Hydrofluoric Acid vs Barium Sulfate: Solubility Characteristics
AUG 26, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
HF and BaSO4 Chemistry Background and Research Objectives
Hydrofluoric acid (HF) represents a unique compound in the realm of inorganic chemistry, distinguished by its exceptional properties that deviate from typical strong mineral acids. First isolated in the 17th century, HF has evolved from a laboratory curiosity to an industrial cornerstone. Unlike other hydrogen halides, HF exhibits weak acid behavior in aqueous solutions due to the strong hydrogen bonding that occurs between fluorine and hydrogen atoms, resulting in incomplete dissociation.
The historical development of HF technology has been closely tied to industrial applications, particularly in semiconductor manufacturing, glass etching, and petroleum refining. The evolution of purification methods and handling protocols has significantly enhanced its utility while addressing its inherently hazardous nature. Current research focuses on developing safer alternatives or containment systems that maintain HF's valuable chemical properties while mitigating risks.
Barium sulfate (BaSO4), conversely, represents an inorganic compound with remarkably different chemical characteristics. Known since the early 18th century as the mineral barite, BaSO4 has been extensively studied for its exceptional insolubility in water and most common solvents. This property has made it particularly valuable in applications requiring chemical stability and resistance to dissolution.
The solubility characteristics of these two compounds present a fascinating contrast in chemical behavior. HF demonstrates high solubility in water with complex equilibrium dynamics, while BaSO4 exhibits one of the lowest solubility products (Ksp ≈ 1.1 × 10^-10 at 25°C) among common inorganic salts. This stark difference in solubility behavior has profound implications for their respective applications and handling requirements.
The primary objective of this technical research is to conduct a comprehensive comparative analysis of the solubility characteristics of hydrofluoric acid and barium sulfate across various conditions. Specifically, we aim to investigate how temperature, pressure, pH, and the presence of common ions affect their dissolution behaviors. Understanding these parameters is crucial for optimizing industrial processes where either compound is utilized.
Additionally, this research seeks to explore the fundamental molecular interactions that govern the solubility properties of both substances. For HF, this includes examining hydrogen bonding networks and ionization equilibria, while for BaSO4, crystal lattice energies and ion-solvent interactions will be investigated. These insights will contribute to developing predictive models for solubility behavior under non-standard conditions.
The technological goal of this investigation extends to identifying novel applications that might leverage the contrasting solubility profiles of these compounds, particularly in separation technologies, controlled release systems, and advanced materials development. By elucidating the underlying principles governing their solubility characteristics, we aim to establish a foundation for innovation in chemical processing and materials science.
The historical development of HF technology has been closely tied to industrial applications, particularly in semiconductor manufacturing, glass etching, and petroleum refining. The evolution of purification methods and handling protocols has significantly enhanced its utility while addressing its inherently hazardous nature. Current research focuses on developing safer alternatives or containment systems that maintain HF's valuable chemical properties while mitigating risks.
Barium sulfate (BaSO4), conversely, represents an inorganic compound with remarkably different chemical characteristics. Known since the early 18th century as the mineral barite, BaSO4 has been extensively studied for its exceptional insolubility in water and most common solvents. This property has made it particularly valuable in applications requiring chemical stability and resistance to dissolution.
The solubility characteristics of these two compounds present a fascinating contrast in chemical behavior. HF demonstrates high solubility in water with complex equilibrium dynamics, while BaSO4 exhibits one of the lowest solubility products (Ksp ≈ 1.1 × 10^-10 at 25°C) among common inorganic salts. This stark difference in solubility behavior has profound implications for their respective applications and handling requirements.
The primary objective of this technical research is to conduct a comprehensive comparative analysis of the solubility characteristics of hydrofluoric acid and barium sulfate across various conditions. Specifically, we aim to investigate how temperature, pressure, pH, and the presence of common ions affect their dissolution behaviors. Understanding these parameters is crucial for optimizing industrial processes where either compound is utilized.
Additionally, this research seeks to explore the fundamental molecular interactions that govern the solubility properties of both substances. For HF, this includes examining hydrogen bonding networks and ionization equilibria, while for BaSO4, crystal lattice energies and ion-solvent interactions will be investigated. These insights will contribute to developing predictive models for solubility behavior under non-standard conditions.
The technological goal of this investigation extends to identifying novel applications that might leverage the contrasting solubility profiles of these compounds, particularly in separation technologies, controlled release systems, and advanced materials development. By elucidating the underlying principles governing their solubility characteristics, we aim to establish a foundation for innovation in chemical processing and materials science.
Market Applications and Demand Analysis for HF and BaSO4
The global market for hydrofluoric acid (HF) is primarily driven by its extensive applications in various industries, with the fluorochemicals sector accounting for the largest share. The demand for HF in the production of refrigerants, particularly hydrofluorocarbons (HFCs) and hydrochlorofluorocarbons (HCFCs), remains substantial despite environmental regulations phasing out certain compounds. This segment is expected to maintain steady growth as newer, more environmentally friendly fluorochemicals are developed.
In the semiconductor industry, high-purity HF serves as a critical etching agent for silicon wafers and cleaning agent for removing oxide residues. With the continuous expansion of the electronics market and increasing complexity of semiconductor devices, the demand for ultra-pure HF continues to rise. Market analysts project a compound annual growth rate (CAGR) of 5.7% for HF in semiconductor applications through 2028.
The petroleum industry represents another significant market for HF, where it is used as a catalyst in alkylation processes for producing high-octane gasoline components. Despite efforts to develop alternative alkylation technologies due to safety concerns, HF alkylation remains economically advantageous in many refineries globally, sustaining demand in this sector.
Barium sulfate (BaSO4) markets demonstrate different demand characteristics, largely driven by its insolubility properties. The medical imaging sector constitutes a premium market segment, where high-purity BaSO4 is used as a contrast agent for X-ray and CT scan procedures. This application commands higher prices and requires specialized grades with strict quality control. The aging global population and increasing diagnostic procedures are expanding this market segment steadily.
The industrial applications of BaSO4 represent the largest volume market, particularly in paints and coatings where it serves as a filler and whitening agent. The construction industry boom in developing economies has significantly increased demand for BaSO4 in architectural coatings. Additionally, the automotive sector utilizes BaSO4 in undercoats and sound-dampening applications, with market growth closely tied to automotive production trends.
Oil and gas drilling operations consume substantial quantities of BaSO4 as a weighting agent in drilling fluids. This application is highly cyclical, fluctuating with oil prices and drilling activity. Recent years have seen increased demand for higher-grade BaSO4 in this sector as drilling operations move to more challenging environments requiring superior performance characteristics.
The contrasting solubility properties of these compounds directly influence their market applications and handling requirements. HF's high solubility necessitates specialized containment systems and safety protocols, adding to operational costs but enabling its use in solution-based processes. Conversely, BaSO4's exceptional insolubility makes it valuable in applications requiring chemical stability and permanence, particularly in harsh environments.
In the semiconductor industry, high-purity HF serves as a critical etching agent for silicon wafers and cleaning agent for removing oxide residues. With the continuous expansion of the electronics market and increasing complexity of semiconductor devices, the demand for ultra-pure HF continues to rise. Market analysts project a compound annual growth rate (CAGR) of 5.7% for HF in semiconductor applications through 2028.
The petroleum industry represents another significant market for HF, where it is used as a catalyst in alkylation processes for producing high-octane gasoline components. Despite efforts to develop alternative alkylation technologies due to safety concerns, HF alkylation remains economically advantageous in many refineries globally, sustaining demand in this sector.
Barium sulfate (BaSO4) markets demonstrate different demand characteristics, largely driven by its insolubility properties. The medical imaging sector constitutes a premium market segment, where high-purity BaSO4 is used as a contrast agent for X-ray and CT scan procedures. This application commands higher prices and requires specialized grades with strict quality control. The aging global population and increasing diagnostic procedures are expanding this market segment steadily.
The industrial applications of BaSO4 represent the largest volume market, particularly in paints and coatings where it serves as a filler and whitening agent. The construction industry boom in developing economies has significantly increased demand for BaSO4 in architectural coatings. Additionally, the automotive sector utilizes BaSO4 in undercoats and sound-dampening applications, with market growth closely tied to automotive production trends.
Oil and gas drilling operations consume substantial quantities of BaSO4 as a weighting agent in drilling fluids. This application is highly cyclical, fluctuating with oil prices and drilling activity. Recent years have seen increased demand for higher-grade BaSO4 in this sector as drilling operations move to more challenging environments requiring superior performance characteristics.
The contrasting solubility properties of these compounds directly influence their market applications and handling requirements. HF's high solubility necessitates specialized containment systems and safety protocols, adding to operational costs but enabling its use in solution-based processes. Conversely, BaSO4's exceptional insolubility makes it valuable in applications requiring chemical stability and permanence, particularly in harsh environments.
Current Solubility Challenges and Technical Limitations
The solubility characteristics of hydrofluoric acid (HF) and barium sulfate (BaSO4) present significant technical challenges in various industrial applications. HF demonstrates exceptional solubility in water, forming a highly corrosive solution that can reach concentrations up to 48% at standard conditions. This high solubility creates substantial handling difficulties due to its extreme reactivity with numerous materials, including glass, many metals, and biological tissues.
Conversely, barium sulfate exhibits remarkably low solubility in water (approximately 0.0025 g/L at 25°C), creating different but equally challenging technical limitations. This near-insolubility makes BaSO4 difficult to process in aqueous environments without specialized techniques or additives, significantly restricting its application potential in certain fields.
Current analytical methods for precisely measuring HF solubility in complex matrices remain inadequate, particularly at low concentrations where traditional titration methods lack sensitivity. The development of reliable sensors for continuous monitoring of HF in solution presents ongoing technical hurdles due to the acid's corrosive nature, which rapidly degrades most sensing elements.
For barium sulfate, the primary technical limitation stems from its extremely low dissolution rate, which hampers efficient processing in applications requiring even minimal solubility. Current methods to enhance BaSO4 solubility, such as temperature manipulation, pressure alteration, or chemical additives, have shown limited effectiveness or introduce undesirable side effects in downstream processes.
The temperature dependence of solubility creates additional complications. While HF solubility increases moderately with temperature, the relationship is non-linear and difficult to predict in mixed solvent systems. BaSO4 exhibits minimal solubility change with temperature variations, making thermal manipulation an ineffective control strategy in most applications.
pH-dependent solubility behavior presents further challenges. HF demonstrates complex equilibrium dynamics between molecular HF and fluoride ions that shift dramatically with pH changes. Meanwhile, BaSO4 maintains its low solubility across a wide pH range, only showing increased dissolution under extremely acidic conditions (pH < 2), which limits processing options.
The contrasting solubility profiles create significant engineering challenges when these compounds must coexist in industrial processes. Separation technologies struggle with the extreme solubility differences, while containment systems must simultaneously address the aggressive nature of dissolved HF and the precipitation tendencies of BaSO4. Current filtration and separation technologies often fail to efficiently handle these opposing solubility characteristics in a single process stream.
Conversely, barium sulfate exhibits remarkably low solubility in water (approximately 0.0025 g/L at 25°C), creating different but equally challenging technical limitations. This near-insolubility makes BaSO4 difficult to process in aqueous environments without specialized techniques or additives, significantly restricting its application potential in certain fields.
Current analytical methods for precisely measuring HF solubility in complex matrices remain inadequate, particularly at low concentrations where traditional titration methods lack sensitivity. The development of reliable sensors for continuous monitoring of HF in solution presents ongoing technical hurdles due to the acid's corrosive nature, which rapidly degrades most sensing elements.
For barium sulfate, the primary technical limitation stems from its extremely low dissolution rate, which hampers efficient processing in applications requiring even minimal solubility. Current methods to enhance BaSO4 solubility, such as temperature manipulation, pressure alteration, or chemical additives, have shown limited effectiveness or introduce undesirable side effects in downstream processes.
The temperature dependence of solubility creates additional complications. While HF solubility increases moderately with temperature, the relationship is non-linear and difficult to predict in mixed solvent systems. BaSO4 exhibits minimal solubility change with temperature variations, making thermal manipulation an ineffective control strategy in most applications.
pH-dependent solubility behavior presents further challenges. HF demonstrates complex equilibrium dynamics between molecular HF and fluoride ions that shift dramatically with pH changes. Meanwhile, BaSO4 maintains its low solubility across a wide pH range, only showing increased dissolution under extremely acidic conditions (pH < 2), which limits processing options.
The contrasting solubility profiles create significant engineering challenges when these compounds must coexist in industrial processes. Separation technologies struggle with the extreme solubility differences, while containment systems must simultaneously address the aggressive nature of dissolved HF and the precipitation tendencies of BaSO4. Current filtration and separation technologies often fail to efficiently handle these opposing solubility characteristics in a single process stream.
Comparative Solubility Methodologies and Solutions
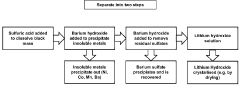
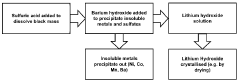
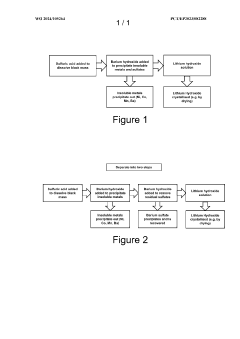
01 Dissolution of barium sulfate using hydrofluoric acid
Hydrofluoric acid can be used to dissolve barium sulfate, which is typically insoluble in most acids. The fluoride ions from hydrofluoric acid form complexes with barium ions, facilitating the dissolution of barium sulfate. This property is particularly useful in industrial applications where barium sulfate scale removal is required, such as in oil and gas production equipment and boilers.- Dissolution of barium sulfate using hydrofluoric acid: Hydrofluoric acid can be used to dissolve barium sulfate deposits due to its ability to break down the sulfate compound. This process is particularly useful in industrial applications where barium sulfate scale removal is necessary. The reaction forms soluble barium fluoride, effectively removing the insoluble barium sulfate deposits. This method is employed in various fields including oil and gas production, where scale formation is a common issue.
- Formulations for enhanced barium sulfate dissolution: Specific formulations combining hydrofluoric acid with other chemicals can enhance the dissolution of barium sulfate. These formulations may include chelating agents, surfactants, or other acids that work synergistically with hydrofluoric acid. The enhanced formulations improve dissolution efficiency while potentially reducing the concentration of hydrofluoric acid needed, thereby decreasing safety risks and environmental concerns associated with strong acid use.
- Applications in oilfield scale treatment: The solubility properties of barium sulfate in hydrofluoric acid are particularly relevant in oilfield operations. Barium sulfate scale commonly forms in oil wells and production equipment, reducing flow efficiency. Treatment methods utilizing hydrofluoric acid or its derivatives can effectively remove these deposits, restoring production rates. These treatments often involve specific protocols for acid concentration, contact time, and subsequent neutralization to protect equipment integrity.
- Safety and environmental considerations: When using hydrofluoric acid for barium sulfate dissolution, significant safety and environmental considerations must be addressed. Modified formulations aim to reduce risks while maintaining effectiveness. These include buffered systems, inhibited acid formulations, and controlled-release mechanisms that minimize exposure hazards. Environmental impact is mitigated through neutralization processes and waste treatment methods that prevent fluoride contamination of water sources.
- Alternative approaches to barium sulfate solubilization: Beyond direct hydrofluoric acid application, alternative approaches have been developed for barium sulfate solubilization. These include chelating agent systems, conversion reactions that transform barium sulfate into more soluble compounds, and sequential treatment methods that use multiple chemical agents. Some approaches utilize hydrofluoric acid derivatives or precursors that generate hydrofluoric acid in situ, providing controlled release and enhanced safety profiles while maintaining dissolution effectiveness.
02 Barium sulfate recovery from hydrofluoric acid solutions
Methods for recovering barium sulfate from solutions containing hydrofluoric acid involve precipitation techniques. By adjusting pH conditions or adding specific reagents, barium sulfate can be selectively precipitated from hydrofluoric acid solutions. These recovery processes are important in industrial waste treatment and in recycling valuable barium compounds from spent acid solutions.Expand Specific Solutions03 Hydrofluoric acid resistant barium sulfate composites
Specialized composites containing barium sulfate can be formulated to resist hydrofluoric acid corrosion. These materials typically incorporate polymeric matrices or other protective compounds that shield the barium sulfate from direct contact with hydrofluoric acid. Such composites find applications in chemical storage containers, reaction vessels, and protective coatings where resistance to hydrofluoric acid is required.Expand Specific Solutions04 Controlled solubility of barium sulfate in hydrofluoric acid mixtures
The solubility of barium sulfate in hydrofluoric acid can be controlled by adjusting the concentration of acid, temperature, and by adding other chemical agents. By manipulating these parameters, the dissolution rate of barium sulfate can be optimized for specific applications. This controlled solubility is particularly important in applications such as oil well stimulation, where precise dissolution rates are required.Expand Specific Solutions05 Applications utilizing hydrofluoric acid and barium sulfate interactions
The interaction between hydrofluoric acid and barium sulfate is utilized in various industrial applications. These include scale removal in industrial equipment, etching of glass and ceramics, preparation of specialized chemicals, and in certain medical imaging techniques. The unique solubility properties of barium sulfate in hydrofluoric acid enable these diverse applications across multiple industries.Expand Specific Solutions
Leading Industry Players in Fluorochemicals and Barium Compounds
The hydrofluoric acid and barium sulfate solubility market is in a mature growth phase with specialized applications driving steady expansion. The global market size for these chemicals is estimated at $3-4 billion annually, with projected growth of 4-5% CAGR through 2027. Technologically, the field shows varying maturity levels across applications. Leading players like Honeywell International and Merck Patent GmbH focus on high-purity formulations for semiconductor applications, while DAIKIN INDUSTRIES and DuPont de Nemours dominate fluorochemical production. Solvay and BASF have established strong positions in barium sulfate applications, particularly in medical imaging. Chinese manufacturers including Jiujiang Tianci and Do-Fluoride are rapidly expanding market share through cost-competitive production and increasing R&D investments in novel applications.
Honeywell International Technologies Ltd.
Technical Solution: Honeywell has developed advanced fluorine chemistry solutions focusing on the differential solubility properties of hydrofluoric acid and barium sulfate. Their technology leverages the high solubility of HF in water (completely miscible) versus the extremely low solubility of barium sulfate (0.0025 g/L at 25°C). This contrast is utilized in their industrial separation processes and pollution control systems. Honeywell's proprietary treatment methods employ controlled precipitation reactions where barium compounds are used to neutralize and immobilize hydrofluoric acid waste streams, forming highly insoluble barium fluoride precipitates. Their process achieves fluoride removal efficiencies exceeding 99.5% while maintaining effluent concentrations below 2 ppm, significantly under regulatory requirements for industrial discharge.
Strengths: Exceptional efficiency in fluoride waste treatment with near-complete removal capabilities; systems can handle variable concentration inputs while maintaining consistent output quality. Weaknesses: Requires careful pH control and monitoring; treatment process generates solid waste that requires proper disposal; relatively high operational costs compared to simpler treatment methods.
Merck Patent GmbH
Technical Solution: Merck has pioneered analytical techniques for precise characterization of solubility differences between hydrofluoric acid and barium sulfate, particularly important in pharmaceutical applications. Their technology includes specialized titration methods for HF quantification in complex matrices and advanced particle characterization systems for barium sulfate suspensions. Merck's proprietary "SolubilityPro" platform enables real-time monitoring of dissolution kinetics, critical for pharmaceutical formulations where barium sulfate serves as a radiopaque contrast agent. Their research has established that while HF demonstrates complete miscibility in water with strong temperature dependence, barium sulfate maintains consistent low solubility (approximately 2.45 mg/L at 20°C) across a wide temperature range. This stability makes it ideal for controlled-release drug formulations and diagnostic imaging applications where predictable behavior is essential.
Strengths: Highly precise analytical methods with detection limits in the ppb range; comprehensive solubility data across various conditions enables predictive modeling for formulation development. Weaknesses: Specialized equipment requirements increase implementation costs; methods require highly trained personnel; some techniques have limited applicability outside pharmaceutical contexts.
Key Scientific Literature on HF-BaSO4 Interactions
method
PatentWO2024105264A1
Innovation
- A multi-step precipitation method involving acid treatment followed by sequential addition of bases to precipitate out insoluble metals and conjugate acid compounds, allowing for the isolation of lithium hydroxide with reduced contaminants and lower reagent costs, and potential recycling of by-products.
Process for production of etching or cleaning fluids
PatentInactiveUS20060178282A1
Innovation
- A method involving the mixing of hydrofluoric acid with heteroatom-containing organic solvents and nitrogen-containing basic components, followed by filtration, to create solutions with fluoride or bifluoride salts, achieving high concentrations of ammonium bifluoride or other bifluorides, which are stable and suitable for etching and cleaning processes.
Environmental Impact and Safety Considerations
The environmental impact and safety considerations of hydrofluoric acid (HF) and barium sulfate (BaSO₄) differ significantly due to their contrasting chemical properties. Hydrofluoric acid presents severe environmental hazards when released into ecosystems. Its high water solubility enables rapid dispersion in aquatic environments, potentially causing widespread acidification of water bodies and devastating impacts on aquatic life. The acid can persist in soil, altering pH levels and affecting nutrient availability for plants, while also potentially contaminating groundwater resources.
From a safety perspective, hydrofluoric acid represents one of the most dangerous industrial chemicals. Unlike other acids that cause immediate burns, HF can penetrate skin and tissues without immediate pain, leading to delayed recognition of exposure. Once absorbed, it binds with calcium and magnesium in the body, potentially causing hypocalcemia, cardiac arrhythmias, and even death in severe cases. Inhalation of HF vapors can cause severe respiratory damage, while eye contact may result in permanent vision loss.
Barium sulfate, conversely, demonstrates significantly lower environmental impact due to its extremely low solubility in water. When released into the environment, it tends to remain inert and localized, with minimal migration through soil or water systems. This insolubility prevents barium ions from becoming bioavailable to organisms, substantially reducing ecological risk. The compound typically settles as sediment in aquatic environments without significant chemical interactions.
Safety protocols for handling these substances reflect their distinct risk profiles. HF requires extensive safety measures including specialized chemical-resistant gloves, face shields, respiratory protection, and immediate access to calcium gluconate as an antidote. Facilities using HF must implement rigorous containment systems, neutralization capabilities, and emergency response protocols. Workers require specialized training on HF-specific hazards and emergency procedures.
Regulatory frameworks worldwide classify hydrofluoric acid as a highly hazardous substance subject to strict reporting requirements, transportation restrictions, and usage limitations. In contrast, barium sulfate's regulatory burden is considerably lighter due to its low toxicity profile when used in its insoluble form, though precautions against dust inhalation remain important during handling.
The stark contrast in environmental and safety profiles between these compounds has driven significant research into alternatives to hydrofluoric acid in industrial applications, with many industries seeking to replace HF with safer compounds where technically feasible, even at higher operational costs, to mitigate the substantial risks associated with its use.
From a safety perspective, hydrofluoric acid represents one of the most dangerous industrial chemicals. Unlike other acids that cause immediate burns, HF can penetrate skin and tissues without immediate pain, leading to delayed recognition of exposure. Once absorbed, it binds with calcium and magnesium in the body, potentially causing hypocalcemia, cardiac arrhythmias, and even death in severe cases. Inhalation of HF vapors can cause severe respiratory damage, while eye contact may result in permanent vision loss.
Barium sulfate, conversely, demonstrates significantly lower environmental impact due to its extremely low solubility in water. When released into the environment, it tends to remain inert and localized, with minimal migration through soil or water systems. This insolubility prevents barium ions from becoming bioavailable to organisms, substantially reducing ecological risk. The compound typically settles as sediment in aquatic environments without significant chemical interactions.
Safety protocols for handling these substances reflect their distinct risk profiles. HF requires extensive safety measures including specialized chemical-resistant gloves, face shields, respiratory protection, and immediate access to calcium gluconate as an antidote. Facilities using HF must implement rigorous containment systems, neutralization capabilities, and emergency response protocols. Workers require specialized training on HF-specific hazards and emergency procedures.
Regulatory frameworks worldwide classify hydrofluoric acid as a highly hazardous substance subject to strict reporting requirements, transportation restrictions, and usage limitations. In contrast, barium sulfate's regulatory burden is considerably lighter due to its low toxicity profile when used in its insoluble form, though precautions against dust inhalation remain important during handling.
The stark contrast in environmental and safety profiles between these compounds has driven significant research into alternatives to hydrofluoric acid in industrial applications, with many industries seeking to replace HF with safer compounds where technically feasible, even at higher operational costs, to mitigate the substantial risks associated with its use.
Regulatory Framework for Hazardous Chemical Handling
The regulatory landscape governing the handling of hydrofluoric acid (HF) and barium sulfate presents distinct frameworks due to their significantly different hazard profiles. Hydrofluoric acid, classified as an extremely hazardous substance, falls under stringent regulatory control in most jurisdictions worldwide. In the United States, HF handling is regulated by multiple agencies including OSHA under 29 CFR 1910.1200 (Hazard Communication Standard) and EPA under CERCLA and EPCRA, with mandatory reporting thresholds as low as 100 pounds.
The European Union regulates HF under REACH (Registration, Evaluation, Authorization and Restriction of Chemicals) and CLP (Classification, Labeling and Packaging) regulations, requiring extensive documentation, exposure scenarios, and safety data sheets. Additionally, facilities storing significant quantities of HF must comply with the Seveso III Directive for major accident prevention.
In contrast, barium sulfate's regulatory burden is substantially lighter due to its low solubility and reduced toxicity profile. While still classified as a chemical requiring proper handling, it is exempt from many of the stringent controls applied to HF. Most regulatory frameworks classify barium sulfate as a non-hazardous substance when in pure form, though contaminated materials may require special consideration.
Personal protective equipment (PPE) requirements illustrate this regulatory divergence clearly. HF handling necessitates comprehensive protection including chemical-resistant full-body coverage, specialized gloves (typically neoprene or butyl rubber), face shields, and often respiratory protection. Emergency response protocols for HF exposure are highly specific, requiring immediate specialized medical intervention and calcium gluconate treatment.
Transportation regulations further highlight these differences. HF is designated as a Class 8 Corrosive under UN transportation codes, requiring specialized containers, placarding, and shipping documentation. Carriers must maintain hazardous materials endorsements and follow strict routing guidelines. Barium sulfate typically requires only standard industrial transportation protocols without specialized hazardous materials designations.
Waste disposal regulations complete this regulatory contrast. HF waste is classified as hazardous under RCRA (Resource Conservation and Recovery Act) in the US and similar frameworks globally, requiring specialized treatment, neutralization, and disposal through licensed hazardous waste handlers. Barium sulfate waste, unless contaminated with other hazardous substances, can generally be disposed of through conventional industrial waste channels, though local regulations regarding mineral waste may apply.
This regulatory disparity directly impacts laboratory and industrial operations, with HF requiring dedicated storage facilities, specialized handling equipment, regular employee training, medical surveillance programs, and detailed emergency response planning that are not typically necessary for barium sulfate operations.
The European Union regulates HF under REACH (Registration, Evaluation, Authorization and Restriction of Chemicals) and CLP (Classification, Labeling and Packaging) regulations, requiring extensive documentation, exposure scenarios, and safety data sheets. Additionally, facilities storing significant quantities of HF must comply with the Seveso III Directive for major accident prevention.
In contrast, barium sulfate's regulatory burden is substantially lighter due to its low solubility and reduced toxicity profile. While still classified as a chemical requiring proper handling, it is exempt from many of the stringent controls applied to HF. Most regulatory frameworks classify barium sulfate as a non-hazardous substance when in pure form, though contaminated materials may require special consideration.
Personal protective equipment (PPE) requirements illustrate this regulatory divergence clearly. HF handling necessitates comprehensive protection including chemical-resistant full-body coverage, specialized gloves (typically neoprene or butyl rubber), face shields, and often respiratory protection. Emergency response protocols for HF exposure are highly specific, requiring immediate specialized medical intervention and calcium gluconate treatment.
Transportation regulations further highlight these differences. HF is designated as a Class 8 Corrosive under UN transportation codes, requiring specialized containers, placarding, and shipping documentation. Carriers must maintain hazardous materials endorsements and follow strict routing guidelines. Barium sulfate typically requires only standard industrial transportation protocols without specialized hazardous materials designations.
Waste disposal regulations complete this regulatory contrast. HF waste is classified as hazardous under RCRA (Resource Conservation and Recovery Act) in the US and similar frameworks globally, requiring specialized treatment, neutralization, and disposal through licensed hazardous waste handlers. Barium sulfate waste, unless contaminated with other hazardous substances, can generally be disposed of through conventional industrial waste channels, though local regulations regarding mineral waste may apply.
This regulatory disparity directly impacts laboratory and industrial operations, with HF requiring dedicated storage facilities, specialized handling equipment, regular employee training, medical surveillance programs, and detailed emergency response planning that are not typically necessary for barium sulfate operations.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!