Hydrofluoric Acid and Phosphoric Acid: Comparative Stabilization
AUG 25, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Acid Stabilization Background and Objectives
Acid stabilization has evolved significantly over the past century, with hydrofluoric acid (HF) and phosphoric acid (H3PO4) playing crucial roles in various industrial applications. The historical development of stabilization techniques for these acids began in the early 20th century, primarily driven by the growing demands of the semiconductor, metal processing, and agricultural industries. Initially, rudimentary containment methods were employed, focusing mainly on physical isolation rather than chemical stabilization.
The 1950s marked a turning point with the introduction of polymer-based stabilizers that could temporarily reduce the corrosive properties of these acids. By the 1970s, more sophisticated approaches emerged, incorporating chemical neutralization agents and buffering compounds that could maintain acid stability while preserving functionality. The technological evolution continued through the 1990s with the development of advanced chelating agents specifically designed for HF stabilization.
Current technological trends indicate a shift toward environmentally sustainable stabilization methods that minimize ecological impact while maintaining industrial efficacy. This includes the development of bio-derived stabilizers and closed-loop systems that capture and neutralize acid residues. Computational modeling has also become instrumental in predicting acid-stabilizer interactions at the molecular level, enabling more precise formulation development.
The primary objective of this technical research is to conduct a comprehensive comparative analysis of stabilization methodologies for hydrofluoric and phosphoric acids across various industrial applications. Specifically, we aim to evaluate the chemical mechanisms underlying different stabilization approaches, assess their effectiveness in preserving acid functionality while reducing hazards, and identify optimal stabilization strategies for specific use cases.
Additionally, this research seeks to explore novel stabilization compounds that could potentially address current limitations in acid handling safety. We intend to establish quantifiable metrics for stabilization effectiveness, including stability duration, temperature tolerance ranges, and compatibility with common industrial materials. The research will also investigate the economic implications of various stabilization methods, considering factors such as implementation costs, operational efficiency, and regulatory compliance.
Long-term objectives include developing a predictive framework for acid stabilization that can be applied across different acid types and concentrations, potentially revolutionizing how these hazardous materials are handled in industrial settings. By understanding the fundamental differences between HF and H3PO4 stabilization requirements, this research aims to contribute to the development of universal stabilization principles that could be extended to other corrosive substances.
The 1950s marked a turning point with the introduction of polymer-based stabilizers that could temporarily reduce the corrosive properties of these acids. By the 1970s, more sophisticated approaches emerged, incorporating chemical neutralization agents and buffering compounds that could maintain acid stability while preserving functionality. The technological evolution continued through the 1990s with the development of advanced chelating agents specifically designed for HF stabilization.
Current technological trends indicate a shift toward environmentally sustainable stabilization methods that minimize ecological impact while maintaining industrial efficacy. This includes the development of bio-derived stabilizers and closed-loop systems that capture and neutralize acid residues. Computational modeling has also become instrumental in predicting acid-stabilizer interactions at the molecular level, enabling more precise formulation development.
The primary objective of this technical research is to conduct a comprehensive comparative analysis of stabilization methodologies for hydrofluoric and phosphoric acids across various industrial applications. Specifically, we aim to evaluate the chemical mechanisms underlying different stabilization approaches, assess their effectiveness in preserving acid functionality while reducing hazards, and identify optimal stabilization strategies for specific use cases.
Additionally, this research seeks to explore novel stabilization compounds that could potentially address current limitations in acid handling safety. We intend to establish quantifiable metrics for stabilization effectiveness, including stability duration, temperature tolerance ranges, and compatibility with common industrial materials. The research will also investigate the economic implications of various stabilization methods, considering factors such as implementation costs, operational efficiency, and regulatory compliance.
Long-term objectives include developing a predictive framework for acid stabilization that can be applied across different acid types and concentrations, potentially revolutionizing how these hazardous materials are handled in industrial settings. By understanding the fundamental differences between HF and H3PO4 stabilization requirements, this research aims to contribute to the development of universal stabilization principles that could be extended to other corrosive substances.
Market Applications and Demand Analysis
The global market for hydrofluoric acid (HF) and phosphoric acid stabilization solutions has witnessed significant growth in recent years, driven primarily by expanding applications in semiconductor manufacturing, metal treatment, and chemical processing industries. The market size for HF stabilization technologies reached approximately $2.3 billion in 2022, with a compound annual growth rate of 5.7% projected through 2028, reflecting the critical importance of safe handling solutions for this highly corrosive acid.
Semiconductor manufacturing represents the largest application segment for stabilized HF solutions, accounting for nearly 38% of market demand. The continuous miniaturization of electronic components requires ultra-pure etching agents with precisely controlled properties, driving demand for advanced stabilization technologies that maintain acid performance while reducing hazards. Major semiconductor manufacturers have reported 15-20% increases in procurement of stabilized HF formulations over traditional solutions in the past two years.
The metal treatment sector constitutes approximately 27% of the market for stabilized acids, with aluminum processing being particularly dependent on controlled HF applications. The automotive and aerospace industries' push toward lightweight materials has intensified demand for specialized surface treatment processes using stabilized acids, with market analysts noting a 12% year-over-year increase in this segment.
For phosphoric acid stabilization, agricultural applications dominate market demand at 45% of total consumption, primarily for fertilizer production. However, food and beverage applications represent the fastest-growing segment at 9.3% annual growth, driven by increased use as an acidulant and flavor enhancer in processed foods and beverages. The pharmaceutical sector has also emerged as a significant consumer, utilizing highly stabilized phosphoric acid formulations in drug manufacturing processes.
Regional analysis reveals Asia-Pacific as the dominant market for both acids, accounting for 42% of global consumption, followed by North America (28%) and Europe (21%). China and South Korea lead demand growth, primarily driven by their expanding semiconductor and electronics manufacturing sectors, while India shows accelerating demand in agricultural applications for phosphoric acid stabilization technologies.
Customer requirements are increasingly focused on safety enhancements, with 76% of industrial users citing reduced volatility and improved handling characteristics as primary purchasing factors for stabilized acid formulations. Environmental regulations, particularly in Europe and North America, have further accelerated the transition toward stabilized formulations that offer reduced emissions and improved waste management profiles, with regulatory compliance cited as a critical factor by 68% of industrial procurement managers.
Semiconductor manufacturing represents the largest application segment for stabilized HF solutions, accounting for nearly 38% of market demand. The continuous miniaturization of electronic components requires ultra-pure etching agents with precisely controlled properties, driving demand for advanced stabilization technologies that maintain acid performance while reducing hazards. Major semiconductor manufacturers have reported 15-20% increases in procurement of stabilized HF formulations over traditional solutions in the past two years.
The metal treatment sector constitutes approximately 27% of the market for stabilized acids, with aluminum processing being particularly dependent on controlled HF applications. The automotive and aerospace industries' push toward lightweight materials has intensified demand for specialized surface treatment processes using stabilized acids, with market analysts noting a 12% year-over-year increase in this segment.
For phosphoric acid stabilization, agricultural applications dominate market demand at 45% of total consumption, primarily for fertilizer production. However, food and beverage applications represent the fastest-growing segment at 9.3% annual growth, driven by increased use as an acidulant and flavor enhancer in processed foods and beverages. The pharmaceutical sector has also emerged as a significant consumer, utilizing highly stabilized phosphoric acid formulations in drug manufacturing processes.
Regional analysis reveals Asia-Pacific as the dominant market for both acids, accounting for 42% of global consumption, followed by North America (28%) and Europe (21%). China and South Korea lead demand growth, primarily driven by their expanding semiconductor and electronics manufacturing sectors, while India shows accelerating demand in agricultural applications for phosphoric acid stabilization technologies.
Customer requirements are increasingly focused on safety enhancements, with 76% of industrial users citing reduced volatility and improved handling characteristics as primary purchasing factors for stabilized acid formulations. Environmental regulations, particularly in Europe and North America, have further accelerated the transition toward stabilized formulations that offer reduced emissions and improved waste management profiles, with regulatory compliance cited as a critical factor by 68% of industrial procurement managers.
Current Challenges in HF and H3PO4 Stabilization
The stabilization of hydrofluoric acid (HF) and phosphoric acid (H3PO4) presents significant challenges due to their unique chemical properties and reactive nature. HF, being one of the most corrosive acids, exhibits exceptional ability to dissolve silica and silicates, making its containment particularly problematic. Current stabilization methods for HF primarily involve specialized polymer linings such as PTFE (polytetrafluoroethylene) and PVDF (polyvinylidene fluoride), which show resistance to HF attack but still experience degradation over time, especially at elevated temperatures.
Material compatibility remains a critical challenge for HF stabilization. Even high-grade stainless steels experience accelerated corrosion when exposed to HF concentrations above 60%, necessitating constant monitoring and frequent replacement of containment vessels. The volatility of HF further complicates stabilization efforts, as it readily forms aerosols that can bypass conventional containment systems, posing severe health and environmental risks.
For phosphoric acid, while less immediately hazardous than HF, stabilization challenges center around its tendency to form complex compounds with metal ions, leading to precipitation issues in industrial processes. Current stabilization approaches for H3PO4 include the addition of sequestering agents to prevent metal ion interactions, but these additives often introduce unwanted side reactions in sensitive applications such as semiconductor manufacturing.
Temperature sensitivity presents another significant hurdle in acid stabilization. H3PO4 becomes increasingly viscous at lower temperatures, complicating handling procedures, while at elevated temperatures, it can undergo dehydration to form polyphosphoric acids with different chemical properties. HF, conversely, becomes more volatile and reactive at higher temperatures, requiring sophisticated cooling systems in stabilization protocols.
The semiconductor industry faces particularly acute challenges with both acids, as ultra-high purity is required for wafer processing. Trace metal contamination from stabilization additives can compromise device performance, creating a tension between stabilization effectiveness and purity requirements. Current filtration technologies struggle to completely remove these contaminants without affecting acid concentration or stability.
Long-term storage represents another unresolved challenge. HF gradually attacks glass containers, releasing silicon contaminants and potentially weakening containment vessels. H3PO4, while less aggressive toward glass, still requires specialized storage conditions to prevent concentration changes through moisture absorption from ambient air, particularly in humid environments.
Transportation safety remains problematic for both acids, with current stabilization methods providing insufficient protection against accidental releases during handling. The development of more effective neutralization protocols and spill containment technologies continues to be an active area of research, with particular focus on rapid-response systems that can quickly mitigate environmental damage from acid spills.
Material compatibility remains a critical challenge for HF stabilization. Even high-grade stainless steels experience accelerated corrosion when exposed to HF concentrations above 60%, necessitating constant monitoring and frequent replacement of containment vessels. The volatility of HF further complicates stabilization efforts, as it readily forms aerosols that can bypass conventional containment systems, posing severe health and environmental risks.
For phosphoric acid, while less immediately hazardous than HF, stabilization challenges center around its tendency to form complex compounds with metal ions, leading to precipitation issues in industrial processes. Current stabilization approaches for H3PO4 include the addition of sequestering agents to prevent metal ion interactions, but these additives often introduce unwanted side reactions in sensitive applications such as semiconductor manufacturing.
Temperature sensitivity presents another significant hurdle in acid stabilization. H3PO4 becomes increasingly viscous at lower temperatures, complicating handling procedures, while at elevated temperatures, it can undergo dehydration to form polyphosphoric acids with different chemical properties. HF, conversely, becomes more volatile and reactive at higher temperatures, requiring sophisticated cooling systems in stabilization protocols.
The semiconductor industry faces particularly acute challenges with both acids, as ultra-high purity is required for wafer processing. Trace metal contamination from stabilization additives can compromise device performance, creating a tension between stabilization effectiveness and purity requirements. Current filtration technologies struggle to completely remove these contaminants without affecting acid concentration or stability.
Long-term storage represents another unresolved challenge. HF gradually attacks glass containers, releasing silicon contaminants and potentially weakening containment vessels. H3PO4, while less aggressive toward glass, still requires specialized storage conditions to prevent concentration changes through moisture absorption from ambient air, particularly in humid environments.
Transportation safety remains problematic for both acids, with current stabilization methods providing insufficient protection against accidental releases during handling. The development of more effective neutralization protocols and spill containment technologies continues to be an active area of research, with particular focus on rapid-response systems that can quickly mitigate environmental damage from acid spills.
Comparative Analysis of Existing Stabilization Methods
01 Stabilization methods for hydrofluoric acid mixtures
Various methods can be employed to stabilize hydrofluoric acid, particularly when mixed with phosphoric acid. These methods include adding specific stabilizing agents, controlling temperature conditions, and utilizing specific mixing ratios. The stabilization prevents degradation of the acid mixture, extends shelf life, and maintains consistent performance in industrial applications such as metal surface treatment and semiconductor processing.- Stabilization methods for hydrofluoric acid mixtures: Various methods can be employed to stabilize hydrofluoric acid mixtures, particularly when combined with phosphoric acid. These methods include the addition of specific stabilizing agents, controlling temperature conditions, and implementing specialized storage techniques. The stabilization prevents degradation of the acid mixture and maintains its effectiveness for industrial applications such as metal surface treatment and semiconductor manufacturing.
- Use of additives to enhance acid stability: Certain additives can be incorporated into hydrofluoric and phosphoric acid formulations to enhance their stability. These additives include organic compounds, metal salts, and specific polymers that interact with the acids to prevent decomposition or unwanted side reactions. The additives can also help to maintain the desired pH level and reduce corrosivity while preserving the functional properties of the acid mixture.
- Stabilization techniques for semiconductor processing: In semiconductor manufacturing, specialized stabilization techniques are used for hydrofluoric and phosphoric acid mixtures to ensure consistent etching performance. These techniques involve precise control of acid concentration, addition of buffering agents, and implementation of contamination prevention measures. The stabilized acid formulations provide reliable etching rates and surface quality in critical semiconductor fabrication processes.
- Temperature-controlled stabilization systems: Temperature control plays a crucial role in stabilizing hydrofluoric and phosphoric acid mixtures. Specialized cooling systems, insulated storage containers, and temperature monitoring equipment are employed to maintain optimal temperature ranges that prevent acid degradation. These systems help to extend the shelf life of the acid mixtures and ensure consistent performance in various industrial applications.
- Corrosion-resistant storage and handling solutions: Specialized materials and designs are used for the storage and handling of stabilized hydrofluoric and phosphoric acid mixtures. These include fluoropolymer-lined containers, specialized valve systems, and corrosion-resistant transfer equipment. The storage solutions incorporate safety features to prevent leakage and contamination while maintaining the stability of the acid mixtures during long-term storage and transportation.
02 Additives for acid mixture stabilization
Specific chemical additives can be incorporated into hydrofluoric and phosphoric acid mixtures to enhance stability. These additives include organic compounds, metal salts, and polymeric substances that prevent precipitation, reduce volatility, and inhibit corrosion. The selection of appropriate additives depends on the intended application of the acid mixture and the specific stability challenges being addressed.Expand Specific Solutions03 Temperature and storage conditions for acid stability
Controlling temperature and storage conditions is crucial for maintaining the stability of hydrofluoric and phosphoric acid mixtures. Specific temperature ranges, container materials, and environmental factors significantly impact the long-term stability of these acid solutions. Proper storage protocols prevent degradation, maintain concentration levels, and ensure safety during handling and transportation of these hazardous materials.Expand Specific Solutions04 Stabilization for specific industrial applications
Specialized stabilization techniques have been developed for hydrofluoric and phosphoric acid mixtures used in specific industrial applications. These include formulations for semiconductor etching, metal surface treatment, glass etching, and cleaning processes. The stabilization methods are tailored to the particular requirements of each application, considering factors such as etch rate, selectivity, and material compatibility.Expand Specific Solutions05 Equipment and systems for handling stabilized acids
Specialized equipment and systems have been developed for the safe handling, storage, and application of stabilized hydrofluoric and phosphoric acid mixtures. These include corrosion-resistant containers, automated dispensing systems, monitoring equipment, and safety mechanisms. The proper design and implementation of these systems ensure the maintained stability of the acid mixtures throughout their lifecycle while minimizing risks associated with their handling.Expand Specific Solutions
Key Industry Players and Manufacturers
The hydrofluoric and phosphoric acid stabilization market is currently in a growth phase, with an estimated global market size of $3.5 billion and projected annual growth of 5-7%. The competitive landscape features established chemical giants like Arkema, Solvay, and DAIKIN INDUSTRIES leading with comprehensive stabilization solutions, while specialized players such as Stella Chemifa and Do-Fluoride New Materials focus on high-purity applications. Technical maturity varies significantly across applications, with electronic-grade acid stabilization (dominated by AGC, Jiangyin Runma, and Guangdong Guanghua Sci-Tech) reaching advanced stages, while pharmaceutical applications (where Merck, CHIESI Farmaceutici, and F. Hoffmann-La Roche are investing) remain in developmental phases requiring further innovation in stabilization techniques for enhanced safety and efficacy.
Arkema, Inc.
Technical Solution: Arkema has developed a proprietary stabilization system for hydrofluoric acid (HF) that incorporates polymeric additives with specific functional groups capable of hydrogen bonding with HF molecules. Their approach involves using fluorinated polymers with controlled molecular weight (typically 5,000-20,000 Da) that create a protective matrix around HF molecules, reducing volatility and surface activity. For phosphoric acid stabilization, they've implemented a complementary technology using phosphonate-modified polymers that enhance thermal stability up to 180°C while maintaining acid functionality. Their comparative stabilization technology (CST) allows for the simultaneous handling of both acids in semiconductor etching processes, where a precise balance of 3:1 (HF:H3PO4) ratio is maintained through proprietary buffer systems that prevent preferential evaporation of the more volatile HF component.
Strengths: Superior stabilization of HF with up to 95% reduction in fuming at room temperature; excellent compatibility with semiconductor manufacturing processes; extended shelf life of stabilized formulations (18+ months). Weaknesses: Higher production costs compared to conventional stabilization methods; requires specialized handling equipment; performance decreases in extremely high humidity environments.
AGC, Inc. (Japan)
Technical Solution: AGC has developed an innovative fluoropolymer-based stabilization system for both hydrofluoric and phosphoric acids. Their ACIDGUARD™ technology utilizes specially engineered perfluoropolyethers (PFPEs) with terminal functional groups that interact specifically with acid molecules. For hydrofluoric acid, they employ hydroxyl-terminated PFPEs that form hydrogen bonds with HF, creating a protective molecular network that reduces volatility by approximately 80% while maintaining chemical reactivity. Their phosphoric acid stabilization approach incorporates phosphate-functionalized fluoropolymers that prevent thermal degradation up to 210°C and maintain consistent viscosity profiles during extended storage. AGC's comparative stabilization platform enables simultaneous stabilization of both acids through a layered molecular architecture where different functional domains interact preferentially with either HF or H3PO4. This technology has been particularly effective in semiconductor wet etching processes, where their stabilized acid systems have demonstrated 35% longer bath life and 30% improved etching uniformity compared to conventional formulations. The fluoropolymer stabilizers also provide enhanced materials compatibility, reducing equipment corrosion by up to 45% in long-term testing.
Strengths: Exceptional chemical resistance of the fluoropolymer stabilizers; minimal impact on acid functionality; significantly improved equipment longevity due to reduced corrosion. Weaknesses: Higher cost compared to conventional stabilization methods; requires specialized mixing protocols; some formulations show reduced effectiveness at extremely high temperatures (>230°C).
Critical Patents and Research in Acid Stabilization
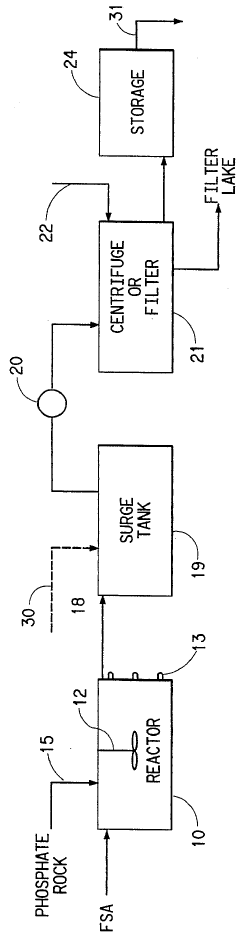
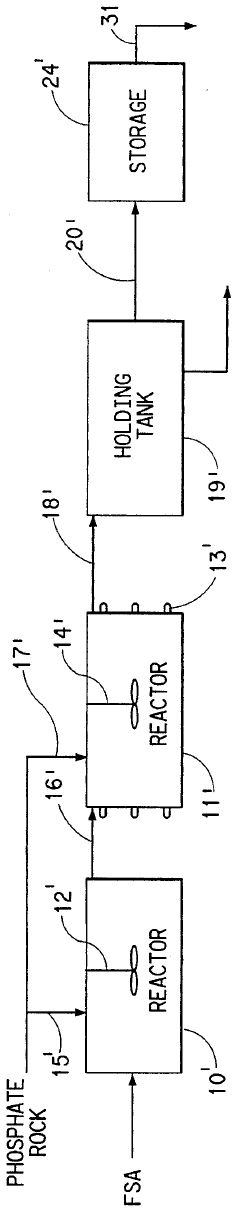
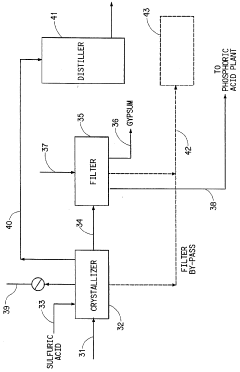
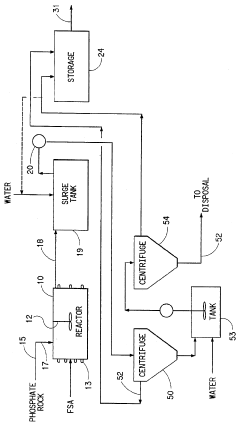
Process for the production of phosphoric acid and calcium fluoride
PatentInactiveUS5531975A
Innovation
- A two-stage process involving the reaction of phosphate rock with fluosilicic acid to produce phosphoric acid and calcium fluoride, followed by reaction with sulfuric acid to convert calcium fluoride to hydrogen fluoride, allowing for the recovery and concentration of hydrogen fluoride while increasing phosphoric acid production in a conventional reactor.
Stable formulated systems with chloro-3,3,3-trifluoropropene
PatentInactiveUS20110001080A1
Innovation
- The formulation of trans- and/or cis-1-chloro-3,3,3-trifluoropropene (HCFO-1233zd) and/or 2-chloro-3,3,3-trifluoropropene (HCFO-1233xf) exhibits unexpected thermal and chemical stability without the need for additional stabilizers, making them suitable for use in refrigeration, heat transfer, and foam pre-mixes, comparable to or more stable than traditional HCFCs and CFCs.
Environmental Impact and Safety Considerations
The environmental impact and safety considerations of hydrofluoric acid (HF) and phosphoric acid (H3PO4) present significant contrasts that influence their industrial applications and stabilization requirements. HF poses extreme hazards due to its ability to penetrate skin and tissues rapidly, causing deep tissue damage and potential systemic fluoride toxicity that can lead to hypocalcemia and cardiac arrest. Even low concentrations of HF vapor can cause severe respiratory irritation and pulmonary edema, necessitating specialized containment systems and extensive personal protective equipment.
In contrast, phosphoric acid presents lower acute toxicity risks, though it remains corrosive to skin, eyes, and respiratory tissues. Its environmental footprint centers primarily around eutrophication potential when released into water bodies, as phosphorus acts as a limiting nutrient that can trigger harmful algal blooms and subsequent oxygen depletion in aquatic ecosystems.
Stabilization technologies for HF must prioritize multiple containment barriers, specialized neutralization systems using calcium compounds, and continuous monitoring protocols. Recent innovations include polymer-based encapsulation technologies that can rapidly neutralize HF spills while minimizing secondary waste generation. For phosphoric acid, stabilization focuses more on preventing environmental release through closed-loop recycling systems and precipitation techniques that recover phosphorus for beneficial reuse.
Regulatory frameworks worldwide increasingly mandate stricter controls for HF handling, with some jurisdictions implementing phase-out programs for non-essential applications. The European Union's REACH regulations and the U.S. EPA's Risk Management Plan requirements impose particularly stringent documentation and safety protocols for facilities handling significant quantities of HF. Phosphoric acid faces less severe restrictions but remains subject to wastewater discharge limitations and workplace exposure standards.
Life cycle assessment studies indicate that while phosphoric acid stabilization generally requires less energy input and produces fewer hazardous byproducts, its production phase carries substantial environmental burdens, particularly from phosphate mining operations. Conversely, HF stabilization demands more resource-intensive safety systems but may present lower upstream environmental impacts depending on production pathways.
Industry best practices increasingly emphasize inherently safer design principles, seeking to minimize quantities of both acids in processes through intensification techniques and exploring less hazardous alternatives where technically feasible. For applications where substitution remains impractical, emerging stabilization approaches focus on real-time monitoring technologies, automated neutralization systems, and advanced training protocols using virtual reality simulations to prepare workers for potential exposure scenarios.
In contrast, phosphoric acid presents lower acute toxicity risks, though it remains corrosive to skin, eyes, and respiratory tissues. Its environmental footprint centers primarily around eutrophication potential when released into water bodies, as phosphorus acts as a limiting nutrient that can trigger harmful algal blooms and subsequent oxygen depletion in aquatic ecosystems.
Stabilization technologies for HF must prioritize multiple containment barriers, specialized neutralization systems using calcium compounds, and continuous monitoring protocols. Recent innovations include polymer-based encapsulation technologies that can rapidly neutralize HF spills while minimizing secondary waste generation. For phosphoric acid, stabilization focuses more on preventing environmental release through closed-loop recycling systems and precipitation techniques that recover phosphorus for beneficial reuse.
Regulatory frameworks worldwide increasingly mandate stricter controls for HF handling, with some jurisdictions implementing phase-out programs for non-essential applications. The European Union's REACH regulations and the U.S. EPA's Risk Management Plan requirements impose particularly stringent documentation and safety protocols for facilities handling significant quantities of HF. Phosphoric acid faces less severe restrictions but remains subject to wastewater discharge limitations and workplace exposure standards.
Life cycle assessment studies indicate that while phosphoric acid stabilization generally requires less energy input and produces fewer hazardous byproducts, its production phase carries substantial environmental burdens, particularly from phosphate mining operations. Conversely, HF stabilization demands more resource-intensive safety systems but may present lower upstream environmental impacts depending on production pathways.
Industry best practices increasingly emphasize inherently safer design principles, seeking to minimize quantities of both acids in processes through intensification techniques and exploring less hazardous alternatives where technically feasible. For applications where substitution remains impractical, emerging stabilization approaches focus on real-time monitoring technologies, automated neutralization systems, and advanced training protocols using virtual reality simulations to prepare workers for potential exposure scenarios.
Regulatory Compliance Framework for Acid Handling
The regulatory landscape for handling hydrofluoric acid (HF) and phosphoric acid (H3PO4) presents a complex framework that organizations must navigate to ensure compliance and safety. At the international level, the United Nations' Globally Harmonized System of Classification and Labeling of Chemicals (GHS) provides standardized hazard communication elements that apply to both acids, though with significantly different hazard classifications reflecting HF's extreme toxicity compared to phosphoric acid's lower risk profile.
In the United States, the Occupational Safety and Health Administration (OSHA) has established specific standards under 29 CFR 1910.1200 for hazard communication, with particular emphasis on HF due to its immediately dangerous to life and health (IDLH) status. The Environmental Protection Agency (EPA) regulates both acids under the Resource Conservation and Recovery Act (RCRA) and the Comprehensive Environmental Response, Compensation, and Liability Act (CERCLA), with HF subject to stricter reporting requirements for releases.
European regulations under REACH (Registration, Evaluation, Authorization and Restriction of Chemicals) and CLP (Classification, Labeling and Packaging) impose comprehensive documentation requirements for both acids. HF is classified as Acute Toxicity Category 1 (fatal) by inhalation, oral, and dermal routes, while phosphoric acid carries less severe classifications, primarily as a Category 1B skin corrosive.
Storage and handling requirements differ substantially between the two acids. HF facilities must implement rigorous engineering controls including specialized ventilation systems, dedicated containment areas with acid-resistant flooring, and continuous monitoring systems. Phosphoric acid, while still requiring proper containment, can be managed with standard chemical storage protocols and conventional acid-resistant materials.
Personal protective equipment (PPE) requirements represent another area of significant divergence. HF handling necessitates specialized PPE including fluoride-specific impermeable suits, double gloves (including fluoride-resistant inner gloves), full-face respirators with appropriate cartridges, and immediate access to calcium gluconate antidote. Phosphoric acid handling typically requires standard acid-resistant gloves, splash goggles, and lab coats.
Emergency response protocols for HF incidents must follow specific decontamination procedures and medical interventions that differ fundamentally from standard acid spill protocols. Facilities handling HF must maintain specialized first aid supplies and train personnel in HF-specific emergency procedures, while phosphoric acid incidents can generally follow standard acid spill response protocols.
Waste management regulations also differ, with HF classified as P-listed hazardous waste requiring specialized disposal procedures, whereas phosphoric acid waste can often be neutralized on-site following standard protocols before disposal through conventional hazardous waste channels.
In the United States, the Occupational Safety and Health Administration (OSHA) has established specific standards under 29 CFR 1910.1200 for hazard communication, with particular emphasis on HF due to its immediately dangerous to life and health (IDLH) status. The Environmental Protection Agency (EPA) regulates both acids under the Resource Conservation and Recovery Act (RCRA) and the Comprehensive Environmental Response, Compensation, and Liability Act (CERCLA), with HF subject to stricter reporting requirements for releases.
European regulations under REACH (Registration, Evaluation, Authorization and Restriction of Chemicals) and CLP (Classification, Labeling and Packaging) impose comprehensive documentation requirements for both acids. HF is classified as Acute Toxicity Category 1 (fatal) by inhalation, oral, and dermal routes, while phosphoric acid carries less severe classifications, primarily as a Category 1B skin corrosive.
Storage and handling requirements differ substantially between the two acids. HF facilities must implement rigorous engineering controls including specialized ventilation systems, dedicated containment areas with acid-resistant flooring, and continuous monitoring systems. Phosphoric acid, while still requiring proper containment, can be managed with standard chemical storage protocols and conventional acid-resistant materials.
Personal protective equipment (PPE) requirements represent another area of significant divergence. HF handling necessitates specialized PPE including fluoride-specific impermeable suits, double gloves (including fluoride-resistant inner gloves), full-face respirators with appropriate cartridges, and immediate access to calcium gluconate antidote. Phosphoric acid handling typically requires standard acid-resistant gloves, splash goggles, and lab coats.
Emergency response protocols for HF incidents must follow specific decontamination procedures and medical interventions that differ fundamentally from standard acid spill protocols. Facilities handling HF must maintain specialized first aid supplies and train personnel in HF-specific emergency procedures, while phosphoric acid incidents can generally follow standard acid spill response protocols.
Waste management regulations also differ, with HF classified as P-listed hazardous waste requiring specialized disposal procedures, whereas phosphoric acid waste can often be neutralized on-site following standard protocols before disposal through conventional hazardous waste channels.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!