How to Determine Hydrofluoric Acid Quality in Industrial Use
AUG 25, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
HF Acid Quality Assessment Background and Objectives
Hydrofluoric acid (HF) has emerged as a critical chemical compound in various industrial applications, including semiconductor manufacturing, glass etching, metal surface treatment, and petroleum refining. The evolution of HF acid usage can be traced back to the early 19th century, with significant advancements occurring during the industrial revolution. Over the past decades, the requirements for HF acid quality have become increasingly stringent, particularly in high-precision industries where even minor impurities can lead to substantial product defects or process inefficiencies.
The technological trajectory of HF acid quality assessment has progressed from basic chemical titration methods to sophisticated analytical techniques incorporating spectroscopy, chromatography, and electrochemical analysis. This evolution reflects the growing industrial demand for higher purity standards and more reliable quality control protocols. Current industry trends indicate a continued push toward real-time monitoring capabilities and non-destructive testing methodologies that can be integrated into production environments.
The primary objective of this technical research is to comprehensively evaluate existing and emerging methodologies for determining hydrofluoric acid quality in industrial applications. This includes identifying key quality parameters that significantly impact industrial processes, analyzing the sensitivity and reliability of various detection techniques, and establishing standardized protocols that can be implemented across different industrial sectors.
Additionally, this research aims to address the critical challenges associated with HF acid quality assessment, including the detection of trace contaminants, stability monitoring during storage and transportation, and the development of safer testing procedures that minimize exposure risks to personnel. The ultimate goal is to establish a framework for quality determination that balances analytical precision with practical implementation considerations in industrial settings.
From a global perspective, the standards for HF acid quality vary significantly across regions and industries, creating challenges for international trade and technology transfer. This research will also examine regional variations in quality requirements and testing methodologies, with the intention of contributing to more harmonized international standards that can facilitate global industrial collaboration and innovation in HF-dependent technologies.
The technological objectives extend beyond mere quality assessment to encompass predictive capabilities that can anticipate quality degradation before it impacts industrial processes. This forward-looking approach aligns with Industry 4.0 principles, where data-driven decision-making and preventive maintenance strategies are becoming increasingly central to manufacturing excellence and operational efficiency.
The technological trajectory of HF acid quality assessment has progressed from basic chemical titration methods to sophisticated analytical techniques incorporating spectroscopy, chromatography, and electrochemical analysis. This evolution reflects the growing industrial demand for higher purity standards and more reliable quality control protocols. Current industry trends indicate a continued push toward real-time monitoring capabilities and non-destructive testing methodologies that can be integrated into production environments.
The primary objective of this technical research is to comprehensively evaluate existing and emerging methodologies for determining hydrofluoric acid quality in industrial applications. This includes identifying key quality parameters that significantly impact industrial processes, analyzing the sensitivity and reliability of various detection techniques, and establishing standardized protocols that can be implemented across different industrial sectors.
Additionally, this research aims to address the critical challenges associated with HF acid quality assessment, including the detection of trace contaminants, stability monitoring during storage and transportation, and the development of safer testing procedures that minimize exposure risks to personnel. The ultimate goal is to establish a framework for quality determination that balances analytical precision with practical implementation considerations in industrial settings.
From a global perspective, the standards for HF acid quality vary significantly across regions and industries, creating challenges for international trade and technology transfer. This research will also examine regional variations in quality requirements and testing methodologies, with the intention of contributing to more harmonized international standards that can facilitate global industrial collaboration and innovation in HF-dependent technologies.
The technological objectives extend beyond mere quality assessment to encompass predictive capabilities that can anticipate quality degradation before it impacts industrial processes. This forward-looking approach aligns with Industry 4.0 principles, where data-driven decision-making and preventive maintenance strategies are becoming increasingly central to manufacturing excellence and operational efficiency.
Market Demand Analysis for High-Purity HF Acid
The global market for high-purity hydrofluoric acid (HF) continues to expand significantly, driven primarily by the semiconductor industry's growing demand for ultra-pure chemicals. Current market valuations place the high-purity HF sector at approximately 3.2 billion USD in 2023, with projections indicating a compound annual growth rate of 6.8% through 2030.
Electronics manufacturing represents the largest consumption segment, accounting for nearly 42% of high-purity HF demand. The semiconductor industry specifically requires HF with purity levels exceeding 99.99% for critical processes including silicon wafer cleaning, etching, and surface preparation. As chip manufacturers continue to develop more advanced nodes at 3nm and below, the specifications for chemical purity become increasingly stringent.
The photovoltaic industry emerges as another significant growth driver, with solar panel production requiring high-purity HF for texturing silicon wafers and removing damage caused during the wafer slicing process. Market analysis indicates that solar applications now represent approximately 18% of high-purity HF consumption, with this percentage expected to increase as global renewable energy installations accelerate.
Pharmaceutical manufacturing constitutes a smaller but premium market segment, utilizing high-purity HF in the synthesis of various fluorinated compounds and active pharmaceutical ingredients. This sector demands exceptional quality control and traceability, often paying premium prices for guaranteed purity levels.
Regional analysis reveals Asia-Pacific as the dominant market, accounting for over 65% of global high-purity HF consumption. This concentration aligns with the region's manufacturing footprint in electronics and semiconductor production. North America and Europe follow with approximately 18% and 12% market share respectively, primarily driven by specialty applications in pharmaceuticals and advanced materials.
Customer requirements are evolving beyond mere purity specifications to include comprehensive quality parameters. End-users increasingly demand detailed impurity profiles, particle counts, and metal contamination analyses. This shift reflects the growing recognition that trace contaminants, even at parts-per-billion levels, can significantly impact production yields in high-precision manufacturing processes.
Supply chain security has emerged as a critical market factor following recent global disruptions. Many industrial consumers are now willing to pay premium prices for guaranteed supply continuity and quality consistency, creating opportunities for producers who can demonstrate robust quality determination methodologies and stable manufacturing processes.
Electronics manufacturing represents the largest consumption segment, accounting for nearly 42% of high-purity HF demand. The semiconductor industry specifically requires HF with purity levels exceeding 99.99% for critical processes including silicon wafer cleaning, etching, and surface preparation. As chip manufacturers continue to develop more advanced nodes at 3nm and below, the specifications for chemical purity become increasingly stringent.
The photovoltaic industry emerges as another significant growth driver, with solar panel production requiring high-purity HF for texturing silicon wafers and removing damage caused during the wafer slicing process. Market analysis indicates that solar applications now represent approximately 18% of high-purity HF consumption, with this percentage expected to increase as global renewable energy installations accelerate.
Pharmaceutical manufacturing constitutes a smaller but premium market segment, utilizing high-purity HF in the synthesis of various fluorinated compounds and active pharmaceutical ingredients. This sector demands exceptional quality control and traceability, often paying premium prices for guaranteed purity levels.
Regional analysis reveals Asia-Pacific as the dominant market, accounting for over 65% of global high-purity HF consumption. This concentration aligns with the region's manufacturing footprint in electronics and semiconductor production. North America and Europe follow with approximately 18% and 12% market share respectively, primarily driven by specialty applications in pharmaceuticals and advanced materials.
Customer requirements are evolving beyond mere purity specifications to include comprehensive quality parameters. End-users increasingly demand detailed impurity profiles, particle counts, and metal contamination analyses. This shift reflects the growing recognition that trace contaminants, even at parts-per-billion levels, can significantly impact production yields in high-precision manufacturing processes.
Supply chain security has emerged as a critical market factor following recent global disruptions. Many industrial consumers are now willing to pay premium prices for guaranteed supply continuity and quality consistency, creating opportunities for producers who can demonstrate robust quality determination methodologies and stable manufacturing processes.
Current State and Challenges in HF Quality Control
The global hydrofluoric acid (HF) quality control landscape presents a complex mix of established methodologies and emerging challenges. Currently, the predominant analytical techniques include titration methods, ion-selective electrode analysis, spectrophotometric determination, and ion chromatography. These methods vary significantly in precision, with titration offering ±0.5% accuracy while more advanced spectroscopic techniques can achieve ±0.1% under optimal conditions.
Industry standards for HF quality control remain fragmented across regions, with the American Society for Testing and Materials (ASTM) and International Organization for Standardization (ISO) providing the most widely adopted frameworks. However, implementation consistency varies substantially between developed and developing markets, creating quality assurance challenges in global supply chains.
A significant technical challenge in HF quality assessment involves the detection and quantification of trace metal contaminants, particularly iron, nickel, and chromium, which can significantly impact downstream applications even at concentrations below 10 ppm. Current detection limits using conventional methods often prove inadequate for high-purity applications in semiconductor manufacturing and pharmaceutical processing.
The highly corrosive nature of HF presents persistent sampling and handling difficulties, requiring specialized equipment and safety protocols that increase testing complexity and cost. This has created a technological bottleneck where accuracy requirements conflict with practical implementation constraints, especially in continuous monitoring scenarios.
Real-time monitoring capabilities remain underdeveloped, with most quality control processes still relying on batch sampling rather than continuous assessment. This creates blind spots in production environments where quality fluctuations between sampling intervals may go undetected, potentially compromising product integrity.
Emerging challenges include the increasing demand for ultra-high-purity HF (>99.999%) in advanced electronics manufacturing, which exceeds the detection capabilities of many conventional analytical methods. Additionally, environmental regulations are driving requirements for more sensitive detection of HF in waste streams and emissions, necessitating new analytical approaches.
The digitalization gap in HF quality control is notable, with limited integration of data analytics and predictive quality models. While other chemical processes have benefited from Industry 4.0 technologies, HF quality assessment remains largely dependent on discrete measurements rather than integrated data systems that could identify trends and predict quality deviations before they occur.
Industry standards for HF quality control remain fragmented across regions, with the American Society for Testing and Materials (ASTM) and International Organization for Standardization (ISO) providing the most widely adopted frameworks. However, implementation consistency varies substantially between developed and developing markets, creating quality assurance challenges in global supply chains.
A significant technical challenge in HF quality assessment involves the detection and quantification of trace metal contaminants, particularly iron, nickel, and chromium, which can significantly impact downstream applications even at concentrations below 10 ppm. Current detection limits using conventional methods often prove inadequate for high-purity applications in semiconductor manufacturing and pharmaceutical processing.
The highly corrosive nature of HF presents persistent sampling and handling difficulties, requiring specialized equipment and safety protocols that increase testing complexity and cost. This has created a technological bottleneck where accuracy requirements conflict with practical implementation constraints, especially in continuous monitoring scenarios.
Real-time monitoring capabilities remain underdeveloped, with most quality control processes still relying on batch sampling rather than continuous assessment. This creates blind spots in production environments where quality fluctuations between sampling intervals may go undetected, potentially compromising product integrity.
Emerging challenges include the increasing demand for ultra-high-purity HF (>99.999%) in advanced electronics manufacturing, which exceeds the detection capabilities of many conventional analytical methods. Additionally, environmental regulations are driving requirements for more sensitive detection of HF in waste streams and emissions, necessitating new analytical approaches.
The digitalization gap in HF quality control is notable, with limited integration of data analytics and predictive quality models. While other chemical processes have benefited from Industry 4.0 technologies, HF quality assessment remains largely dependent on discrete measurements rather than integrated data systems that could identify trends and predict quality deviations before they occur.
Existing HF Quality Determination Techniques
01 Purification methods for hydrofluoric acid
Various purification techniques are employed to improve the quality of hydrofluoric acid. These methods include distillation, filtration, and chemical treatments to remove impurities such as metal ions, particulates, and other contaminants. Advanced purification processes can achieve high-purity hydrofluoric acid suitable for semiconductor manufacturing and other precision applications where impurity levels must be strictly controlled.- Purification methods for hydrofluoric acid: Various purification techniques are employed to enhance the quality of hydrofluoric acid. These methods include distillation processes, filtration systems, and chemical treatments to remove impurities such as metal ions, particulates, and other contaminants. Advanced purification methods can achieve high-purity hydrofluoric acid suitable for semiconductor and electronics manufacturing applications where ultra-pure reagents are required.
- Quality control and analysis techniques: Specialized analytical methods are used to assess and maintain the quality of hydrofluoric acid. These techniques include spectroscopic analysis, chromatography, and electrochemical methods to detect and quantify impurities. Quality control protocols involve regular testing for specific contaminants, concentration verification, and stability monitoring to ensure the acid meets industry standards and specifications for various applications.
- Production processes for high-quality hydrofluoric acid: Manufacturing processes significantly impact the quality of hydrofluoric acid. Advanced production methods focus on optimizing reaction conditions, using high-grade raw materials, and implementing precise process controls. Innovations in production technology include continuous flow reactors, improved catalyst systems, and enhanced reaction monitoring to produce consistent, high-quality hydrofluoric acid with minimal impurities.
- Storage and handling to maintain quality: Proper storage and handling practices are essential for maintaining hydrofluoric acid quality over time. Specialized containers made from materials resistant to HF corrosion, such as certain polymers or specially treated metals, are used. Environmental factors like temperature control, moisture exclusion, and protection from contaminants are critical. Stabilizing additives may be incorporated to extend shelf life and preserve acid quality during storage and transportation.
- Applications requiring specific quality grades: Different applications demand specific quality grades of hydrofluoric acid. Semiconductor manufacturing requires ultra-high-purity acid with extremely low levels of metallic impurities. Glass etching applications need consistent concentration and minimal particulates. Chemical synthesis applications may require specific pH ranges and stability characteristics. The quality requirements are tailored to the end-use, with specialized formulations developed for critical applications in electronics, pharmaceuticals, and materials processing.
02 Quality control and analysis techniques
Specialized analytical methods are used to assess and maintain the quality of hydrofluoric acid. These techniques include spectroscopic analysis, chromatography, and electrochemical methods to detect and quantify impurities. Quality control protocols involve regular testing and monitoring of acid batches to ensure they meet specified purity standards and performance requirements for industrial applications.Expand Specific Solutions03 Production processes affecting quality
The manufacturing process significantly impacts the quality of hydrofluoric acid. Key factors include raw material selection, reaction conditions, and post-production handling. Optimized production methods can minimize the formation of byproducts and contaminants. Innovations in production technology focus on improving yield while maintaining or enhancing the purity level of the final hydrofluoric acid product.Expand Specific Solutions04 Storage and handling to maintain quality
Proper storage and handling procedures are essential for maintaining hydrofluoric acid quality over time. This includes using appropriate container materials resistant to HF corrosion, temperature-controlled storage environments, and specialized transfer systems. Degradation of acid quality can occur through contamination, exposure to moisture, or reaction with container materials, necessitating specific protocols for industrial and laboratory settings.Expand Specific Solutions05 Application-specific quality requirements
Different applications of hydrofluoric acid require specific quality parameters. For semiconductor manufacturing, ultra-high purity acid with minimal metal contamination is essential. In metal surface treatment, the concentration and specific impurity profile may be more important than absolute purity. Quality specifications are tailored to end-use requirements, with varying tolerances for particular impurities depending on how they affect performance in specific applications.Expand Specific Solutions
Key Industry Players in HF Production and Analysis
The hydrofluoric acid quality determination market is currently in a growth phase, driven by increasing demand in semiconductor manufacturing, glass etching, and metal processing industries. The global market size is estimated to exceed $2 billion, with a projected CAGR of 5-7% through 2027. Technologically, the field shows varying maturity levels across different applications. Leading players like Do-Fluoride New Materials and Stella Chemifa have established advanced analytical techniques for high-purity acid quality control, while companies such as Runma Electronic Material and Hubei Sinophorus focus on ultra-pure grades for semiconductor applications. Research institutions including Shandong University and CEA are developing next-generation quality assessment methodologies, while established industrial giants like Siemens and Honeywell offer integrated quality monitoring systems.
Stella Chemifa Corp.
Technical Solution: Stella Chemifa has developed an advanced quality control system specifically for ultra-high-purity hydrofluoric acid used in semiconductor manufacturing. Their technology integrates automated sampling systems with multiple analytical techniques including ICP-MS for metal impurity detection at sub-ppb levels, ion chromatography for anion analysis, and particle counters for particulate contamination assessment. The company's proprietary "HF-Scan" technology employs near-infrared spectroscopy combined with chemometric algorithms to provide real-time concentration monitoring without sample extraction. Stella Chemifa has also pioneered a novel electrochemical sensor array that can simultaneously detect multiple quality parameters including acid concentration, oxidation-reduction potential, and specific metal contaminants, allowing for comprehensive quality profiling with minimal sample volume requirements.
Strengths: Industry-leading detection limits for semiconductor-grade HF; integrated automation reduces human exposure to hazardous materials; comprehensive multi-parameter analysis in a single system. Weaknesses: Solutions are primarily optimized for ultra-high-purity applications and may be overengineered for standard industrial uses; significant capital investment required for implementation.
Ecolab USA, Inc.
Technical Solution: Ecolab has developed the "HF-Quality Assurance System," a comprehensive solution for industrial hydrofluoric acid quality determination. Their approach combines traditional analytical methods with digital monitoring technologies to create a holistic quality management framework. The system features automated sampling stations that extract HF samples at predetermined intervals, coupled with multi-parameter analyzers that measure concentration, density, conductivity, and pH simultaneously. Ecolab's proprietary algorithms correlate these measurements to detect quality deviations before they impact production processes. Their technology includes specialized fluoropolymer-lined sensors capable of withstanding HF's corrosive properties while providing accurate readings in real-time. The system also incorporates cloud-based data management that enables trend analysis, predictive maintenance, and remote monitoring capabilities, allowing facilities to optimize HF usage while maintaining strict quality parameters.
Strengths: Comprehensive integration with existing industrial systems; robust data analytics for predictive quality management; excellent technical support network. Weaknesses: Subscription-based service model increases long-term costs; system requires regular calibration and maintenance to maintain accuracy.
Critical Technologies for HF Purity Assessment
Apparatus and method for determining hydrofluoric acid in aqueous solutions
PatentInactiveEP0293663A3
Innovation
- A portable device with a silicon electrode and platinum counter-electrode arrangement that utilizes anodic oxidation of silicon oxide to detect hydrofluoric acid, indicated by a light-emitting diode, allowing for qualitative assessment and precise measurement using calibration solutions, and further quantification with an ammeter.
Process for the production of HCFC-1233zd
PatentPendingEP4461716A2
Innovation
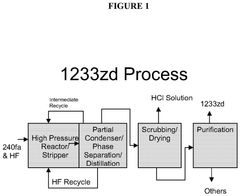
- A process involving a high-pressure liquid phase reaction of HCC-240fa and HF in a reactor, followed by partial condensation, phase separation, recycling of HF, scrubbing and recovery of HCl, and distillation to produce HCFC-1233zd with a pressure range of 150 psig to 600 psig, utilizing catalysts like TiCl4 and SbCl5, and employing various reactor designs such as stirred-tank, plug flow, and static mixers, to achieve commercial product specifications.
Safety Protocols for HF Handling and Testing
Handling hydrofluoric acid (HF) requires stringent safety protocols due to its highly corrosive nature and ability to cause severe tissue damage upon contact. All personnel involved in HF quality determination must undergo comprehensive training before handling this hazardous substance. Training should cover emergency response procedures, proper use of personal protective equipment (PPE), and specific testing methodologies.
The minimum required PPE for HF handling includes chemical-resistant full-face shields, neoprene or butyl rubber gloves with extended cuffs, acid-resistant lab coats or full-body suits, and closed-toe chemical-resistant footwear. Double gloving is strongly recommended, with regular inspection for degradation during use. Respiratory protection with appropriate acid gas cartridges must be available when working with concentrated solutions or in poorly ventilated areas.
Laboratory facilities for HF testing must be equipped with specialized safety infrastructure including calcium gluconate gel stations positioned within arm's reach of all handling areas, dedicated emergency showers and eyewash stations, and HF-specific spill kits. Ventilation systems must maintain negative pressure with a minimum of 10 air changes per hour, and all testing should be conducted within properly functioning fume hoods.
Quality determination procedures must incorporate specific safety checkpoints. These include mandatory buddy systems where no personnel work alone with HF, regular equipment integrity checks before testing begins, and clear documentation of all safety measures taken. Testing protocols should minimize sample volumes to reduce exposure risks, with typical analytical procedures using no more than 5-10 ml of acid when possible.
Emergency response protocols must be prominently displayed in all testing areas, with clear step-by-step instructions for different exposure scenarios. First aid supplies, particularly calcium gluconate gel (2.5% concentration), must be readily available and regularly checked for expiration dates. Facilities should establish direct communication channels with local emergency medical services familiar with HF exposure treatment.
Waste management represents another critical safety component, with all HF-containing waste requiring neutralization before disposal. Typically, this involves careful dilution followed by neutralization with calcium hydroxide to form insoluble calcium fluoride. Verification testing must confirm complete neutralization before disposal according to local regulations.
Regular safety audits and drills should be conducted quarterly to ensure all protocols remain effective and personnel maintain readiness for emergency situations. Documentation of these drills, along with any incidents or near-misses, provides valuable data for continuous improvement of safety measures in HF quality determination processes.
The minimum required PPE for HF handling includes chemical-resistant full-face shields, neoprene or butyl rubber gloves with extended cuffs, acid-resistant lab coats or full-body suits, and closed-toe chemical-resistant footwear. Double gloving is strongly recommended, with regular inspection for degradation during use. Respiratory protection with appropriate acid gas cartridges must be available when working with concentrated solutions or in poorly ventilated areas.
Laboratory facilities for HF testing must be equipped with specialized safety infrastructure including calcium gluconate gel stations positioned within arm's reach of all handling areas, dedicated emergency showers and eyewash stations, and HF-specific spill kits. Ventilation systems must maintain negative pressure with a minimum of 10 air changes per hour, and all testing should be conducted within properly functioning fume hoods.
Quality determination procedures must incorporate specific safety checkpoints. These include mandatory buddy systems where no personnel work alone with HF, regular equipment integrity checks before testing begins, and clear documentation of all safety measures taken. Testing protocols should minimize sample volumes to reduce exposure risks, with typical analytical procedures using no more than 5-10 ml of acid when possible.
Emergency response protocols must be prominently displayed in all testing areas, with clear step-by-step instructions for different exposure scenarios. First aid supplies, particularly calcium gluconate gel (2.5% concentration), must be readily available and regularly checked for expiration dates. Facilities should establish direct communication channels with local emergency medical services familiar with HF exposure treatment.
Waste management represents another critical safety component, with all HF-containing waste requiring neutralization before disposal. Typically, this involves careful dilution followed by neutralization with calcium hydroxide to form insoluble calcium fluoride. Verification testing must confirm complete neutralization before disposal according to local regulations.
Regular safety audits and drills should be conducted quarterly to ensure all protocols remain effective and personnel maintain readiness for emergency situations. Documentation of these drills, along with any incidents or near-misses, provides valuable data for continuous improvement of safety measures in HF quality determination processes.
Environmental Impact and Regulatory Compliance
The industrial use of hydrofluoric acid (HF) carries significant environmental implications that necessitate stringent regulatory oversight. When released into the environment, HF can cause severe ecological damage, particularly to aquatic ecosystems where even low concentrations can be lethal to fish and other organisms. The acid's high water solubility enables rapid dispersion in water bodies, potentially affecting large areas before detection occurs.
Regulatory frameworks governing HF usage vary globally but generally focus on three key areas: emissions control, waste management, and workplace safety. In the United States, the Environmental Protection Agency (EPA) regulates HF under multiple statutes including the Clean Air Act, where it is listed as a Hazardous Air Pollutant, and the Comprehensive Environmental Response, Compensation, and Liability Act (CERCLA), which mandates reporting of releases exceeding reportable quantities.
The European Union's REACH (Registration, Evaluation, Authorization and Restriction of Chemicals) regulation imposes additional requirements for HF manufacturers and importers, including comprehensive safety assessments and risk management measures. Similarly, China has implemented the Measures for Environmental Management of New Chemical Substances, which specifically addresses highly hazardous chemicals including HF.
Quality determination protocols for HF must incorporate compliance verification elements to ensure adherence to these regulations. This includes regular monitoring of emission levels, proper documentation of waste disposal practices, and verification that acid purity meets environmental standards. Many jurisdictions require facilities to implement continuous emission monitoring systems (CEMS) for real-time tracking of HF releases.
The relationship between HF quality and environmental impact is direct and significant. Higher-purity HF typically contains fewer metal contaminants that might otherwise be released into the environment during use or disposal. However, higher concentration also increases potential hazard severity in case of accidental release, necessitating more robust containment systems and emergency response protocols.
Companies utilizing industrial HF increasingly face pressure to adopt circular economy principles, including acid recycling and regeneration technologies. These approaches not only reduce environmental footprint but often improve economic efficiency. Advanced quality determination methods that can accurately assess recycled HF purity have therefore become essential components of environmentally responsible industrial practices.
Compliance with evolving regulatory standards requires ongoing investment in analytical capabilities. Many jurisdictions now mandate regular third-party verification of quality control procedures and environmental management systems, creating additional incentives for developing reliable, standardized quality determination methodologies for industrial HF.
Regulatory frameworks governing HF usage vary globally but generally focus on three key areas: emissions control, waste management, and workplace safety. In the United States, the Environmental Protection Agency (EPA) regulates HF under multiple statutes including the Clean Air Act, where it is listed as a Hazardous Air Pollutant, and the Comprehensive Environmental Response, Compensation, and Liability Act (CERCLA), which mandates reporting of releases exceeding reportable quantities.
The European Union's REACH (Registration, Evaluation, Authorization and Restriction of Chemicals) regulation imposes additional requirements for HF manufacturers and importers, including comprehensive safety assessments and risk management measures. Similarly, China has implemented the Measures for Environmental Management of New Chemical Substances, which specifically addresses highly hazardous chemicals including HF.
Quality determination protocols for HF must incorporate compliance verification elements to ensure adherence to these regulations. This includes regular monitoring of emission levels, proper documentation of waste disposal practices, and verification that acid purity meets environmental standards. Many jurisdictions require facilities to implement continuous emission monitoring systems (CEMS) for real-time tracking of HF releases.
The relationship between HF quality and environmental impact is direct and significant. Higher-purity HF typically contains fewer metal contaminants that might otherwise be released into the environment during use or disposal. However, higher concentration also increases potential hazard severity in case of accidental release, necessitating more robust containment systems and emergency response protocols.
Companies utilizing industrial HF increasingly face pressure to adopt circular economy principles, including acid recycling and regeneration technologies. These approaches not only reduce environmental footprint but often improve economic efficiency. Advanced quality determination methods that can accurately assess recycled HF purity have therefore become essential components of environmentally responsible industrial practices.
Compliance with evolving regulatory standards requires ongoing investment in analytical capabilities. Many jurisdictions now mandate regular third-party verification of quality control procedures and environmental management systems, creating additional incentives for developing reliable, standardized quality determination methodologies for industrial HF.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!