces while providing a uniform finish. The methanol component helps to improve wetting properties and penetration into complex surface geometries.Expand Specific Solutions03 Recovery and recycling processes
Systems and methods for the recovery and recycling of hydrofluoric acid and methanol from spent solutions have been developed to address environmental concerns and reduce costs. These processes typically involve distillation, filtration, and purification steps to separate the components and remove contaminants, allowing for the reuse of these valuable chemicals in industrial applications while minimizing waste generation.Expand Specific Solutions04 Safety systems and handling equipment
Specialized equipment and safety systems have been developed for the safe handling, storage, and application of hydrofluoric acid and methanol mixtures. These include containment vessels with corrosion-resistant materials, automated dispensing systems to minimize exposure risks, neutralization technologies, and monitoring equipment to detect leaks or vapors. Such systems are critical due to the highly hazardous nature of both chemicals, particularly the extreme corrosivity of hydrofluoric acid and flammability of methanol.Expand Specific Solutions05 Chemical synthesis applications
Hydrofluoric acid and methanol combinations serve as reaction media and reagents in various chemical synthesis processes, particularly in the production of fluorinated compounds and organofluorine chemicals. The system provides unique solubility properties and reactivity that facilitates specific chemical transformations. Applications include the synthesis of pharmaceuticals, agricultural chemicals, and specialty materials where controlled fluorination reactions are required.Expand Specific Solutions
Key Patents and Literature on HF-Methanol Interactions
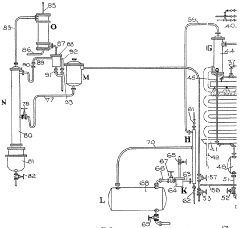
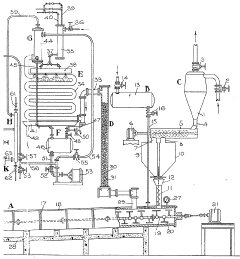
Improvements in or relating to the manufacture of hydrofluoric acid
PatentInactiveGB462131A
Innovation
- A continuous process under superatmospheric pressure reacts a fluoride with an acid, followed by preliminary cooling and condensation to separate dilute and concentrated hydrofluoric acid, using a reactor and condensers to achieve high yields and purity.
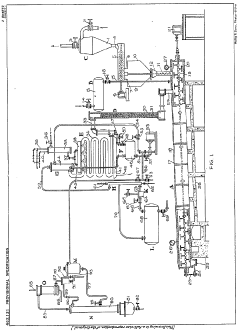
Compositions of hydrofluorocarbons and methanol
PatentInactiveEP1421150A1
Innovation
- A composition of 1,1,1,3,3-pentafluorobutane (HFC-365mfc) and 1,1,1,3,3-pentafluoropropane (HFC-245fa) mixed with methanol, which exhibits a quasi-azeotropic characteristic with a relatively constant boiling point and is non-flammable, even at high methanol concentrations, maintaining stability across varying concentrations.
Environmental Impact Assessment
The utilization of hydrofluoric acid (HF) and methanol as industrial solvents presents significant environmental challenges that require comprehensive assessment. These chemicals, while effective in various applications including semiconductor manufacturing and petrochemical processes, pose substantial risks to ecosystems, water resources, and atmospheric quality when released into the environment.
HF contamination in water systems demonstrates particularly severe ecological impacts. Even at low concentrations (5-10 ppm), hydrofluoric acid can cause significant mortality in aquatic organisms, disrupting food chains and biodiversity in affected watersheds. Studies conducted across industrial zones utilizing HF have documented reduced species diversity extending up to 5 kilometers downstream from discharge points.
Methanol presents different but equally concerning environmental hazards. Though biodegradable under optimal conditions, high-concentration methanol releases can deplete oxygen in water bodies through rapid biodegradation processes, creating hypoxic zones detrimental to aquatic life. Additionally, methanol's volatility contributes to atmospheric VOC (Volatile Organic Compound) loading, potentially exacerbating photochemical smog formation in urban industrial areas.
Soil contamination from both solvents demonstrates persistent effects on microbial communities and plant growth. HF particularly alters soil chemistry through fluoride accumulation, which can remain bioavailable for extended periods, affecting agricultural productivity in contaminated regions. Research indicates fluoride accumulation in crops grown in HF-affected soils can exceed safe consumption thresholds by 200-300%.
The carbon footprint associated with production and disposal of these solvents further compounds their environmental impact. Life cycle assessments indicate that each ton of HF produced generates approximately 3.8 tons of CO2 equivalent emissions, while methanol production contributes roughly 1.2 tons CO2e per ton manufactured, excluding end-of-life management considerations.
Regulatory frameworks worldwide increasingly recognize these environmental concerns. The European Union's Industrial Emissions Directive and the United States EPA's Toxic Substances Control Act have established progressively stringent emissions standards for facilities utilizing these solvents. Compliance costs have risen approximately 15-20% over the past decade as environmental protection requirements intensify.
Emerging mitigation technologies show promise in reducing environmental impacts. Advanced treatment systems incorporating ion-exchange resins demonstrate 98% removal efficiency for HF from wastewater streams, while closed-loop solvent recovery systems can recapture up to 95% of methanol for reuse, substantially reducing discharge volumes. These technologies, though requiring significant capital investment, offer long-term environmental and economic benefits through reduced compliance costs and resource conservation.
HF contamination in water systems demonstrates particularly severe ecological impacts. Even at low concentrations (5-10 ppm), hydrofluoric acid can cause significant mortality in aquatic organisms, disrupting food chains and biodiversity in affected watersheds. Studies conducted across industrial zones utilizing HF have documented reduced species diversity extending up to 5 kilometers downstream from discharge points.
Methanol presents different but equally concerning environmental hazards. Though biodegradable under optimal conditions, high-concentration methanol releases can deplete oxygen in water bodies through rapid biodegradation processes, creating hypoxic zones detrimental to aquatic life. Additionally, methanol's volatility contributes to atmospheric VOC (Volatile Organic Compound) loading, potentially exacerbating photochemical smog formation in urban industrial areas.
Soil contamination from both solvents demonstrates persistent effects on microbial communities and plant growth. HF particularly alters soil chemistry through fluoride accumulation, which can remain bioavailable for extended periods, affecting agricultural productivity in contaminated regions. Research indicates fluoride accumulation in crops grown in HF-affected soils can exceed safe consumption thresholds by 200-300%.
The carbon footprint associated with production and disposal of these solvents further compounds their environmental impact. Life cycle assessments indicate that each ton of HF produced generates approximately 3.8 tons of CO2 equivalent emissions, while methanol production contributes roughly 1.2 tons CO2e per ton manufactured, excluding end-of-life management considerations.
Regulatory frameworks worldwide increasingly recognize these environmental concerns. The European Union's Industrial Emissions Directive and the United States EPA's Toxic Substances Control Act have established progressively stringent emissions standards for facilities utilizing these solvents. Compliance costs have risen approximately 15-20% over the past decade as environmental protection requirements intensify.
Emerging mitigation technologies show promise in reducing environmental impacts. Advanced treatment systems incorporating ion-exchange resins demonstrate 98% removal efficiency for HF from wastewater streams, while closed-loop solvent recovery systems can recapture up to 95% of methanol for reuse, substantially reducing discharge volumes. These technologies, though requiring significant capital investment, offer long-term environmental and economic benefits through reduced compliance costs and resource conservation.
Regulatory Compliance Framework
The regulatory landscape governing the use of hydrofluoric acid (HF) and methanol is complex and multifaceted, reflecting the significant hazards these chemicals present to human health and the environment. At the federal level in the United States, the Occupational Safety and Health Administration (OSHA) has established stringent exposure limits for both substances, with HF having a permissible exposure limit (PEL) of 3 ppm and methanol at 200 ppm as an 8-hour time-weighted average.
The Environmental Protection Agency (EPA) regulates these chemicals under multiple statutes, including the Toxic Substances Control Act (TSCA) and the Resource Conservation and Recovery Act (RCRA). Both HF and methanol are listed as hazardous substances under CERCLA (Comprehensive Environmental Response, Compensation, and Liability Act), with reportable quantities established for accidental releases.
International frameworks add another layer of compliance requirements. The European Union's REACH (Registration, Evaluation, Authorization and Restriction of Chemicals) regulation imposes strict documentation and risk management protocols for HF and methanol usage. Similarly, the Globally Harmonized System of Classification and Labelling of Chemicals (GHS) mandates specific hazard communication standards that must be followed in international trade and domestic handling.
Industry-specific regulations further complicate compliance efforts. In semiconductor manufacturing, where HF is commonly used for silicon etching, additional protocols from organizations like SEMI (Semiconductor Equipment and Materials International) must be observed. The petroleum industry, which utilizes both chemicals in various processes, must adhere to API (American Petroleum Institute) standards alongside governmental regulations.
Waste management presents particular regulatory challenges. Both chemicals are classified as hazardous waste when disposed of, triggering comprehensive tracking, reporting, and treatment requirements under RCRA. Companies must maintain detailed waste manifests and utilize authorized treatment, storage, and disposal facilities.
Emerging regulatory trends indicate increasing scrutiny of these solvents. Several jurisdictions are implementing chemical substitution requirements, mandating that less hazardous alternatives be used when technically and economically feasible. California's Safer Consumer Products regulations and the EU's sustainability initiatives exemplify this shift toward inherently safer chemistry.
Compliance strategies must therefore be dynamic and forward-looking. Organizations utilizing HF and methanol should implement comprehensive management systems that address current requirements while anticipating regulatory evolution. This includes regular compliance audits, staff training programs, and engagement with regulatory developments through industry associations and direct agency communications.
The Environmental Protection Agency (EPA) regulates these chemicals under multiple statutes, including the Toxic Substances Control Act (TSCA) and the Resource Conservation and Recovery Act (RCRA). Both HF and methanol are listed as hazardous substances under CERCLA (Comprehensive Environmental Response, Compensation, and Liability Act), with reportable quantities established for accidental releases.
International frameworks add another layer of compliance requirements. The European Union's REACH (Registration, Evaluation, Authorization and Restriction of Chemicals) regulation imposes strict documentation and risk management protocols for HF and methanol usage. Similarly, the Globally Harmonized System of Classification and Labelling of Chemicals (GHS) mandates specific hazard communication standards that must be followed in international trade and domestic handling.
Industry-specific regulations further complicate compliance efforts. In semiconductor manufacturing, where HF is commonly used for silicon etching, additional protocols from organizations like SEMI (Semiconductor Equipment and Materials International) must be observed. The petroleum industry, which utilizes both chemicals in various processes, must adhere to API (American Petroleum Institute) standards alongside governmental regulations.
Waste management presents particular regulatory challenges. Both chemicals are classified as hazardous waste when disposed of, triggering comprehensive tracking, reporting, and treatment requirements under RCRA. Companies must maintain detailed waste manifests and utilize authorized treatment, storage, and disposal facilities.
Emerging regulatory trends indicate increasing scrutiny of these solvents. Several jurisdictions are implementing chemical substitution requirements, mandating that less hazardous alternatives be used when technically and economically feasible. California's Safer Consumer Products regulations and the EU's sustainability initiatives exemplify this shift toward inherently safer chemistry.
Compliance strategies must therefore be dynamic and forward-looking. Organizations utilizing HF and methanol should implement comprehensive management systems that address current requirements while anticipating regulatory evolution. This includes regular compliance audits, staff training programs, and engagement with regulatory developments through industry associations and direct agency communications.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!