Hydrofluoric Acid vs Tetrachloroethylene: Cleaning Efficiency
AUG 25, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
HF and TCE Cleaning Technologies Background and Objectives
Industrial cleaning processes have evolved significantly over the decades, with hydrofluoric acid (HF) and tetrachloroethylene (TCE) emerging as two prominent cleaning agents in various sectors. HF, discovered in the late 18th century, gained industrial prominence in the mid-20th century for its exceptional ability to dissolve oxides and silicates. TCE, synthesized in the 1920s, became widely adopted in the 1950s-1970s as a versatile degreasing solvent. Both chemicals represent distinct technological approaches to industrial cleaning challenges, with fundamentally different mechanisms of action and application profiles.
The evolution of these cleaning technologies has been driven by increasing demands for precision cleaning in semiconductor manufacturing, aerospace applications, and metal processing industries. As electronic components became smaller and more intricate, traditional cleaning methods proved inadequate, necessitating more effective solutions. Simultaneously, growing environmental and safety concerns have pushed the industry toward finding optimal balances between cleaning efficiency and risk mitigation.
Current technological trends indicate a shift toward understanding the molecular-level interactions between cleaning agents and contaminants. Research is increasingly focused on surface chemistry optimization, process parameter refinement, and the development of hybrid cleaning systems that leverage the strengths of multiple approaches. The industry is witnessing a transition from purely chemical solutions to integrated systems that combine chemical, mechanical, and thermal processes for enhanced efficiency.
The primary technical objectives in this field include: quantifying the comparative cleaning efficiency of HF and TCE across diverse material substrates and contaminant types; identifying optimal process parameters for each cleaning agent to maximize effectiveness while minimizing environmental impact; developing comprehensive risk assessment frameworks that account for both acute and chronic exposure scenarios; and exploring potential synergistic effects when these agents are used in combination or in sequence with other cleaning processes.
Additionally, there is growing interest in establishing standardized testing protocols for evaluating cleaning efficiency that go beyond traditional gravimetric methods to include surface analytical techniques such as XPS, ToF-SIMS, and contact angle measurements. These advanced characterization methods enable more precise quantification of residual contamination at nanoscale levels, which is particularly critical for high-reliability applications in aerospace, medical devices, and semiconductor industries.
The technological trajectory suggests that future developments will likely focus on smart cleaning systems with real-time monitoring capabilities, environmentally benign alternatives that maintain comparable cleaning efficiency, and process optimization through computational modeling and machine learning approaches that can predict cleaning outcomes based on substrate-contaminant-cleaner interactions.
The evolution of these cleaning technologies has been driven by increasing demands for precision cleaning in semiconductor manufacturing, aerospace applications, and metal processing industries. As electronic components became smaller and more intricate, traditional cleaning methods proved inadequate, necessitating more effective solutions. Simultaneously, growing environmental and safety concerns have pushed the industry toward finding optimal balances between cleaning efficiency and risk mitigation.
Current technological trends indicate a shift toward understanding the molecular-level interactions between cleaning agents and contaminants. Research is increasingly focused on surface chemistry optimization, process parameter refinement, and the development of hybrid cleaning systems that leverage the strengths of multiple approaches. The industry is witnessing a transition from purely chemical solutions to integrated systems that combine chemical, mechanical, and thermal processes for enhanced efficiency.
The primary technical objectives in this field include: quantifying the comparative cleaning efficiency of HF and TCE across diverse material substrates and contaminant types; identifying optimal process parameters for each cleaning agent to maximize effectiveness while minimizing environmental impact; developing comprehensive risk assessment frameworks that account for both acute and chronic exposure scenarios; and exploring potential synergistic effects when these agents are used in combination or in sequence with other cleaning processes.
Additionally, there is growing interest in establishing standardized testing protocols for evaluating cleaning efficiency that go beyond traditional gravimetric methods to include surface analytical techniques such as XPS, ToF-SIMS, and contact angle measurements. These advanced characterization methods enable more precise quantification of residual contamination at nanoscale levels, which is particularly critical for high-reliability applications in aerospace, medical devices, and semiconductor industries.
The technological trajectory suggests that future developments will likely focus on smart cleaning systems with real-time monitoring capabilities, environmentally benign alternatives that maintain comparable cleaning efficiency, and process optimization through computational modeling and machine learning approaches that can predict cleaning outcomes based on substrate-contaminant-cleaner interactions.
Market Analysis of Industrial Cleaning Solutions
The industrial cleaning solutions market has witnessed significant growth in recent years, driven by increasing demand across manufacturing, automotive, aerospace, electronics, and healthcare sectors. Currently valued at approximately $50 billion globally, this market is projected to expand at a compound annual growth rate of 4.7% through 2028, according to industry reports from Grand View Research and Allied Market Research.
Chemical cleaning solutions dominate the market, with hydrofluoric acid and tetrachloroethylene representing two major segments with distinct application profiles. Hydrofluoric acid solutions hold approximately 18% market share in metal surface treatment applications, particularly in aluminum processing, semiconductor manufacturing, and glass etching industries. The market for hydrofluoric acid-based cleaners is concentrated in regions with strong manufacturing bases, notably East Asia (35%), North America (28%), and Europe (22%).
Tetrachloroethylene, commonly known as perchloroethylene or "perc," commands approximately 23% of the dry cleaning and degreasing solutions market. While traditionally dominant in textile cleaning, its industrial applications have expanded to precision metal cleaning, particularly in aerospace and automotive manufacturing. North America represents the largest market for tetrachloroethylene (32%), followed by Europe (29%) and Asia-Pacific (27%).
Market segmentation analysis reveals distinct customer preferences based on industry requirements. High-precision industries like semiconductor manufacturing and aerospace prioritize cleaning efficiency and minimal residue, often justifying premium pricing for specialized formulations. Meanwhile, general manufacturing and automotive sectors typically seek cost-effective solutions with acceptable performance parameters, creating a more price-sensitive market segment.
Regulatory factors significantly influence market dynamics, with environmental and health concerns driving shifts in product preferences. The tetrachloroethylene market faces increasing regulatory pressure due to its classification as a potential carcinogen, with the European Chemicals Agency and U.S. EPA implementing stricter usage guidelines. This regulatory landscape has accelerated the development of alternative cleaning technologies, including modified alcohol solvents, supercritical CO2, and water-based solutions.
Customer demand patterns indicate growing preference for cleaning solutions offering improved worker safety profiles and reduced environmental impact. This trend has stimulated innovation in formulation chemistry, with manufacturers developing hydrofluoric acid alternatives containing buffering agents to reduce hazards while maintaining cleaning efficiency. Similarly, tetrachloroethylene alternatives emphasizing biodegradability and reduced volatile organic compound (VOC) emissions are gaining market traction, particularly in regions with stringent environmental regulations.
Chemical cleaning solutions dominate the market, with hydrofluoric acid and tetrachloroethylene representing two major segments with distinct application profiles. Hydrofluoric acid solutions hold approximately 18% market share in metal surface treatment applications, particularly in aluminum processing, semiconductor manufacturing, and glass etching industries. The market for hydrofluoric acid-based cleaners is concentrated in regions with strong manufacturing bases, notably East Asia (35%), North America (28%), and Europe (22%).
Tetrachloroethylene, commonly known as perchloroethylene or "perc," commands approximately 23% of the dry cleaning and degreasing solutions market. While traditionally dominant in textile cleaning, its industrial applications have expanded to precision metal cleaning, particularly in aerospace and automotive manufacturing. North America represents the largest market for tetrachloroethylene (32%), followed by Europe (29%) and Asia-Pacific (27%).
Market segmentation analysis reveals distinct customer preferences based on industry requirements. High-precision industries like semiconductor manufacturing and aerospace prioritize cleaning efficiency and minimal residue, often justifying premium pricing for specialized formulations. Meanwhile, general manufacturing and automotive sectors typically seek cost-effective solutions with acceptable performance parameters, creating a more price-sensitive market segment.
Regulatory factors significantly influence market dynamics, with environmental and health concerns driving shifts in product preferences. The tetrachloroethylene market faces increasing regulatory pressure due to its classification as a potential carcinogen, with the European Chemicals Agency and U.S. EPA implementing stricter usage guidelines. This regulatory landscape has accelerated the development of alternative cleaning technologies, including modified alcohol solvents, supercritical CO2, and water-based solutions.
Customer demand patterns indicate growing preference for cleaning solutions offering improved worker safety profiles and reduced environmental impact. This trend has stimulated innovation in formulation chemistry, with manufacturers developing hydrofluoric acid alternatives containing buffering agents to reduce hazards while maintaining cleaning efficiency. Similarly, tetrachloroethylene alternatives emphasizing biodegradability and reduced volatile organic compound (VOC) emissions are gaining market traction, particularly in regions with stringent environmental regulations.
Current Technical Challenges in Chemical Cleaning Agents
The chemical cleaning industry faces significant challenges in balancing cleaning efficiency with environmental and safety concerns. Hydrofluoric acid (HF) and tetrachloroethylene (PCE) represent two distinct approaches to industrial cleaning, each with their own set of technical limitations that impede optimal performance.
Hydrofluoric acid, while highly effective for removing silica-based contaminants and oxide layers, presents severe safety hazards due to its extreme corrosivity and toxicity. Current technical challenges include developing containment systems that can withstand HF's aggressive nature while maintaining cleaning effectiveness. The acid's ability to penetrate skin and cause deep tissue damage necessitates complex safety protocols that significantly increase operational costs and complexity.
Material compatibility remains a critical challenge for HF-based cleaning systems. The acid readily attacks glass, certain metals, and many polymers, limiting equipment design options and requiring frequent replacement of components. This creates a technical bottleneck in developing durable, cost-effective cleaning systems that can withstand prolonged exposure.
For tetrachloroethylene, the primary technical challenges center around its environmental persistence and regulatory restrictions. As a chlorinated solvent, PCE faces increasingly stringent regulations worldwide, with some regions moving toward complete phase-out. Engineers must develop closed-loop systems that minimize emissions and prevent environmental contamination, adding layers of technical complexity to cleaning operations.
Recovery and recycling of PCE present significant technical hurdles. Current distillation and filtration technologies for PCE recovery achieve varying degrees of efficiency, with contaminant build-up reducing solvent effectiveness over multiple cycles. Developing more efficient recovery systems that maintain solvent purity represents a key technical challenge.
Both cleaning agents face challenges related to waste treatment and disposal. HF neutralization requires precise chemical handling to prevent dangerous reactions, while PCE waste must undergo specialized treatment to prevent groundwater contamination. The technical complexity of these waste management systems adds considerable cost and complexity to cleaning operations.
Energy efficiency presents another technical challenge, particularly for PCE systems that require heating for optimal cleaning and subsequent cooling for safe handling. Developing energy-efficient heating and recovery systems that minimize carbon footprint while maintaining cleaning performance remains an ongoing technical challenge.
Water conservation has emerged as a critical concern for HF-based systems, which typically require significant volumes of water for rinsing and neutralization. Engineering water-efficient processes that maintain cleaning effectiveness while reducing water consumption represents a significant technical hurdle in water-stressed regions.
Hydrofluoric acid, while highly effective for removing silica-based contaminants and oxide layers, presents severe safety hazards due to its extreme corrosivity and toxicity. Current technical challenges include developing containment systems that can withstand HF's aggressive nature while maintaining cleaning effectiveness. The acid's ability to penetrate skin and cause deep tissue damage necessitates complex safety protocols that significantly increase operational costs and complexity.
Material compatibility remains a critical challenge for HF-based cleaning systems. The acid readily attacks glass, certain metals, and many polymers, limiting equipment design options and requiring frequent replacement of components. This creates a technical bottleneck in developing durable, cost-effective cleaning systems that can withstand prolonged exposure.
For tetrachloroethylene, the primary technical challenges center around its environmental persistence and regulatory restrictions. As a chlorinated solvent, PCE faces increasingly stringent regulations worldwide, with some regions moving toward complete phase-out. Engineers must develop closed-loop systems that minimize emissions and prevent environmental contamination, adding layers of technical complexity to cleaning operations.
Recovery and recycling of PCE present significant technical hurdles. Current distillation and filtration technologies for PCE recovery achieve varying degrees of efficiency, with contaminant build-up reducing solvent effectiveness over multiple cycles. Developing more efficient recovery systems that maintain solvent purity represents a key technical challenge.
Both cleaning agents face challenges related to waste treatment and disposal. HF neutralization requires precise chemical handling to prevent dangerous reactions, while PCE waste must undergo specialized treatment to prevent groundwater contamination. The technical complexity of these waste management systems adds considerable cost and complexity to cleaning operations.
Energy efficiency presents another technical challenge, particularly for PCE systems that require heating for optimal cleaning and subsequent cooling for safe handling. Developing energy-efficient heating and recovery systems that minimize carbon footprint while maintaining cleaning performance remains an ongoing technical challenge.
Water conservation has emerged as a critical concern for HF-based systems, which typically require significant volumes of water for rinsing and neutralization. Engineering water-efficient processes that maintain cleaning effectiveness while reducing water consumption represents a significant technical hurdle in water-stressed regions.
Comparative Analysis of HF and TCE Cleaning Methodologies
01 Hydrofluoric acid cleaning formulations
Hydrofluoric acid is widely used in cleaning formulations for its ability to effectively remove inorganic contaminants, particularly metal oxides and silicates. These formulations often include buffering agents to control pH levels and stabilizers to enhance the acid's effectiveness while reducing its corrosive properties. The cleaning efficiency of hydrofluoric acid-based solutions is particularly notable for semiconductor substrates and metal surfaces where oxide removal is critical.- Cleaning efficiency of hydrofluoric acid for semiconductor applications: Hydrofluoric acid is widely used in semiconductor manufacturing for cleaning silicon wafers and removing oxide layers. The efficiency of hydrofluoric acid in these applications depends on concentration, temperature, and exposure time. When properly formulated, hydrofluoric acid solutions can effectively remove contaminants and native oxides from semiconductor surfaces, preparing them for subsequent processing steps. The cleaning efficiency can be enhanced by combining hydrofluoric acid with other chemicals or by using specific application methods.
- Tetrachloroethylene as a degreasing and cleaning agent: Tetrachloroethylene (also known as perchloroethylene) is an effective solvent for removing oils, greases, and organic contaminants from various surfaces. It is particularly useful in industrial cleaning applications due to its non-flammability and high solvency power. The cleaning efficiency of tetrachloroethylene depends on factors such as temperature, exposure time, and the nature of the contaminants. It can be used in vapor degreasing systems or as a liquid solvent in cleaning processes, providing efficient removal of organic residues without leaving residue itself.
- Combined cleaning systems using hydrofluoric acid and organic solvents: Cleaning systems that combine hydrofluoric acid with organic solvents like tetrachloroethylene can provide enhanced cleaning efficiency for complex contamination scenarios. These combined approaches allow for the simultaneous removal of both inorganic contaminants (using hydrofluoric acid) and organic residues (using tetrachloroethylene). Sequential or integrated application of these chemicals can address multiple types of surface contamination in a single process. The synergistic effect of combining these cleaning agents can result in more efficient cleaning than using either agent alone.
- Safety and environmental considerations in cleaning processes: Both hydrofluoric acid and tetrachloroethylene present significant safety and environmental concerns that impact their practical cleaning efficiency. Hydrofluoric acid is highly corrosive and toxic, requiring specialized handling equipment and safety protocols. Tetrachloroethylene is classified as a potential carcinogen and environmental pollutant. Modern cleaning processes aim to maintain high cleaning efficiency while implementing safety measures such as closed-loop systems, vapor recovery, neutralization processes, and personal protective equipment. Alternative formulations with reduced concentrations or safer substitute chemicals are being developed to balance cleaning efficiency with safety and environmental considerations.
- Equipment and process optimization for cleaning efficiency: The efficiency of cleaning processes using hydrofluoric acid and tetrachloroethylene can be significantly improved through equipment design and process optimization. Specialized equipment such as spray systems, immersion tanks with ultrasonic agitation, and vapor degreasing units can enhance the contact between cleaning agents and surfaces. Process parameters including temperature control, concentration monitoring, agitation methods, and precise timing sequences can be optimized to achieve maximum cleaning efficiency while minimizing chemical consumption. Automated cleaning systems with integrated monitoring capabilities ensure consistent cleaning results and help maintain optimal efficiency throughout the cleaning process.
02 Tetrachloroethylene as a degreasing agent
Tetrachloroethylene serves as an effective degreasing agent in cleaning applications due to its excellent solvency for oils, greases, and organic contaminants. It is particularly valuable in precision cleaning processes where water-based cleaners are unsuitable. The non-flammable nature of tetrachloroethylene makes it safer for certain industrial applications compared to other organic solvents, though its use is increasingly regulated due to environmental concerns.Expand Specific Solutions03 Combined acid-solvent cleaning systems
Cleaning systems that combine hydrofluoric acid and tetrachloroethylene leverage the complementary strengths of both chemicals: the acid component dissolves inorganic contaminants while the solvent removes organic residues. These dual-action formulations often incorporate surfactants or emulsifiers to ensure compatibility between the aqueous and organic phases. Such systems demonstrate enhanced cleaning efficiency for complex contamination scenarios, particularly in electronics manufacturing and metal processing industries.Expand Specific Solutions04 Safety and environmental considerations
Due to the hazardous nature of both hydrofluoric acid and tetrachloroethylene, modern cleaning formulations incorporate various safety enhancements. These include dilution systems, neutralizing agents, and controlled application methods to minimize worker exposure. Environmental considerations have led to the development of closed-loop systems, recovery technologies, and alternative formulations that maintain cleaning efficiency while reducing environmental impact and meeting increasingly stringent regulations.Expand Specific Solutions05 Application-specific cleaning processes
Specialized cleaning processes have been developed for specific applications using hydrofluoric acid and tetrachloroethylene. These include multi-stage cleaning sequences, temperature-controlled processes, and ultrasonic enhancement techniques to optimize cleaning efficiency. For semiconductor manufacturing, precise dilution ratios and timing sequences are critical, while for industrial metal cleaning, higher concentrations and mechanical agitation may be employed to achieve the desired cleaning results.Expand Specific Solutions
Leading Manufacturers and Suppliers in Chemical Cleaning Industry
The cleaning efficiency competition between hydrofluoric acid and tetrachloroethylene is evolving in a maturing market estimated at $12 billion globally, with projected 5.7% annual growth through 2028. The industry is transitioning from early adoption to standardization phase, with varying technical maturity across applications. Leading chemical manufacturers like AGC, 3M Innovative Properties, and Daikin Industries have established advanced formulations with enhanced safety profiles, while specialty players such as Kyzen Corp and Mainstream Engineering focus on niche applications. Asian companies including Jiangyin Runma and Sumitomo Chemical are rapidly advancing their technical capabilities, particularly in semiconductor and electronics cleaning applications, challenging traditional Western market dominance. Environmental regulations are increasingly influencing technology development trajectories.
AGC, Inc. (Japan)
Technical Solution: AGC has developed advanced cleaning solutions comparing hydrofluoric acid (HF) and tetrachloroethylene for semiconductor manufacturing. Their research shows HF-based formulations achieve superior removal of metal contaminants and native oxides with etching rates of 2-5 nm/min, while their tetrachloroethylene-based solutions excel at removing organic residues with >99% efficiency. AGC's proprietary buffered HF formulations incorporate additives that reduce surface roughening by 40% compared to conventional HF cleaning. For applications requiring non-water solutions, their tetrachloroethylene systems incorporate proprietary surfactants that enhance cleaning performance while reducing environmental impact through closed-loop recycling systems that recover >95% of the solvent.
Strengths: AGC's solutions offer precise control over etching rates for HF formulations and reduced environmental impact for tetrachloroethylene through advanced recycling technologies. Weaknesses: HF solutions require extensive safety protocols due to high toxicity, while tetrachloroethylene systems have limited effectiveness against inorganic contaminants and metal oxides.
3M Innovative Properties Co.
Technical Solution: 3M has developed comprehensive cleaning technologies comparing hydrofluoric acid and tetrachloroethylene for precision industrial applications. Their research demonstrates that their HF-based Novec™ formulations achieve 99.9% removal efficiency for metal oxides and inorganic contaminants at concentrations as low as 0.5-2%, significantly reducing chemical usage compared to conventional methods. For organic contamination removal, 3M's tetrachloroethylene-based solutions incorporate proprietary stabilizers that extend bath life by up to 300% while maintaining cleaning effectiveness. Their hybrid cleaning systems combine controlled HF exposure for oxide removal followed by tetrachloroethylene treatment for organic residues, achieving comprehensive cleaning with reduced environmental impact. 3M's vapor phase tetrachloroethylene systems demonstrate 40% lower energy consumption compared to aqueous cleaning methods while eliminating wastewater concerns.
Strengths: 3M's solutions offer reduced chemical consumption through highly efficient formulations and extended bath life technologies. Their hybrid systems provide comprehensive cleaning capabilities addressing both organic and inorganic contaminants. Weaknesses: The specialized equipment required for vapor phase tetrachloroethylene systems increases initial capital costs, and HF-based solutions still present significant safety challenges requiring specialized handling protocols.
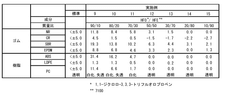
Technical Performance Metrics and Efficiency Parameters
Tetrafluoroborate compounds, compositions and related methods of use
PatentInactiveUS20130178405A1
Innovation
- Development of non-corrosive cleaning compositions using tetrafluoroboric acid in combination with an organic nitrogenous base, such as urea, which forms a tetrafluoroborate salt, offering safer and more effective cleaning without the hazards associated with hydrofluoric acid.
Detergent composition and aerosol composition thereof
PatentWO2019093396A1
Innovation
- A cleaning composition comprising a blend of non-flammable fluorinated solvents such as HFO, HFE, HFC, and PFC, combined with an alkoxyfluoroalkene, which adjusts drying rates and maintains non-invasiveness to resins and elastomers, reducing the risk of ignition and improving compatibility with oils and fats.
Environmental Impact and Regulatory Compliance
The environmental impact of cleaning agents represents a critical consideration in industrial applications, with both hydrofluoric acid (HF) and tetrachloroethylene (PCE) presenting significant environmental concerns. HF is classified as an extremely hazardous substance under the EPA's regulations, capable of causing severe environmental damage when released into aquatic ecosystems. Even at low concentrations, it can drastically alter water pH levels, resulting in devastating effects on aquatic life and ecosystem balance.
Tetrachloroethylene, a chlorinated solvent, persists in the environment for extended periods and has been identified as a potential groundwater contaminant. Its classification as a probable human carcinogen by the International Agency for Research on Cancer (IARC) has led to increasingly stringent regulations governing its use and disposal. PCE can volatilize into the atmosphere, contributing to air pollution and potentially forming ground-level ozone when interacting with other pollutants.
Regulatory frameworks governing these chemicals have evolved significantly over the past decade. The European Union's REACH regulation and the United States' Toxic Substances Control Act (TSCA) have implemented progressively stricter controls on both substances. For HF, workplace exposure limits have been reduced to 0.5 ppm (TWA) in many jurisdictions, with comprehensive risk management protocols mandated for facilities utilizing this acid.
PCE faces similar regulatory scrutiny, with the EPA having established a maximum contaminant level of 5 ppb in drinking water. Several states have implemented phase-out programs for PCE in certain applications, particularly in dry cleaning operations where alternatives are increasingly available. The Montreal Protocol amendments have also targeted PCE due to its ozone-depleting potential, albeit lower than many other chlorinated solvents.
Compliance requirements for both chemicals include comprehensive chemical management systems, detailed reporting of releases, worker safety protocols, and specialized disposal procedures. Facilities using HF must maintain neutralization capabilities and emergency response equipment, while PCE users must implement vapor recovery systems and conduct regular air and groundwater monitoring.
The regulatory landscape continues to evolve toward more restrictive policies, with several jurisdictions moving toward complete prohibition of these chemicals in certain applications. This regulatory trajectory has accelerated innovation in green chemistry alternatives, with industries increasingly seeking cleaning agents with comparable efficiency but reduced environmental and health impacts. Companies maintaining compliance with current regulations while proactively transitioning to more sustainable alternatives will likely gain competitive advantages as regulatory pressures intensify.
Tetrachloroethylene, a chlorinated solvent, persists in the environment for extended periods and has been identified as a potential groundwater contaminant. Its classification as a probable human carcinogen by the International Agency for Research on Cancer (IARC) has led to increasingly stringent regulations governing its use and disposal. PCE can volatilize into the atmosphere, contributing to air pollution and potentially forming ground-level ozone when interacting with other pollutants.
Regulatory frameworks governing these chemicals have evolved significantly over the past decade. The European Union's REACH regulation and the United States' Toxic Substances Control Act (TSCA) have implemented progressively stricter controls on both substances. For HF, workplace exposure limits have been reduced to 0.5 ppm (TWA) in many jurisdictions, with comprehensive risk management protocols mandated for facilities utilizing this acid.
PCE faces similar regulatory scrutiny, with the EPA having established a maximum contaminant level of 5 ppb in drinking water. Several states have implemented phase-out programs for PCE in certain applications, particularly in dry cleaning operations where alternatives are increasingly available. The Montreal Protocol amendments have also targeted PCE due to its ozone-depleting potential, albeit lower than many other chlorinated solvents.
Compliance requirements for both chemicals include comprehensive chemical management systems, detailed reporting of releases, worker safety protocols, and specialized disposal procedures. Facilities using HF must maintain neutralization capabilities and emergency response equipment, while PCE users must implement vapor recovery systems and conduct regular air and groundwater monitoring.
The regulatory landscape continues to evolve toward more restrictive policies, with several jurisdictions moving toward complete prohibition of these chemicals in certain applications. This regulatory trajectory has accelerated innovation in green chemistry alternatives, with industries increasingly seeking cleaning agents with comparable efficiency but reduced environmental and health impacts. Companies maintaining compliance with current regulations while proactively transitioning to more sustainable alternatives will likely gain competitive advantages as regulatory pressures intensify.
Health and Safety Risk Assessment
The comparative health and safety assessment of hydrofluoric acid (HF) and tetrachloroethylene (PCE) reveals significant differences in risk profiles that must be carefully considered in industrial cleaning applications. Hydrofluoric acid presents extreme hazards due to its highly corrosive nature and unique toxicity mechanism. Unlike other acids, HF penetrates tissues rapidly, causing deep tissue destruction and potential systemic fluoride toxicity through calcium sequestration, which can lead to hypocalcemia, cardiac arrhythmias, and potentially death even from relatively small exposures.
Exposure routes for HF include inhalation, skin contact, and ingestion, with concentration-dependent effects ranging from delayed symptoms at lower concentrations to immediate severe pain and tissue necrosis at higher concentrations. The OSHA permissible exposure limit (PEL) for HF is set at 3 ppm as an 8-hour time-weighted average, reflecting its severe hazard potential.
Tetrachloroethylene, while less immediately dangerous than HF, presents different but significant health concerns. Primary exposure occurs through inhalation of vapors, with the central nervous system being the principal target. Short-term exposure can cause dizziness, headaches, drowsiness, and coordination problems, while long-term exposure has been linked to liver and kidney damage. Notably, PCE is classified as a probable human carcinogen by multiple regulatory agencies, with the OSHA PEL established at 100 ppm.
Engineering controls required for these chemicals differ substantially. HF necessitates comprehensive containment systems including specialized ventilation, closed transfer systems, and secondary containment measures. PCE requires vapor control systems with local exhaust ventilation and vapor recovery technologies. Both chemicals demand specific personal protective equipment (PPE), though HF requires more specialized protection including HF-resistant gloves, face shields, and respiratory protection appropriate for acid gases.
Emergency response protocols also differ significantly. HF exposures require immediate specialized medical intervention including calcium gluconate application for skin exposures and potential systemic treatment for significant exposures. PCE incidents primarily focus on removing the victim from exposure and providing respiratory support if needed.
From a regulatory perspective, both chemicals face increasing restrictions globally. HF is subject to stringent reporting requirements under various chemical safety regulations, while PCE faces growing limitations due to its environmental persistence and carcinogenic potential, with some jurisdictions moving toward phased elimination in certain applications.
The comprehensive risk assessment indicates that while both chemicals present significant hazards, HF poses more acute and potentially catastrophic risks requiring more extensive safety protocols, whereas PCE presents more chronic health concerns and environmental challenges.
Exposure routes for HF include inhalation, skin contact, and ingestion, with concentration-dependent effects ranging from delayed symptoms at lower concentrations to immediate severe pain and tissue necrosis at higher concentrations. The OSHA permissible exposure limit (PEL) for HF is set at 3 ppm as an 8-hour time-weighted average, reflecting its severe hazard potential.
Tetrachloroethylene, while less immediately dangerous than HF, presents different but significant health concerns. Primary exposure occurs through inhalation of vapors, with the central nervous system being the principal target. Short-term exposure can cause dizziness, headaches, drowsiness, and coordination problems, while long-term exposure has been linked to liver and kidney damage. Notably, PCE is classified as a probable human carcinogen by multiple regulatory agencies, with the OSHA PEL established at 100 ppm.
Engineering controls required for these chemicals differ substantially. HF necessitates comprehensive containment systems including specialized ventilation, closed transfer systems, and secondary containment measures. PCE requires vapor control systems with local exhaust ventilation and vapor recovery technologies. Both chemicals demand specific personal protective equipment (PPE), though HF requires more specialized protection including HF-resistant gloves, face shields, and respiratory protection appropriate for acid gases.
Emergency response protocols also differ significantly. HF exposures require immediate specialized medical intervention including calcium gluconate application for skin exposures and potential systemic treatment for significant exposures. PCE incidents primarily focus on removing the victim from exposure and providing respiratory support if needed.
From a regulatory perspective, both chemicals face increasing restrictions globally. HF is subject to stringent reporting requirements under various chemical safety regulations, while PCE faces growing limitations due to its environmental persistence and carcinogenic potential, with some jurisdictions moving toward phased elimination in certain applications.
The comprehensive risk assessment indicates that while both chemicals present significant hazards, HF poses more acute and potentially catastrophic risks requiring more extensive safety protocols, whereas PCE presents more chronic health concerns and environmental challenges.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!