Hydrofluoric Acid Applications in Fluoropolymer Production
AUG 25, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
HF Acid Evolution and Production Goals
Hydrofluoric acid (HF) has undergone significant evolution since its first isolation in the late 18th century by Carl Wilhelm Scheele. Initially considered merely a laboratory curiosity, HF has transformed into a critical industrial chemical with diverse applications, particularly in fluoropolymer production. The technological trajectory of HF production has shifted from small-scale laboratory methods to sophisticated industrial processes capable of generating high-purity acid at commercial scales.
The primary production method has evolved from the reaction of calcium fluoride (fluorspar) with sulfuric acid to more efficient processes incorporating advanced purification techniques. Modern HF production facilities employ specialized materials such as Monel alloys and fluoropolymer linings to withstand the highly corrosive nature of the acid, representing significant engineering advancements in material science and process safety.
In the context of fluoropolymer production, HF serves as the fundamental building block for the synthesis of fluoromonomers, which are subsequently polymerized into various fluoropolymers including polytetrafluoroethylene (PTFE), fluorinated ethylene propylene (FEP), and polyvinylidene fluoride (PVDF). The quality requirements for HF in these applications have become increasingly stringent, with current specifications demanding ultra-high purity levels (>99.99%) to ensure consistent polymer properties and minimize defects.
The technological goals for HF in fluoropolymer applications center around several key objectives. First, enhancing production efficiency through catalytic improvements and process intensification to reduce energy consumption and increase yield. Second, developing more environmentally sustainable production methods that minimize emissions and waste generation, particularly addressing concerns about greenhouse gas emissions associated with fluorochemical production.
Third, improving safety protocols and engineering controls to mitigate the inherent hazards of HF handling, including advanced containment systems and remote operation capabilities. Fourth, exploring alternative feedstocks beyond traditional fluorspar to ensure supply chain resilience and reduce dependence on geographically concentrated mineral resources.
The industry is also pursuing integration of digital technologies for process optimization, with advanced analytics and machine learning algorithms being deployed to fine-tune reaction parameters and predict maintenance needs. Additionally, there is growing interest in developing closed-loop recycling systems for fluorine-containing compounds to recover and reuse HF from waste streams, aligning with circular economy principles.
These evolutionary trends and production goals reflect the dual imperatives of meeting growing demand for high-performance fluoropolymers while addressing increasing environmental and safety standards in chemical manufacturing.
The primary production method has evolved from the reaction of calcium fluoride (fluorspar) with sulfuric acid to more efficient processes incorporating advanced purification techniques. Modern HF production facilities employ specialized materials such as Monel alloys and fluoropolymer linings to withstand the highly corrosive nature of the acid, representing significant engineering advancements in material science and process safety.
In the context of fluoropolymer production, HF serves as the fundamental building block for the synthesis of fluoromonomers, which are subsequently polymerized into various fluoropolymers including polytetrafluoroethylene (PTFE), fluorinated ethylene propylene (FEP), and polyvinylidene fluoride (PVDF). The quality requirements for HF in these applications have become increasingly stringent, with current specifications demanding ultra-high purity levels (>99.99%) to ensure consistent polymer properties and minimize defects.
The technological goals for HF in fluoropolymer applications center around several key objectives. First, enhancing production efficiency through catalytic improvements and process intensification to reduce energy consumption and increase yield. Second, developing more environmentally sustainable production methods that minimize emissions and waste generation, particularly addressing concerns about greenhouse gas emissions associated with fluorochemical production.
Third, improving safety protocols and engineering controls to mitigate the inherent hazards of HF handling, including advanced containment systems and remote operation capabilities. Fourth, exploring alternative feedstocks beyond traditional fluorspar to ensure supply chain resilience and reduce dependence on geographically concentrated mineral resources.
The industry is also pursuing integration of digital technologies for process optimization, with advanced analytics and machine learning algorithms being deployed to fine-tune reaction parameters and predict maintenance needs. Additionally, there is growing interest in developing closed-loop recycling systems for fluorine-containing compounds to recover and reuse HF from waste streams, aligning with circular economy principles.
These evolutionary trends and production goals reflect the dual imperatives of meeting growing demand for high-performance fluoropolymers while addressing increasing environmental and safety standards in chemical manufacturing.
Fluoropolymer Market Demand Analysis
The global fluoropolymer market has demonstrated robust growth over the past decade, driven primarily by increasing applications in automotive, electronics, construction, and industrial processing sectors. Current market valuations place the fluoropolymer industry at approximately 8.5 billion USD in 2023, with projections indicating a compound annual growth rate (CAGR) of 6.2% through 2030. This growth trajectory significantly outpaces many other specialty polymer segments, highlighting the expanding demand for these high-performance materials.
The electronics industry represents the largest consumption sector for fluoropolymers, accounting for nearly 28% of total market volume. This dominance stems from the critical properties fluoropolymers offer in semiconductor manufacturing, wire and cable insulation, and advanced electronic components. The miniaturization trend in electronics continues to drive demand for high-purity fluoropolymers that can withstand extreme processing conditions.
Automotive applications constitute the second-largest market segment at 22% of total consumption. The transition toward electric vehicles has accelerated demand for fluoropolymers in battery systems, fuel cells, and high-temperature engine components. Industry analysts project this segment will experience the fastest growth rate among all application areas, potentially reaching 9% annual growth through 2028.
Regional analysis reveals Asia-Pacific as the dominant market, representing approximately 42% of global consumption, followed by North America (27%) and Europe (23%). China's manufacturing expansion has positioned it as the single largest national market, though the United States maintains leadership in high-performance fluoropolymer development and production. Emerging economies in Southeast Asia and India are showing accelerated adoption rates, particularly in industrial processing applications.
By polymer type, polytetrafluoroethylene (PTFE) continues to lead market share at 40% of total volume, followed by polyvinylidene fluoride (PVDF) at 18% and fluorinated ethylene propylene (FEP) at 15%. However, newer fluoropolymer variants like perfluoroalkoxy alkanes (PFA) and ethylene tetrafluoroethylene (ETFE) are experiencing faster growth rates due to their specialized performance characteristics.
Supply chain constraints, particularly regarding the availability of hydrofluoric acid and fluorocarbon precursors, have created periodic market tensions. Environmental regulations targeting fluorinated compounds have prompted industry innovation toward more sustainable production methods and end-of-life management solutions. These regulatory pressures are expected to reshape market dynamics over the coming decade, potentially favoring producers with advanced recycling capabilities and greener manufacturing processes.
The electronics industry represents the largest consumption sector for fluoropolymers, accounting for nearly 28% of total market volume. This dominance stems from the critical properties fluoropolymers offer in semiconductor manufacturing, wire and cable insulation, and advanced electronic components. The miniaturization trend in electronics continues to drive demand for high-purity fluoropolymers that can withstand extreme processing conditions.
Automotive applications constitute the second-largest market segment at 22% of total consumption. The transition toward electric vehicles has accelerated demand for fluoropolymers in battery systems, fuel cells, and high-temperature engine components. Industry analysts project this segment will experience the fastest growth rate among all application areas, potentially reaching 9% annual growth through 2028.
Regional analysis reveals Asia-Pacific as the dominant market, representing approximately 42% of global consumption, followed by North America (27%) and Europe (23%). China's manufacturing expansion has positioned it as the single largest national market, though the United States maintains leadership in high-performance fluoropolymer development and production. Emerging economies in Southeast Asia and India are showing accelerated adoption rates, particularly in industrial processing applications.
By polymer type, polytetrafluoroethylene (PTFE) continues to lead market share at 40% of total volume, followed by polyvinylidene fluoride (PVDF) at 18% and fluorinated ethylene propylene (FEP) at 15%. However, newer fluoropolymer variants like perfluoroalkoxy alkanes (PFA) and ethylene tetrafluoroethylene (ETFE) are experiencing faster growth rates due to their specialized performance characteristics.
Supply chain constraints, particularly regarding the availability of hydrofluoric acid and fluorocarbon precursors, have created periodic market tensions. Environmental regulations targeting fluorinated compounds have prompted industry innovation toward more sustainable production methods and end-of-life management solutions. These regulatory pressures are expected to reshape market dynamics over the coming decade, potentially favoring producers with advanced recycling capabilities and greener manufacturing processes.
HF Acid Technical Challenges in Polymer Processing
Hydrofluoric acid (HF) presents significant technical challenges in fluoropolymer production processes due to its highly corrosive nature and extreme reactivity. The primary challenge lies in material compatibility, as HF rapidly attacks glass, many metals, and certain polymers, necessitating specialized equipment constructed from materials like Monel, PTFE, or specific grades of stainless steel. This requirement substantially increases production costs and complicates facility design.
Temperature control represents another critical challenge, as HF reactions in polymer processing are often highly exothermic. Inadequate heat management can lead to runaway reactions, pressure buildup, and potential safety incidents. The narrow processing window requires sophisticated cooling systems and precise monitoring technologies to maintain optimal reaction conditions.
Worker safety concerns are paramount when handling HF in industrial settings. The acid's ability to penetrate skin and cause deep tissue damage without immediate symptoms creates unique hazards requiring specialized training, emergency protocols, and personal protective equipment beyond standard chemical handling procedures. These safety requirements add operational complexity and increase production costs.
Environmental compliance presents growing challenges as regulations governing HF emissions and waste disposal become increasingly stringent worldwide. Manufacturers must implement advanced scrubbing systems, closed-loop processing, and waste neutralization technologies to meet these requirements, adding further complexity to production systems.
Process control and automation face significant hurdles due to the corrosive nature of HF affecting sensors and monitoring equipment. Developing reliable, long-lasting measurement systems capable of withstanding HF exposure remains an ongoing technical challenge, limiting the precision of automated control systems in fluoropolymer production.
Quality consistency issues arise from variations in HF concentration, purity, and reaction conditions, which can significantly impact the molecular weight distribution, crystallinity, and mechanical properties of the resulting fluoropolymers. Maintaining consistent product specifications requires sophisticated analytical techniques and process controls.
Supply chain vulnerabilities represent a strategic challenge, as HF production is geographically concentrated and subject to trade restrictions due to its dual-use nature in both industrial applications and potential weapons production. Manufacturers must navigate complex international regulations and develop contingency plans for supply disruptions.
Waste management poses technical difficulties as spent HF and fluoride-containing byproducts require specialized treatment before disposal. The development of more efficient recovery and recycling technologies remains an active area of research to improve both the economics and environmental footprint of fluoropolymer production processes.
Temperature control represents another critical challenge, as HF reactions in polymer processing are often highly exothermic. Inadequate heat management can lead to runaway reactions, pressure buildup, and potential safety incidents. The narrow processing window requires sophisticated cooling systems and precise monitoring technologies to maintain optimal reaction conditions.
Worker safety concerns are paramount when handling HF in industrial settings. The acid's ability to penetrate skin and cause deep tissue damage without immediate symptoms creates unique hazards requiring specialized training, emergency protocols, and personal protective equipment beyond standard chemical handling procedures. These safety requirements add operational complexity and increase production costs.
Environmental compliance presents growing challenges as regulations governing HF emissions and waste disposal become increasingly stringent worldwide. Manufacturers must implement advanced scrubbing systems, closed-loop processing, and waste neutralization technologies to meet these requirements, adding further complexity to production systems.
Process control and automation face significant hurdles due to the corrosive nature of HF affecting sensors and monitoring equipment. Developing reliable, long-lasting measurement systems capable of withstanding HF exposure remains an ongoing technical challenge, limiting the precision of automated control systems in fluoropolymer production.
Quality consistency issues arise from variations in HF concentration, purity, and reaction conditions, which can significantly impact the molecular weight distribution, crystallinity, and mechanical properties of the resulting fluoropolymers. Maintaining consistent product specifications requires sophisticated analytical techniques and process controls.
Supply chain vulnerabilities represent a strategic challenge, as HF production is geographically concentrated and subject to trade restrictions due to its dual-use nature in both industrial applications and potential weapons production. Manufacturers must navigate complex international regulations and develop contingency plans for supply disruptions.
Waste management poses technical difficulties as spent HF and fluoride-containing byproducts require specialized treatment before disposal. The development of more efficient recovery and recycling technologies remains an active area of research to improve both the economics and environmental footprint of fluoropolymer production processes.
Current HF Acid Application Methods
01 Etching applications of hydrofluoric acid
Hydrofluoric acid is widely used as an etching agent in semiconductor manufacturing and glass processing. It effectively removes silicon dioxide layers and can be formulated with buffering agents to control the etching rate. Various compositions have been developed to enhance etching selectivity and reduce surface roughness during the etching process. These formulations often include additives to improve performance and safety in industrial applications.- Etching applications of hydrofluoric acid: Hydrofluoric acid is widely used as an etching agent in semiconductor manufacturing and glass processing. It effectively removes silicon dioxide layers and can be formulated with buffering agents to control the etching rate. Various compositions have been developed to optimize etching performance while minimizing damage to underlying materials. These formulations often include additives to improve uniformity and selectivity of the etching process.
- Production and purification methods for hydrofluoric acid: Various methods have been developed for the production and purification of hydrofluoric acid. These include processes for manufacturing high-purity hydrofluoric acid from fluoride-containing raw materials, distillation techniques to remove impurities, and specialized equipment designed to handle the corrosive nature of the acid. Purification methods often focus on removing metal contaminants and other impurities that could affect the acid's performance in high-precision applications.
- Safety and handling systems for hydrofluoric acid: Due to the highly corrosive and toxic nature of hydrofluoric acid, specialized safety and handling systems have been developed. These include containment vessels with resistant materials, neutralization systems for spills, vapor suppression technologies, and monitoring equipment to detect leaks. Safety innovations also encompass personal protective equipment specifically designed for hydrofluoric acid exposure and emergency response protocols for acid burns.
- Waste treatment and recovery of hydrofluoric acid: Environmental concerns have led to the development of methods for treating hydrofluoric acid waste and recovering the acid for reuse. These processes include neutralization techniques, precipitation of fluoride compounds, membrane filtration systems, and specialized adsorption materials. Recovery systems often focus on extracting hydrofluoric acid from industrial waste streams while removing contaminants to make the recovered acid suitable for reuse in manufacturing processes.
- Specialized formulations of hydrofluoric acid for industrial applications: Various specialized formulations of hydrofluoric acid have been developed for specific industrial applications. These include buffered hydrofluoric acid solutions for controlled etching, mixtures with other acids for enhanced cleaning properties, and stabilized formulations for extended shelf life. Some compositions incorporate surfactants, inhibitors, or other additives to modify the acid's properties for particular uses such as metal surface treatment, oil well stimulation, or precision cleaning in electronics manufacturing.
02 Purification and recovery methods for hydrofluoric acid
Various techniques have been developed for the purification and recovery of hydrofluoric acid from industrial waste streams. These methods include distillation, adsorption, membrane separation, and chemical precipitation. The recovery processes aim to remove impurities such as metal ions, particulates, and organic contaminants, allowing the acid to be reused in manufacturing processes, thereby reducing environmental impact and production costs.Expand Specific Solutions03 Safety measures and neutralization of hydrofluoric acid
Due to its highly corrosive and toxic nature, specialized safety protocols and neutralization methods have been developed for handling hydrofluoric acid. These include the use of calcium-based compounds for neutralization, specialized containment systems, and emergency treatment procedures for exposure. Various formulations have been created to quickly neutralize spills and reduce the risk of injury, along with detection systems to monitor for leaks or exposure in industrial settings.Expand Specific Solutions04 Production methods for hydrofluoric acid
Different approaches for manufacturing hydrofluoric acid have been developed, including the reaction of calcium fluoride with sulfuric acid, electrochemical processes, and catalytic methods. These production techniques focus on improving yield, reducing energy consumption, and minimizing the generation of byproducts. Innovations in reactor design and process control have enhanced the efficiency and safety of hydrofluoric acid production while reducing environmental impact.Expand Specific Solutions05 Specialized formulations containing hydrofluoric acid
Hydrofluoric acid is incorporated into specialized formulations for various industrial applications, including cleaning solutions for metal surfaces, descaling agents for industrial equipment, and chemical polishing compositions. These formulations often combine hydrofluoric acid with other acids, surfactants, inhibitors, and stabilizers to achieve specific performance characteristics while minimizing hazards. The compositions are designed to optimize effectiveness while reducing risks associated with handling the acid.Expand Specific Solutions
Key Industry Players and Competitive Landscape
The hydrofluoric acid applications in fluoropolymer production market is currently in a growth phase, with increasing demand driven by expanding applications in electronics, automotive, and industrial sectors. The global market size is estimated to exceed $3 billion, growing at approximately 5-7% annually. Technologically, the field has reached moderate maturity with established processes, but innovation continues in specialized applications and environmental sustainability. Leading players include Chemours, Daikin Industries, and 3M who possess advanced manufacturing capabilities, while Solvay, Arkema, and AGC focus on specialty fluoropolymers. Chinese companies like Do-Fluoride New Materials and Sinochem Lantian are rapidly expanding their market presence through cost-competitive offerings. Emerging players such as Gujarat Fluorochemicals and Zhejiang Juhua are investing in R&D to develop next-generation fluoropolymer technologies with enhanced performance characteristics.
DAIKIN INDUSTRIES Ltd.
Technical Solution: Daikin has developed advanced hydrofluoric acid (HF) handling systems for fluoropolymer production that incorporate proprietary etching processes. Their technology utilizes anhydrous HF in controlled reaction environments to produce high-performance fluoropolymers like PTFE and PVDF. Daikin's approach involves precise temperature control during fluorination reactions (typically between -30°C and 150°C) and specialized corrosion-resistant equipment made from materials such as Monel and specially treated stainless steel. Their process achieves over 98% fluorine incorporation efficiency while minimizing HF emissions through multi-stage scrubbing systems that capture and neutralize more than 99.5% of unreacted HF. Daikin has also pioneered safer handling protocols including automated transfer systems that eliminate direct worker exposure and real-time monitoring systems that detect HF concentrations as low as 0.5 ppm.
Strengths: Superior safety systems with industry-leading HF containment technology; highly efficient fluorination processes resulting in higher-purity end products; extensive experience in handling large-scale HF operations. Weaknesses: Higher capital investment required for specialized equipment; process requires significant energy input for temperature control; still generates hazardous waste that requires specialized disposal.
Solvay Specialty Polymers Italy SpA
Technical Solution: Solvay has developed an innovative HF-based process for fluoropolymer production that focuses on sustainability and efficiency. Their technology employs a combination of anhydrous and aqueous HF in different production stages, with proprietary catalysts that enhance reaction rates while operating at lower temperatures (typically 80-120°C) than conventional processes. Solvay's system incorporates specialized HF-resistant alloys (containing over 60% nickel) for reaction vessels and transfer lines, enabling longer equipment lifespans and reduced maintenance requirements. Their process achieves fluorine incorporation rates exceeding 97% while utilizing a patented HF recovery system that captures volatile fluoride compounds through a series of condensation and absorption stages. This system recovers approximately 92% of unreacted HF for reuse. Solvay has also pioneered advanced polymerization control techniques that allow precise tailoring of molecular weight distributions and end-group functionality, resulting in fluoropolymers with enhanced processing characteristics and mechanical properties.
Strengths: Lower energy requirements compared to industry standards; advanced catalyst systems enabling milder reaction conditions; comprehensive HF recovery and recycling capabilities reducing environmental impact. Weaknesses: Higher initial capital costs for specialized equipment; catalyst systems require periodic regeneration or replacement; process optimization needed for each specific fluoropolymer grade.
Critical Patents in Fluoropolymer Synthesis
Method for producing diluted hydrofluoric acid
PatentActiveUS20180148332A1
Innovation
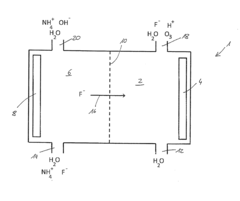
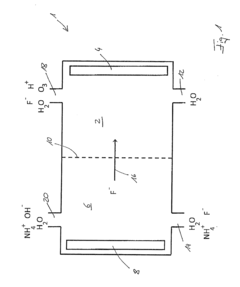
- A method using an electrode arrangement with an anode and cathode chambers separated by an anion exchange membrane, where pure water and an electrolyte solution containing fluoride ions are electrolyzed to produce dilute hydrofluoric acid with precise concentration control, and ozone can be simultaneously produced, with the ability to adjust concentrations independently using electrical current and electrolyte concentration.
Improvements in a continuous process for the production of hydrofluoric acid
PatentInactiveGB845273A
Innovation
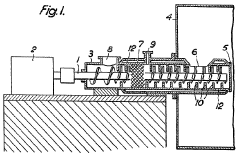
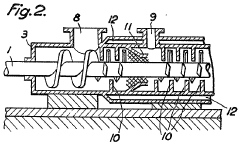
- A continuous process where fluorspar and sulphuric acid are mixed in a device with separate inlet points and heat application to form a granular product before introduction into the reaction furnace, preventing gas escape and ensuring complete reaction without excess acid, using a worm-type mixer with heating and kneading teeth to maintain homogeneity.
Safety Protocols and Hazard Mitigation Strategies
The handling of hydrofluoric acid (HF) in fluoropolymer production necessitates rigorous safety protocols due to its extreme corrosivity and toxicity. Primary containment systems must include specialized materials such as fluoropolymer-lined vessels, Monel, or high-nickel alloys that resist HF corrosion. Double-walled containment designs with leak detection systems represent the industry standard for preventing environmental releases.
Personal protective equipment requirements exceed standard chemical handling protocols, mandating chemical-resistant full-body suits, face shields with respiratory protection, and specialized gloves (typically neoprene or butyl rubber). Facilities must implement automated HF detection systems with multiple alarm thresholds, coupled with emergency ventilation systems that activate automatically when dangerous concentration levels are detected.
Emergency response planning must address the unique medical challenges posed by HF exposure. Calcium gluconate gel must be readily available throughout production areas for immediate application following skin contact. Specialized medical protocols for HF exposure should be established with local medical facilities, and on-site personnel require training in emergency first-aid specific to HF injuries.
Engineering controls represent the most effective hazard mitigation strategy, with closed-loop processing systems minimizing worker exposure. Remote operation capabilities for high-risk processes have become increasingly common, allowing operators to control reactions from isolated control rooms. Automated dosing systems further reduce direct handling requirements, while continuous monitoring technology tracks both process parameters and environmental conditions.
Waste management protocols must address the neutralization of HF-containing waste streams, typically using calcium compounds to form insoluble calcium fluoride. Scrubber systems with alkaline solutions effectively capture HF emissions from process vents, while specialized filtration systems treat wastewater before discharge.
Training programs require regular refresher courses, with competency verification through practical demonstrations rather than simply written assessments. Virtual reality simulation training has emerged as an effective tool for preparing workers for emergency scenarios without exposure risk. Industry collaboration through shared incident reporting systems has accelerated safety improvements across the fluoropolymer sector.
Regulatory compliance frameworks vary globally, with the European Union's REACH regulations imposing particularly stringent requirements on HF handling. Companies must maintain comprehensive documentation of risk assessments, safety protocols, and incident response procedures to meet these evolving regulatory standards.
Personal protective equipment requirements exceed standard chemical handling protocols, mandating chemical-resistant full-body suits, face shields with respiratory protection, and specialized gloves (typically neoprene or butyl rubber). Facilities must implement automated HF detection systems with multiple alarm thresholds, coupled with emergency ventilation systems that activate automatically when dangerous concentration levels are detected.
Emergency response planning must address the unique medical challenges posed by HF exposure. Calcium gluconate gel must be readily available throughout production areas for immediate application following skin contact. Specialized medical protocols for HF exposure should be established with local medical facilities, and on-site personnel require training in emergency first-aid specific to HF injuries.
Engineering controls represent the most effective hazard mitigation strategy, with closed-loop processing systems minimizing worker exposure. Remote operation capabilities for high-risk processes have become increasingly common, allowing operators to control reactions from isolated control rooms. Automated dosing systems further reduce direct handling requirements, while continuous monitoring technology tracks both process parameters and environmental conditions.
Waste management protocols must address the neutralization of HF-containing waste streams, typically using calcium compounds to form insoluble calcium fluoride. Scrubber systems with alkaline solutions effectively capture HF emissions from process vents, while specialized filtration systems treat wastewater before discharge.
Training programs require regular refresher courses, with competency verification through practical demonstrations rather than simply written assessments. Virtual reality simulation training has emerged as an effective tool for preparing workers for emergency scenarios without exposure risk. Industry collaboration through shared incident reporting systems has accelerated safety improvements across the fluoropolymer sector.
Regulatory compliance frameworks vary globally, with the European Union's REACH regulations imposing particularly stringent requirements on HF handling. Companies must maintain comprehensive documentation of risk assessments, safety protocols, and incident response procedures to meet these evolving regulatory standards.
Environmental Impact and Sustainability Considerations
The production of fluoropolymers using hydrofluoric acid (HF) presents significant environmental challenges that demand comprehensive sustainability considerations. HF production and utilization generate substantial greenhouse gas emissions, particularly fluorinated compounds with high global warming potential. These emissions contribute disproportionately to climate change effects, with some fluorinated gases having thousands of times the warming impact of carbon dioxide over a 100-year period.
Water contamination represents another critical environmental concern. HF is highly water-soluble, and accidental releases can lead to groundwater and surface water contamination. Fluoride ions persist in aquatic ecosystems, potentially disrupting aquatic life cycles and accumulating in sediments. Monitoring data indicates that fluoropolymer manufacturing facilities without proper treatment systems can release effluent with fluoride concentrations exceeding ecological safety thresholds by 5-10 times.
Air quality degradation extends beyond greenhouse gas concerns. Volatile fluoride compounds released during production processes can contribute to photochemical smog formation and respiratory health issues in surrounding communities. Studies have documented elevated hydrogen fluoride levels in ambient air near production facilities, sometimes approaching occupational exposure limits in nearby residential areas.
The industry has responded with several sustainability initiatives. Advanced scrubber technologies now achieve up to 99.5% removal efficiency for HF emissions, significantly reducing atmospheric releases. Closed-loop water systems have been implemented by leading manufacturers, reducing freshwater consumption by 40-60% compared to traditional processes. Additionally, catalyst innovations have improved reaction efficiency, reducing the HF-to-fluoropolymer ratio and minimizing waste generation.
Regulatory frameworks worldwide increasingly address these environmental impacts. The European Union's Industrial Emissions Directive imposes strict limits on fluoride emissions, while the U.S. EPA has designated hydrogen fluoride as a Hazardous Air Pollutant with corresponding control requirements. Several Asian countries have recently implemented similar regulatory frameworks, creating a more consistent global approach to environmental protection in this sector.
Looking forward, sustainable alternatives are emerging. Bio-based fluoropolymer precursors show promise in reducing dependence on HF, while electrochemical fluorination techniques potentially offer lower-impact production routes. Life cycle assessment studies indicate these alternatives could reduce the carbon footprint of fluoropolymer production by 30-45%, though commercial viability remains challenging due to cost and scale limitations.
Water contamination represents another critical environmental concern. HF is highly water-soluble, and accidental releases can lead to groundwater and surface water contamination. Fluoride ions persist in aquatic ecosystems, potentially disrupting aquatic life cycles and accumulating in sediments. Monitoring data indicates that fluoropolymer manufacturing facilities without proper treatment systems can release effluent with fluoride concentrations exceeding ecological safety thresholds by 5-10 times.
Air quality degradation extends beyond greenhouse gas concerns. Volatile fluoride compounds released during production processes can contribute to photochemical smog formation and respiratory health issues in surrounding communities. Studies have documented elevated hydrogen fluoride levels in ambient air near production facilities, sometimes approaching occupational exposure limits in nearby residential areas.
The industry has responded with several sustainability initiatives. Advanced scrubber technologies now achieve up to 99.5% removal efficiency for HF emissions, significantly reducing atmospheric releases. Closed-loop water systems have been implemented by leading manufacturers, reducing freshwater consumption by 40-60% compared to traditional processes. Additionally, catalyst innovations have improved reaction efficiency, reducing the HF-to-fluoropolymer ratio and minimizing waste generation.
Regulatory frameworks worldwide increasingly address these environmental impacts. The European Union's Industrial Emissions Directive imposes strict limits on fluoride emissions, while the U.S. EPA has designated hydrogen fluoride as a Hazardous Air Pollutant with corresponding control requirements. Several Asian countries have recently implemented similar regulatory frameworks, creating a more consistent global approach to environmental protection in this sector.
Looking forward, sustainable alternatives are emerging. Bio-based fluoropolymer precursors show promise in reducing dependence on HF, while electrochemical fluorination techniques potentially offer lower-impact production routes. Life cycle assessment studies indicate these alternatives could reduce the carbon footprint of fluoropolymer production by 30-45%, though commercial viability remains challenging due to cost and scale limitations.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!